Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
CT&F - Ciencia, Tecnología y Futuro
versão impressa ISSN 0122-5383versão On-line ISSN 2382-4581
C.T.F Cienc. Tecnol. Futuro v.4 n.2 Bucaramanga jul./dez. 2010
e-mail: marthajparra@gmail.com adan.leon@ecopetrol.com.co
(Received, Feb. 17, 2010; Accepted, Nov. 30, 2010)
* To whom correspondence may be addressed
ABSTRACT
The Instituto Colombiano del Petróleo (ICP), has implemented a methodology for separating vacuum residue fractions using the technique of supercritical fluid extraction at the pilot scale. The present study evaluates the efficiency of extraction of fractions of a typical vacuum residue in the Barrancabermeja refinery. The extraction test was carried out with n-hexane under supercritical conditions of temperature and pressure of the 265°C in the range from 450 to 1250 psi, respectively. Finally, each of the fractions were analyzed for their density, viscosity, sulfur content, Conradson Carbon Residue (CCR) content, SARA compositional analysis, and metals.
Key words: supercritical extraction, vacuum residues, physical properties.
RESUMEN
El Instituto Colombiano del Petróleo (ICP) ha implementando una metodología de separación de fracciones de fondos de vacío mediante la técnica de extracción con fluidos supercríticos a escala piloto. El presente estudio evalúa el rendimiento de extracción de fracciones de un fondo de vacío típico de la refinería de Barrancabermeja. La prueba de extracción se realizó con n-hexano bajo condiciones supercríticas de temperatura y presión de 265°C y 450 a 1250 psi, respectivamente. Finalmente, cada una de las fracciones se les determinó su densidad, viscosidad, contenido de azufre, residuo Carbón Conradson, análisis composicional SARA y metales.
Palabras Clave: extracción supercrítica, fondos de vacio, propiedades físicoquímicas.
1. INTRODUCTION
A supercritical fluid is defined as a pure component found in the region with temperature and pressure greater than or equal to the critical conditions, Tr ≥ 1,0, Pr ≥ 10, where liquid and vapor states are not differentiable. Supercritical fluids (SF) have properties similar to the liquid form (i.e. density) and properties similar to the gaseous form (i.e. diffusivity, viscosity, and zero surface tension). These properties vary with changes in temperature and pressure, which substantially affect the mass transfer, (Han, Yang, Ke, Mao, & Yan, 1998; Gallego & Cardona, 2004).
The solvent power of supercritical fluids depends on its density. These fluids are able to dissolve weakly volatile substances and easily penetrate solid matrices (respective properties of traditional liquid solvents and gases when they reach values close to those of a liquid under supercritical conditions. Thus, supercritical fluids are considered to be effective solvents.
The advantages of supercritical fluids compared with those of other solvents are: low power consumption, easy separation of the solvent and the extracted fraction, high selectivity, and low resistance to mass transfer. The supercritical extraction technique began in the XIX century with the research done by Hannay and Hogarth (1897) in the study of solubility of inorganic salts (KI, KBr, and CaCl2) in supercritical ethanol, methane, ethane and carbon dioxide, Hannay and Hogarth (1897). With the release in 1930 of vapor-liquid diagrams (VLE) of hydrocarbons at high pressures, it was possible to develop the first industrial application of solvent deasphalting of crude in 1943, (Messmore, 1943; Northup & Sloan, 1986).
The supercritical fluid extraction technique has impacted several sectors in industry since the 1970's and 1980's. Many authors have evaluated the effect of variations in temperature, pressure, time, flow and nature of the solvent in the extraction of natural essences, such as pesticides from solid waste and refined oils, (Gallego & Cardona, 2004 and Esquivel & Vargas, 2007).
Subramanian and Hanson (1998) proposed a method for supercritical fluid extraction in oil sands using propane as a solvent. Along the same lines, Masaka, Motonobu, & Tsutomu (1997) and Suoqui, Zhiming, Chunming, Keng, & Chung (2005) applied the extraction technique to waste oils, in order to get free fractions of asphaltene fractions using n-pentane as solvent. Sheng- Li, Sheng-Sheng, Yun-Hua, & Suo-Qi (1984); Tie-Pan, Yun-Xiang, Tong, & Ren-An (1997) and Guangua and Ren-An (1999) characterized the fractions obtained from heavy crude oil, and vacuum residue with Propane, Butane and Pentane in supercritical conditions. Swanson, Dollimore, & Diehl (1985) evaluated the conversion efficiency and extraction conditions of light fractions present in coal by supercritical extraction with solvents such as pentane, hexane, heptane, octane and cyclohexane.
With the steady depletion of reserves of light crude, refineries have been forced to rethink their processes with new feed mixtures, based on heavy crude oil with a projected in high increase atmospheric and vacuum residue. However, to adjust the refining process residue vacuum is essential to know their physicochemical properties, because this characterization allows to increase the efficiency of conversion processes.
To make a broad characterization and estimation of physicochemical properties such as density, viscosity and molecular weight among others, is not an easy task because to get significant amounts of these fractions involves a complicated task with traditional methods such as the SARA separation method.
Ecopetrol S.A. in the Instituto Colombiano del Petróleo (ICP), has built a pilot unit of supercritical fluid extraction and fractionation with two purposes: first, to extract significant quantities of a large number of fractions of a different nature from vacuum residue for further characterization. Second, deepening on the behavior of heavier petroleum fractions to improve and integrate new and future refining schemes.
Although there are a variety of works in the area of supercritical extraction solvent paraffinic from propane to pentane, this research was selected over other n-hexane solvent, due to the fact that the solubility in supercritical conditions on high molecular weight hydrocarbons tends to be higher, as indicated by Swanson et al. (1985).
The purpose of this study is to evaluate the methodology of supercritical extraction implemented by Ecopetrol S. A., in a vacuum residue typical refinery in Barrancabermeja using as solvent n-hexane under operating conditions of 265°C at pressure range between 550 and 1250 psi.
2. EXPERIMENTAL METHODOLOGY
Materials
Vacuum residue typical Barrancabermeja refinery, and n-hexane (density at 25°C = 0,655 g/ml, Pc = 436,9 psi, Tc = 234,7°C).
Description of the extraction process
The extraction unit consists of two high-pressure pumps for solvent and vacuum residue (2, 6), an extraction column (3), a pressure regulator (4), a container to decompress the solvent and collect the samples at each pressure condition (5), and a solvent drum to recover the solvent (1), as shown in Figure 1.
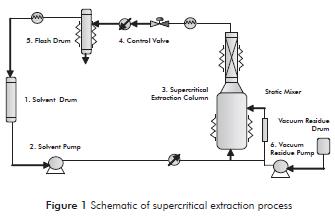
The process begins by heating and transporting a quantity of vacuum residue (900 grams) to the supercritical extraction column (3) with the appropriate pump (6). After reaching the operating conditions of temperature and pressure defined through electrical resistance and pressure regulator for regulating the pressure drop (4), begins the extraction of fractions of vacuum residue are carried by the solvent and led to flash drum (5).
The fraction accumulates in the flash drum for subsequent removal. The solvent is released by decompression and is recovered by condensation using a heat exchanger. The recovered solvent is stored again in the solvent drum (1) and restart the process of recirculation. Throughout the process there is a continuous solvent renewal and recovery.
After extracting for approximately 90 minutes, other operating conditions are adjusted and the extraction procedure is repeated. The extraction process ends when the last set pressure value is reached or until securing significant traces of fractions in the flash drum is no longer possible. Finally, the supercritical extraction column is depressurized and cooled gently to drain the residue contents and conduct the respective mass balance.
The above work was conducted in two stages. Initially, the methodology of supercritical extraction fractionation with n-hexane (operating conditions: 265°C and 450 to 1250 psi) was evaluated. Secondly, a characterization of the fractions was made.
The fractions obtained in the flash drum were collected in a glass Erlenmeyer flask with 24/40 groundglass head, and purified in a rotary vacuum distilled. A nitrogen flow is then used eliminate traces of the solvent. Mass balances end efficiency were calculated using the above quantities.
3. RESULTS
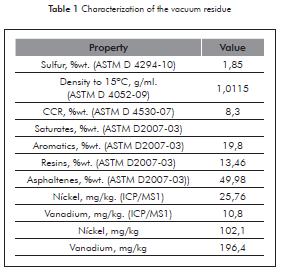
The characterization vacuum residue and the fractions obtained was performed according to ASTM methods described in Table 1.
Efficiency of the extraction process
Table 1 reports an extraction yield of 82,6% compared to the total of vacuum residue used in the test. However, if we consider the extraction yield in relation to the vacuum residue without the fraction of asphaltenes, is possible to extract high yields of species maltenes fraction distributed in a large number of fractions.
Table 2 shows the amounts of the fractions obtained by supercritical fluid extraction and fraction SFEF.
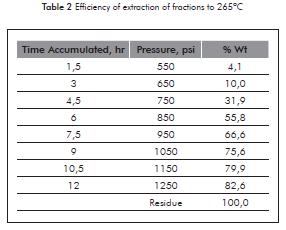
In view of the results, close to 92,6 % of the free fractions of asphaltenes from the selected vacuum residue were separated with n-hexane when maintaining the temperature of the supercritical extraction process at 265°C (far from the decomposition temperature of the vacuum residue).
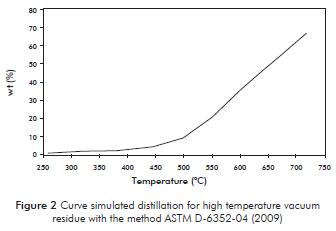
Comparing the results obtained by Simulated distillation of high temperature according to ASTM D6352-04 (Figure 2) with the results of the technique of supercritical extraction can be said that the technique of simulated distillation requires temperatures near 720°C to recover for 67% of vacuum residue, while the supercritical extraction technique is possible to extract about 92,6%.
The results show that the technique of supercritical extraction is a very interesting tool for obtaining high yields of deep fractions from vacuum residue of varying nature. In Figure 3, illustrates the appearance of the fractions obtained by supercritical extraction technique.

The supercritical extraction method with variation of pressure at constant temperature, presents the advantage to obtain a wide separation of fractions with sufficient amounts that may be used for a broad characterization.
The supercritical extraction can be extended in future work, in order to evaluate the performance of extraction and characterization of the fractions obtained from crudes of the different nature, based on the study variables and operating conditions such as nature of solvent, temperature and pressure.
Figure 4, illustrates the physicochemical properties of the fractions obtained by supercritical extraction from vacuum residue typical of the Barrancabermeja Refinery. The results indicate that as the condition is increased pressure to extract fractions at constant temperature increases the density and viscosity, which indicates that the fractions become heavier.
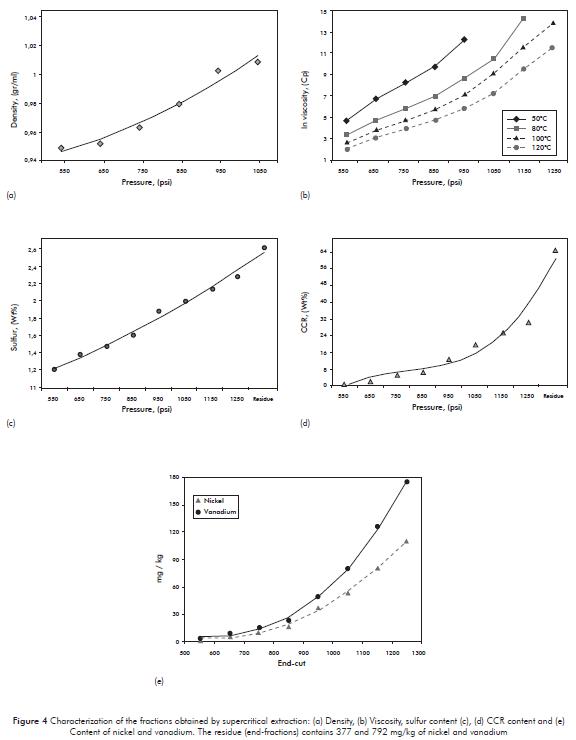
The distributions of sulfur, CCR, nickel, and vanadium indicate that the concentration of these species increases as the fractions become heavier. Researchers like Tie-Pan et al. (1997), and Suoqui et al. (2005) found a very similar behavior of the species tested in the fractions obtained from heavy oil of at different nature by supercritical extraction process with fractionation.
This may indicate that as larger fractions become heavier, their complexity increases and they present a greater tendency to form structures of high molecular weight with heteroatoms. For example, one of the most common ways to find sulfur in complex petroleum structures is in functional groups such as thiols, sulfides, disulfides, thiophenes, and their derivatives. Moreover there is a high probability of finding metals such as nickel and vanadium in shaped porfirines. (Wauquier, 2004 and Helle, 2005).
The results illustrated in Figure 5 indicate that the saturated fraction is concentrated in the first fractions and then begins to decrease its concentration with increasing pressure at constant temperature. The resin content in the fractions shows an inverse trend compared with the saturated concentration. A possible reason for this is due to increased solvency power of the solvent. Aromatic content distribution has a tendency with little variation in the range of 50 to 55%. This observation is related to the high aromatic content of about 50% in the vacuum residue (Table 1). The cut of residue is mainly composed of asphaltenes and resins.
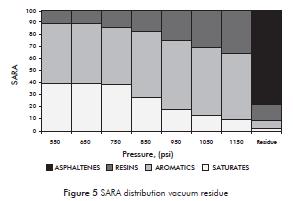
The high content of aromatics and resins in the fractions obtained show that as the fractions are made heavier its complexity also increases particularly for the contribution of aromatics polycondensates.
The technique of supercritical extraction is a highimpact tool to generate information useful in the characterization of fractions obtained from the heavier crude of the nature variable that was not previously had easy access. The new information helps to broaden understanding of the behavior of the heavier fractions of crude oil refining schemes.
With the basic characterization of the deep fractions obtained from the vacuum residue and to the accompaniment of NMR spectroscopic techniques, UV-VIS, NIR and molecular reconstruction, is possible adjust the base Assays of crude Colombia, and strengthen models of interest included in the simulators and optimizers Ecopetrol's S. A. Refining.
4. CONCLUSIONS
- The technique of supercritical extraction is a highimpact tool to generate information useful in the characterization of fractions obtained from the heavier crude of the nature variable that was not previously had easy access. The new information helps to broaden understanding of the behavior of the heavier fractions of crude oil refining schemes.
- Under the operating conditions of temperature and pressure established in this research, it is possible to recover high percentages of light fractions or maltenes that are part of the vacuum residue (saturated, aromatics and resins).
- The density, viscosity, distribution of sulfur, and CCR, indicate the possibility to obtain fractions of different nature from heavy oil and vacuum residue under the specified operating conditions.
- The fractionation of the vacuum residue with pressure increases at constant temperature, allows for fractions become heavier with increasing density, viscosity, sulfur content and CCR. Therefore, the distribution profile of heteroatoms, saturated, aromatics and resins indicates that the complexity of the fractions obtained increases.
- Methodology developed by the Instituto Colombiano del Petróleo (ICP), will expand in the near future knowledge of thof the characterization of the crude base in Colombia.
ACKNOWLEDGEMENTS
The authors express their gratitude to the Instituto Colombiano del Petróleo, ECOPETROL-ICP, for their financial, scientific and human support for the exposition and development of this work.
REFERENCES
ASTM D4294-10. Standard Test Method for Sulfur in Petroleum and Petroleum Products by Energy Dispersive X-ray Fluorescence Spectrometry. Anual Book of Standard, ASTM International, http://www.astm.org., West Conshohocken, PA, 2010. [ Links ]
ASTM D4052-09. Standard Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter. Anual Book of Standard, ASTM International, http://www.astm.org., West Conshohocken, PA, 2009. [ Links ]
ASTM D6352-04. Standard Test Method for Boiling Range Distribution of Petroleum Distillates in Boiling Range from 174 to 700°C by Gas Chromatography. Anual Book of Standard, ASTM International, http://www.astm.org., West Conshohocken, PA, 2009. [ Links ]
ASTM D2007-03 . Standard Test Method for Characteristic Groups in Rubber Extender and Processing Oils and Other Petroleum-Derived Oils by the Clay-Gel Absorption Chromatographic Method. Anual Book of Standard, ASTM International, http://www.astm.org., West Conshohocken, PA, 2008. [ Links ]
ASTM D4530-07. Standard Test Method for Determination of Carbon Residue (Micro Method). Anual Book of Standard, ASTM International, http://www.astm.org., West Conshohocken, PA, 2007. [ Links ]
Esquivel, A., & Vargas, P. (2007). Using essential oils extracted by supercritical fluids for the development of functional foods. Tech. in Motion. 20 (4): 41-50. [ Links ]
Gallego, L. C., & Cardona, C. A. (2004.). Industrial applications of supercritical fluids (and III). Chem. Eng., 413, 210-219. [ Links ]
Guangua, Y., & Ren-An, W. (1999). The supercritical fluid extractive fractionation and the characterization of heavy oils and petroleum residue. J. of petrol. Science & Engineering, 22, 47-52. [ Links ]
Han, B. X., Yang, G., Ke, J., Mao, C., & Yan, H. (1998). Phase Equilibria of Supercritical Propane-Fengcheng Bitumen System, and the Density and Viscosity of the Liquid Phase. Fluid Phase Equilib. 143 (1): 205-211. [ Links ]
Hannay, J. B., & Hogart, J. (1897). On the Solubility of Solids in Gases. J. Proceedings of the Royal Society of London, 30, 178-188. [ Links ]
Helle, H. P. E. (2005). Corrosion control in crude units. Corrosion Control, 3, 21-23. [ Links ]
Masaka, S., Motonobu, G., & Tsutomu, H. (1997). Supercritcal fluid extraction reveals resid properties. Oil and Gas J., 66-69 pp. [ Links ]
Messmore, H. E. (1943). Process for producing asphaltic materials. Phillips Petroleum Co., 2420185, United States. http://www.freepatentsonline.com/2420185.html [ Links ]
Northup, A. H., & Sloan, H. D. (1986). Advances in solvent deasphalting technology. NPRA 1996 Annual Meeting, San Antonio, TX, 3/17-19/96, Paper AM-96-55, 21. [ Links ]
Sheng-Li, C., Sheng-Sheng, J., Yun-Hua, L., & Suo-Qi, Z. (1984). Mild cracking solvent deasphalting: a new methd for upgrading petroleum residue. Fuel. 73, 439-442. [ Links ]
Subramanian, M., & Hanson, F. V. (1998). Supercritical fluid extraction of bitumens from UTA oil sands. Fuel Processing Technology, 55, 35-53. [ Links ]
Suoqi, Z., Zhiming, X., Chunming, X., & Keng, H., Chung, Renan Wang (2005). Systematic characterization of petroleum residue based on SFEF. Fuel. 84, 635-645. [ Links ]
Swanson, M. L, Dollimore, E. S., & Diehl, J. W. (1985). Extraction of a North Dakota lignite with supercritical aliphatic solvents. Conference: 190. American Chemical Society National Meeting, Chicago, IL, USA, 8 Sep, 1985. [ Links ]
Tie-Pan, S., Yung-Xiang, H., Tong, S., & Ren-An, W. (1997). Characterization petroleum vacuum residue by supercritical fluid extraction and fractionation. Ind. Eng.Chem. Res. 36, 3988-3992. [ Links ]
Wauquier, J. P. (2004). Petroleum Refining Crude Oil. (317-322). Editions Technip: Paris, FR. [ Links ]














