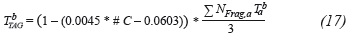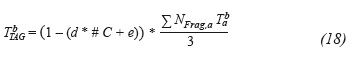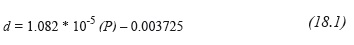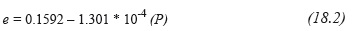Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383
C.T.F Cienc. Tecnol. Futuro vol.5 no.1 Bucaramanga July/Dec. 2012
CALCULATION OF THERMOPHYSICAL PROPERTIES OF OILS AND TRIACYLGLYCEROLS USING AN EXTENDED CONSTITUENT FRAGMENTS APPROACH
CÁLCULO DE PROPIEDADES TERMOFÍSICAS DE ACEITES Y TRIACILGLICEROLES EMPLEANDO UNA METODOLOGÍA EXTENDIDA DE FRAGMENTOS
Diana-Carolina Cruz-Forero1, Oscar-Andrés González-Ruiz1 and Luis-Javier López-Giraldo1*
1Universidad Industrial de Santander, Bucaramanga, Santander, Colombia
E-mail: ljlopez@uis.edu.co
(Received Jul. 05, 2012; Accepted Nov. 09, 2012)
*To whom correspondence should be addressed
ABSTRACT
This paper validates and implements an Extended Constituent Fragments methodology (ECF) for the calculation of thermophysical properties of vegetable oils considering the latter as triglyceride (TAG's) mixtures, both homogeneous and heterogeneous. For this purpose, three different vegetables oils were chosen (soybean oil, canola and olive) and their TAG's profiles were estimated using the ECN 42 generalized method. The ECF methodology estimates the properties of TAG's from their fragment composition and specific parameters of each property, which are adjusted using experimental information available in literature. The average relative errors of calculated properties were between 1 and 32% depending on the oil and the property. These errors were significantly lower than those obtained using the Aspen HYSYS commercial software, which oscillates between 70 and 100%. Additionally, by extrapolating the constituent fragments methodology a method for calculating boiling temperatures of TAG's with average relative errors of ∼1% was proposed. The calculations of properties for the ECF method were performed using the OIL-CALPROP software developed specifically for this purpose.
Keywords: Vegetable oils, Triglycerides, Prediction, Thermodynamic properties, Calorific capacity, Boiling temperature, Simulation, Software.
RESUMEN
En este trabajo se valida e implementa la metodología de Fragmentos Constituyentes Extendida (FCE) al cálculo de propiedades termofísicas de aceites vegetales considerando a éstos últimos como mezclas de triglicéridos (TAG's) tanto homogéneos como heterogéneos. Para tal fin, se seleccionan tres aceites modelo (aceite de soja, canola y oliva) a los cuales se les estimaron los perfiles en TAG´s, a partir de su perfil en ácidos grasos empleando el método ECN 42 generalizado. Con el método de FCE se estiman las propiedades de los TAG's a partir de la composición en fragmentos y parámetros específicos de cada propiedad, los cuales son ajustados empleando información experimental disponible en la literatura. Los errores relativos promedio de las propiedades calculadas fluctúan entre el 1 y el 32% dependiendo del aceite y la propiedad. Estos errores son significativamente menores que los obtenidos empleando el software comercial Aspen HYSYS los cuales oscilan entre 70 y 100%.Adicionalmente, extrapolando la metodología de fragmentos constituyentes, se propuso un método que permite el cálculo de temperaturas de ebullición de TAG's con errores relativos promedio de ∼1%. Los cálculos de propiedades para el método FCE se realizaron usando el software OIL-CALPROP desarrollado específicamente para este propósito.
Palabras clave: Aceites vegetales, Triglicéridos, Predicción, Propiedades termodinámicas, Capacidad calorífica, Temperatura de ebullición, Simulación, Software.
RESUMO
Neste trabalho se valida e implementa a metodologia de Fragmentos Constituintes Estendida (FCE) ao cálculo de propriedades termofísicas de óleos vegetais considerando a estes últimos como misturas de triglicerídeos (TAG's) tanto homogêneos como heterogêneos. Para tal fim, selecionam-se três óleos modelo (óleo de soja, canola e oliva) aos quais foram estimados os perfis em TAG´s, a partir do seu perfil em ácidos grassos empregando o método ECN 42 generalizado. Com o método de FCE se estimam as propriedades dos TAG's a partir da composição em fragmentos e parâmetros específicos de cada pro-priedade, os quais são ajustados empregando informação experimental disponível na literatura. Os erros relativos médio das propriedades calculadas flutuam entre 1 e 32% dependendo do óleo e a propriedade. Estes erros são significativamente menores que os obtidos empregando o software comercial Aspen HYSYS os quais oscilam entre 70 e 100%. Adicionalmente, extrapolando a metodologia de fragmentos constituintes, foi proposto um método que permite o cálculo de temperaturas de ebulição de TAG's com erros relativos médio de ∼1%. Os cálculos de propriedades para o método FCE foram realizados usando o software OIL-CALPROP desenvolvido especificamente para este propósito.
Palavras chave: Óleos vegetais, Triglicerídeos, Predição, Propriedades termodinâmicas, Capacidade calorífica, Temperatura de ebulição, Simulação, Software.
1. INTRODUCTION
The growth of population and technological development has made recent years those of greater energy increases in history. This growth has begun to generate concern due to the prospect of depletion, in the near future, of existing oil reserves. Fortunately, despite being the most widely used, fossil energy is not the only source of energy available to meet this demand. Biodiesel, for example, has become one of the most highly researched energy options in recent years, and it has proven to be a viable and cost-effective alternative. In the specific case of Colombia, between 2009 and 2010, its production had a 126% increase (United States Energy Information Administration, 2011). Bio-diesel can be produced from more than 300 species of different oilseeds. However, soil conditions, climate, yield, oil content and the need to mechanize production, limit the potential of obtaining vegetable oils to a few species among which palm, rapeseed, sunflower, soybean and microalgae stand out (Ministerio de Agricultura y Desarrollo Rural, 2010; Chisti, 2007).
Despite its potential, the implementation of a process for large scale production of biodiesel must be validated through its simulation, for which it is necessary to know the thermodynamic and transport properties of the compounds involved. However, many of these thermodynamic properties are not available for the oils that are commonly used for the production of biodiesel.
To overcome this limitation, predictive methods and thermodynamic models that allow for calculating the properties needed for the simulation of process trends have been used (Díaz-Tovar, Gani & Sarup, 2011). One of the most commonly used simulators for the calculation of properties and modeling of processes in the industry is Aspen HYSYS®.
Despite the fact that this software is recommended especially for the hydrocarbon industry, Aspen H Y-SYS® is commonly used for the simulation of processes for biodiesel production (among other bio-fuels) a fact widely evident in available literature (West, Posarac & Ellis, 2008; Martinho et al., 2008; Santana et al., 2010; Lee, Posarac & Ellis, 2011; Anitescu & Bruno, 2012; Santori, di Nicola, Moglie & Polonara, 2012). However, this simulation software has no databases containing the properties of oils or the triglycerides that constitute them, forcing the assumption that the single compound defined in this data base (triolein) is the constituent of the oil which process is being simulated. An alternative method of calculation is the Constituent Fragments model (CF) (Zong, Ramanathan & Chen, 2010a; Zong, Ramanathan & Chen, 2010b) that considers triglycer-ides as the grouping of a central column (glycerol) and three carbonated chains (fatty acids). However, in their work Zong et al. (2010a) considered oils as a mixture of homogeneous triglycerides.
As a result, one of the objectives of this work is to evaluate the impact of homogeneous and heterogeneous TAG's on the accuracy of estimates of the thermophysical and transport properties of oils (specific calorific capacity, density, viscosity and vapor pressure), designing a computational tool for their calculation. In addition, extrapolating the CF methodology, we propose a method for the calculation of TAG's boiling temperatures (Tb).
2. METHODOLOGY
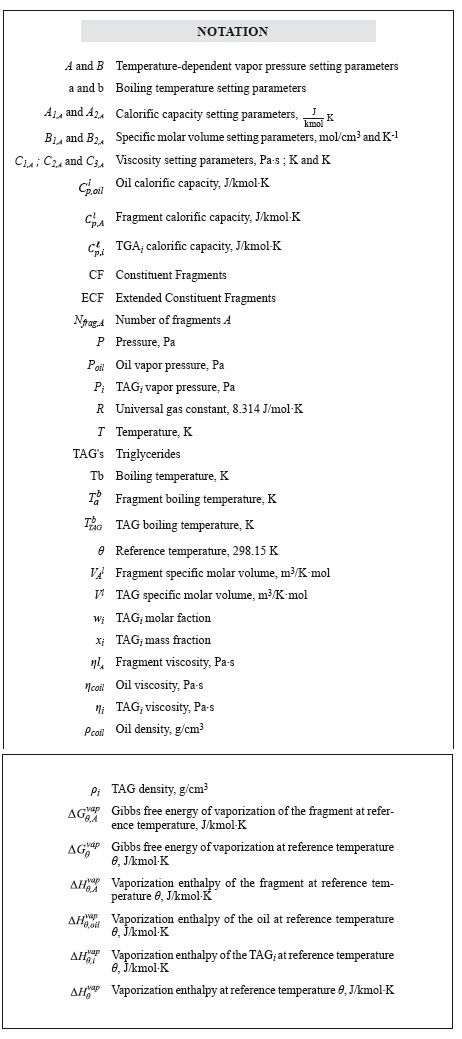
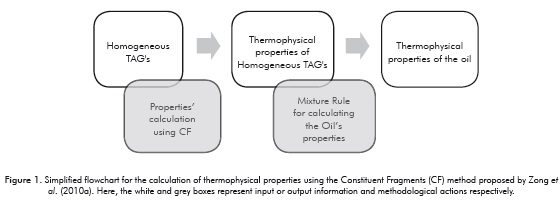
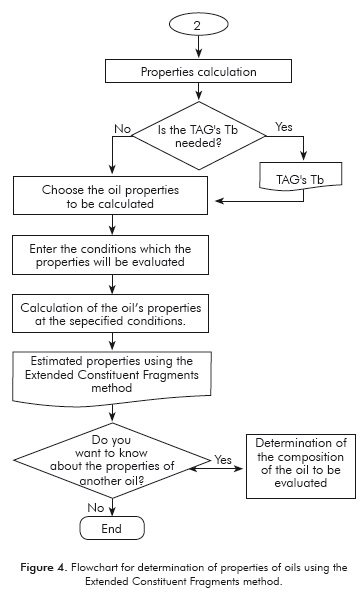
Although the CF method allows for calculating the thermophysical properties of heterogeneous TAG's, Zong et al. (2010a) only validated his work using oils defined by homogeneous TAG's profiles. As shown in Figures 1 and 2, data entry is the main difference between the methods. Since the ECF method supports starting the calculation from the fatty acids profile of a vegetable oil, it allows for greater flexibility and an expanded TAG's spectrum, which in theory would lead to more accurate results. Furthermore, the methodology proposed here offers the possibility of calculating the boiling temperatures of the TAG's that constitute the oil.
A computational tool (OIL-CALPROP, version 1.0) that supports the estimation of the thermophysical properties (i.e. calorific capacity, density, viscosity, vapor pressure) of oils constituted by mixtures of both homogeneous and heterogeneous TAG's was initially designed to validate the reliability of the results obtained using the above mentioned ECF method.
Once the values for the thermophysical properties of soybean, canola and olive oils were estimated, they were compared to the values obtained using commercial software (Aspen HYSYS®, version 7.2), to the values calculated using the original method proposed by Zong et al. (2010a) and to experimental data reported in the literature. It should be noted that calculations obtained using the Aspen HYSYS® simulator had a purely comparative function in the reliability study of the results obtained by the ECF method. The estimation of TAG´s profiles from its fatty acids composition was carried out using the ECN 42 method (Figure 2) (Panreac Química S.A., 1999).
For the scope of this article only the results obtained for the properties of soybean oil will be reported.
Proposal and Development of the Computational Tool for Calculation of the Thermophysical Properties of Oils (OIL-CALPROP, version 1.0)
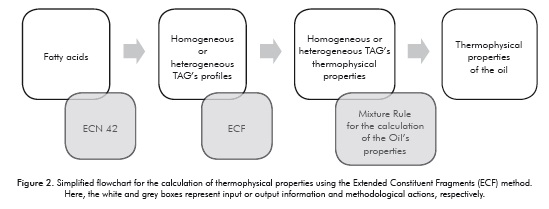
The VISUAL BASIC® STUDIO 2010 simulation interface (Ultimate version) was used for proposal and development of the tool. The logical sequence used is shown in Figures 3 and 4. Both the sequence and the program are divided into two processes; the first is the entry of data that will allow for defining the oil to be evaluated, and the second is the calculation of properties using the ECF method.
Characterization of the TAG's Prof le for Each Oil
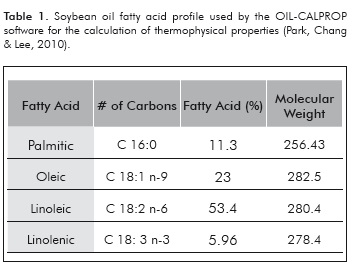
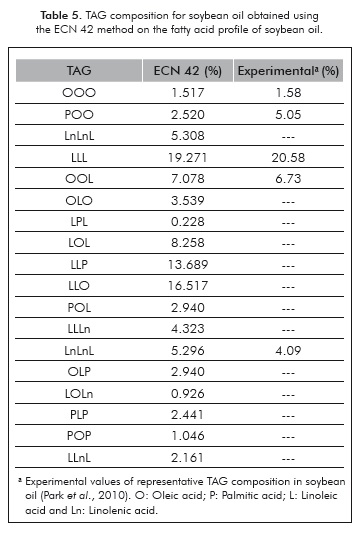
Despite the fact that the soybean oil TAG's profile is relatively easy to find in the literature that information is not available for the oils used in the production of biodiesel. However, it is very common to have available their fatty acids' profiles, from which it is possible to reconstruct the TAG's composition using the ECN 42 method (Panreac Química S.A, 1999). As a result, this methodology was used for implementation of a computational routine which allows for calculating the approximate TAG's composition of oils from their fatty acid content. As an example, the soybean oil's representative (greater than 5%) fatty acid profile is shown in Table 1.
Calculation of TAG's' Thermophysical Properties Using the ECF Method
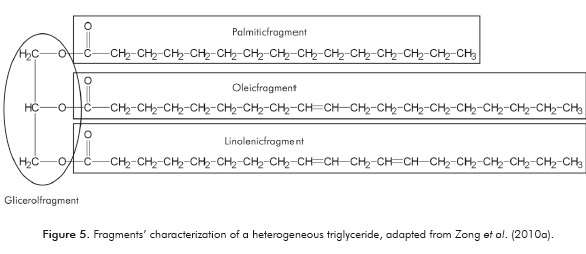
Unlike the CF method proposed by Zong et al. (2010a) the ECF methodology proposed here considers TAG's as molecules formed by fragments of not necessarily equal fatty acids and glycerol (Figure 5).
In addition, the adjustment parameters of the equations in Table 2 were recalculated, using, in some cases, more recent experimental values than those employed by Zong et al. (2010a).
Boiling Temperature In addition to extending the CF methodology, this work employs the semi-empirical approach proposed by Zong et al. (2010a) to propose, for the first time, a strategy that allows for the calculation of the TAG's boiling temperature based on their constituent fragments.
For this purpose, a relationship is proposed between the boiling temperature values of each of the TAG's fragments (acids and glycerol) and the pressure at which this property is measured. Initially, the experimental data (Niir, 2002) on the temperature of each of the fragments were adjusted to Equation 10:
Where a and b are the adjustment parameters of each fragment, Tab is the boiling temperature of the fragment α (K) and P the pressure (mmHg).
Knowing the Tab for each one of the fragments, it is possible to calculate the TAG's Tb value using Equation 11.
Where, TbTAG is the boiling temperature of the TAG (K) and NFrag is the number of fragments α.
Calculation of the Thermophysical Properties of Oils
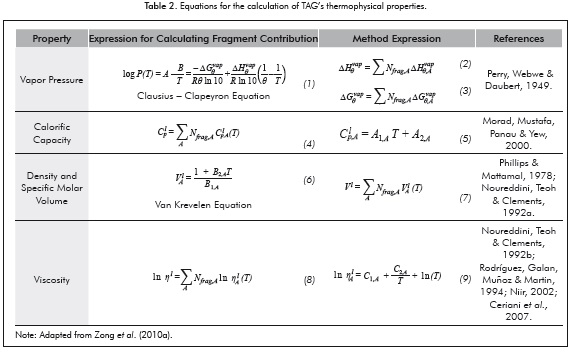
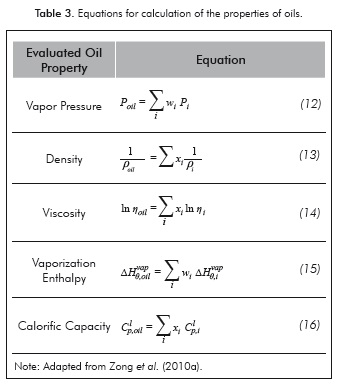
Oils are characterized by a mixture of fatty acids, monoglycerides, diglycerides, triglycerides and minor constituents (carotenes, sterols, etc.). However, TAG's constitute the more representative portion of the mix (75-98% by weight) (Gunstone, 2004). Therefore, for the calculation of their properties, it will be assumed that the oil is formed exclusively by triglycerides. The relationships used for calculating each of the considered properties are listed in Table 3.
3. RESULTS
Adjusted Equation Parameter
Table 4 shows the adjusted values of each of the parameters that will be used for the calculation of thermo-physical properties using the ECF method. In this table all adjustable parameters, with exception of density, were determinate employing a linear function which considerate to carbon number of fragment as independent variable.
Evaluation of the Accuracy Degree of the Predicted Thermophysical Properties of Soybean Oil
Table 5 shows the results of the TAG's composition profile predicted by the ECN 42 method, which will be used for the calculation of the thermophysical properties of soybean oil. When the predicted composition values are compared with the available experimental data, it can be seen that the predictive method has relative estimation errors of less than 7% for three out of the five compared TAG's.
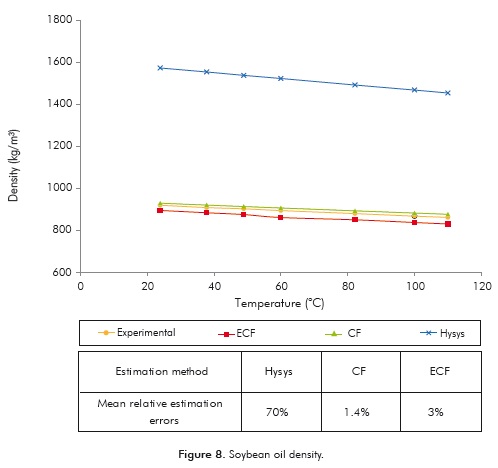
The degree of prediction of each thermophysical property evaluated using the HYSYS ® Simulator, the CF method and the method proposed here are shown in Figures 6 to 9. In the case of the ECF method, results were obtained using the OIL-CALPROP (version 1.0) software and unless otherwise stated, these were calculated from the fatty acid profile.
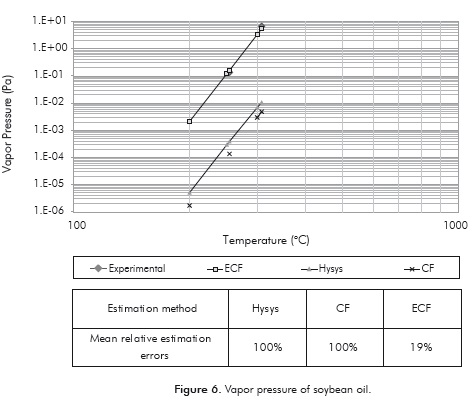
Vapor Pressure
The results in Figure 6 clearly show that the ECF method improves the predictive capacity of the soybean oil vapor pressure. This result was expected, since the ECF method considers a greater number of TAG's than the CF method and consequently the profile is closer to the oils' characteristics. Generally, the ECF method leads to an average relative error of 19 ± 1.5%, whereas the use of HYSYS (Peng-Robinson) or the CF method results in mean relative errors that are close to 100 ± 0.029%. In addition, the vapor pressure values predicted by these two last methods are underestimated by up to 3 orders of magnitude (Figure 6).
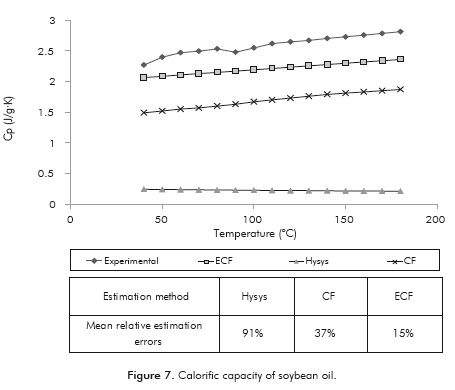
Calorifc Capacity
As in the previous case, the results obtained by the ECF method have a higher degree of accuracy than those obtained by the other two methods (Figure 7). In this particular case the mean relative error obtained with the ECF method is only 15 ± 0.019%, while for the CF method and HYSYS ® (Peng-Robinson) these errors were 37 ± 0.022 and 93 ± 0.005%, respectively. The reason for this behavior is probably that the ECF method considers that a greater number of TAG's constitute the oil, consequently improving the approximation of the simulated matrix. In addition, when the experimental TAG profile of soybean oil (Cunha & Oliveira, 2006) is considered for the calculation of calorific capacity, the average relative error is ∼ 14%.
Density
As shown in Figure 8, the results obtained with the ECF and CF methods are equivalent and in both cases predict the density value with high accuracy (mean relative errors between 1.4 ± 0.42 and 3 ± 0.25%). In the case of the values predicted by HYSYS ®, they lack physical significance for all the assessed range, a result associated with the initial assumption that triolein is the oil's only constituent. In addition, the predictive capacity of the ECF method was evaluated when starting with the experimental TAG profile determined by Cunha and Oliveira (2006) and the mean relative error obtained was ∼ 3%.
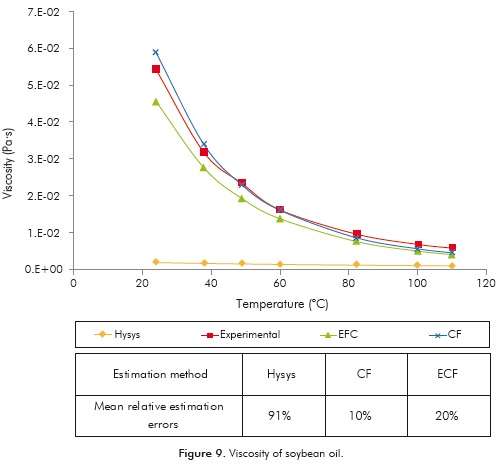
Viscosity
The predictive accuracy of each one of the methods used for the calculation of the viscosity of soybean oil is shown in Figure 9.
The figure shows that both constituent fragments methods, either with and without modifications, have the best predictive accuracy for this property. However, the best adjustment level is obtained using the unaltered constituent fragments method, which produces a mean relative error of 10 ± 8% v r. the 20 ± 7% obtained when using the ECF method. This unexpected behavior may be associated with the predictive errors of the TAG profile calculated using the ECN 42 method.
Consequently, the property value was recalculated using a triglyceride experimental profile of soybean oil (Cunha & Oliveira, 2006). The mean relative error obtained using the ECF method after using the experimental TAG's profile was reduced from 20 ± 7 to 9 ± 3%.
Boiling Temperature
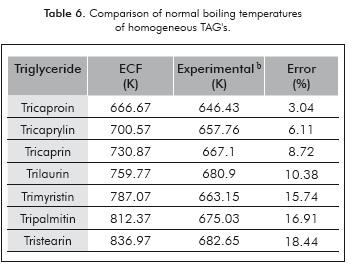
The results obtained for the TTbAG are shon in Table 6.
A direct relationship between error values and the number of carbons in the TAG is observed when the results obtained by this method are compared with experimental data, suggesting that calculations can be corrected. The following expressions are obtained using a graph of the error values against the number of carbons in the TAG's:
Boiling temperature at 760 mmHg:
For pressures lower than 760 mmHg:
Where, d and e are pressure-dependent setting parameters P [mmHg] and #C is the number of carbons in the TAG:
In this way, knowing the number of carbons of a tri-glyceride and the pressure at which boiling temperature is required is enough to calculate it.
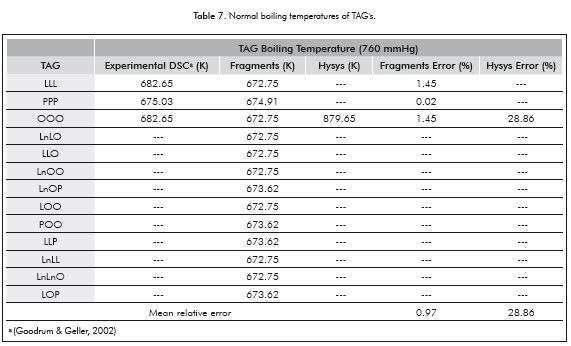
While there are few data for comparison (see Table 7), the proposed correlation can estimate the boiling temperature with mean relative errors close to 1%. In addition, these new approach have a close relation with additive relation of Gibbs which has been employed previously for predicts thermophysical properties (Zong et al., 2010a-2010b).
On the other hand, using the only TAG in the Aspen HYSYS ® database the relative error obtained is 29%. In addition, the proposed expression can be applicable for the calculation of heterogeneous TAG's Tb when experimental data are not available. However, Equations 17 and 18 are limited to the values of the adjusted fragments (see Table 4).
4. DISCUSSION
In general, it can be seen that the results obtained by the ECF method have smaller estimation errors than those obtained using the commercial Aspen HYSYS simulation tool and the CF method. While the mean relative errors obtained when using the ECF method are between 1-20% those obtained using HYSYS ® and CF varied between 70-100 and 1.4-100%, respectively, depending on the calculated property. For example, the ECF method was superior to the CF method in calculating the thermodynamic properties, regardless of whether the latter are calculated from the fatty acids' or TAG's profiles. This seems to indicate that thermodynamic properties (heat capacity and vapor pressure) are less sensitive to inaccuracies in the TAG profile than transport properties. On the other hand, in the case of viscosity, the mean relative error obtained by the ECF method when using the fatty acids profile was greater than the calculated error when the starting point is the TAG's profile (20% from fatty acids vs. 9% from TAG's). Consequently, it can be inferred that the ECF method has a greater predictive capacity than its predecessor (CF) and that this capacity depends on the degree of confidence or knowledge of the characterized lipid matrix.
These results were expected, since the ECF method considers the oil as a mixture of TAG's, both homogeneous and heterogeneous, while estimates of thermo-dynamic properties for vegetable oils using CF and Aspen HYSYS® are performed under the assumption that only homogenous TAG's constitute the oil (Mar-tinho et al., 2008).
Although the commonly used approach to simulate processes involving oils as raw material has been to consider them as triolein, the method validated in this work emerges as a viable and simple solution to improve the results of these simulations.
If this option is considered, the problems associated with the definition of new triglycerides in Aspen HYSYS® (Santana et al., 2010) are solved.
Regarding the calculation of boiling temperatures, the proposed correlation based on the Constituent Fragments methodology is capable of estimating TAG's boiling temperatures with mean relative errors close to 1%, whereas for the only TAG present in the Aspen HYSYS ® database the relative error is ∼ 29%. In addition, this correlation can be easily applicable in the calculation of boiling temperatures of heterogeneous TAG's where no experimental data is available.
5. CONCLUSIONS
- From the results obtained in this work it is concluded that the introduction of a routine that allows for estimating the triglyceride (both homogeneous and heterogeneous) composition of oils improves the accuracy level of the predictions of thermodynamic properties. For example, when the ECF method is compared with the CF method, the average relative errors in the prediction of calorifc capacity and vapor pressure are reduced in 22 and 81%, respectively. Furthermore, the inclusion of such prediction routine makes the ECF method more fexible than the CF method, since in the specialized literature the characterization of oils in terms of fatty acids profles is more common than their characterization in TAG profles.
- Regarding the prediction of transport properties (viscosity and density) the ECF and CF methods are equivalent if the starting point for the prediction of the properties is the TAG's profle.
- In addition, the methodology proposed in this paper provides a new tool for calculation of triglycerides' boiling temperatures. In these regard, the results show that predictive errors do not exceed 1%.
- In general, it can be observed that the maximum error obtained by the ECF method is 20% (percentage obtained in oil viscosity calculations).
ACKNOWLEDGEMENTS
The authors are grateful to Vicerrectoría de Investigación y Extensión of the Universidad Industrial de Santander for its financial support throughout project number 5452.
REFERENCES
Anitescu, G. & Bruno, T. (2012). Fluid properties needed in supercritical transesterification of triglyceride feedstocks to biodiesel fuels for efficient and clean combustion - A review. J. of Supercritical Fluids, 63: 133-149. [ Links ]
Ceriani, R., Gonçalves, C. B., Rabelo, J., Caruso, M., Cunha, A. C. C., Cavaleri, F. W., Batista, E. A. C. & Meirelles A. J. A. (2007). Group contribution model for predicting viscosity of fatty compounds. J. Chem. Eng. Data, 52(3), 965-972. [ Links ]
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnol. Adv., 25(3), 294-306. [ Links ]
Cunha, S. & Oliveira, M. B. P. P. (2006). Discrimination of vegetable oils by triacylglycerols evaluation of profile using HPLC/ELSD. Food Chem., 95(3), 518-524. [ Links ]
Díaz-Tovar, C., Gani, R. & Sarup, B. (2011). Lipid technology: Property prediction and process design/analysis in the edible oil and biodiesel industries. Fluid Phase Equilibria, 302(1-2), 284-293. [ Links ]
Goodrum, J. W. & Geller, D. P. (2002). Rapid thermogravi-metric measurements of boiling points and vapor pressure of saturated medium-and long-chain triglycerides. Bioresource Technol., 84(1), 75-80. [ Links ]
Gunstone, F. (2004). The chemistry of oils and fats sources, composition, properties and uses. Australia: Blackwell Pusblishing. [ Links ]
Lee, S., Posarac, D. & Ellis, N. (2011). Process simulation and economic analysis of biodiesel production processes using fresh and waste vegetable oil and supercritical methanol. Chem. Eng. Research and Design, 89(12), 2626-2642. [ Links ]
Martinho, A., Matos, H., Gani, R., Sarup, B. & Youngreenc, W. (2008). Modelling and simulation of vegetable oil processes. Food Bioprod. Process., 86(2), 87-95. [ Links ]
Ministerio de Agricultura y Desarrollo Rural. (2010). Empresarización de actividades agropecuarias. Bogota, Colombia. [ Links ]
Morad, N. A., Mustafa, A., Panau, F. & Yew, T. W. (2000). Liquid specific heat capacity estimation for fatty acids, triacylglycerols, and vegetable oils based on their fatty acid composition. J. Am. Oil Chem. Soc., 77(9), 1001-1005. [ Links ]
Niir, B. (2002). Modern technology of oils, fats and its derivatives. New Delhi: Asia Pacific Business Press Inc. [ Links ]
Noureddini, H., Teoh, B. & Clements, L. D. (1992a). Densities of vegetable oils and fatty acids. J. Am. Oil Chem. Soc., 69(12), 1184-1188. [ Links ]
Noureddini, H., Teoh, B. & Clements, L. D. (1992b). Viscosities of vegetable oils and fatty acids. J. Am. Oil Chem. Soc.,69(12), 1189-1191. [ Links ]
Panreac Química S.A. (1999). Métodos analíticos en alimentaria ácidos y grasas. Barcelona: Centre Telemamactic Editorial. [ Links ]
Park, Y., Chang, P. & Lee, J. (2010). Application of triacylglycerol and fatty acid analyses to discriminate blended sesame oil with soybean oil. Food Chem., 123(2), 377-383. [ Links ]
Perry, E., Webwe, W. & Daubert, B. (1949). Vapor pressures of phlegmatic liquids. I. Simple and mixed triglycerides. J. Am.Chem. Soc., 71(11), 3720-3726. [ Links ]
Phillips, J. C. & Mattamal, G. J. (1978). Effect of number of carboxyl groups on liquid density of esters of alkylcar-boxylic acids. J. Chem. Eng. Data, 23(1), 1-6. [ Links ]
Rodríguez, M., Galan, M., Muñoz, M. & Martin, R. (1994). Viscosity of triglycerides + alcohols from 278 to 313 K. J. Chem. Eng. Data, 39(1), 102-105. [ Links ]
Santana, G., Martins, P., de Lima, N., Batistella, C., Maciel, R. & Wolf, M. M. (2010). Simulation and cost estimate for biodiesel production using castor oil. Chem. Eng. Res. Des., 88(5), 626-632. [ Links ]
Santori, G., di Nicola, G., Moglie, M. & Polonara, F. (2012). A review analyzing the industrial biodiesel production practice starting from vegetable oil refining. Applied Energy, 92: 109-132. [ Links ]
United States Energy Information Administration. (2011). IndexMundi, world biodiesel consumption by year. United States of America. [ Links ]
West, A. H., Posarac, D. & Ellis, N. (2008). Assessment of four biodiesel production processes using HYSYS ®. Plant. Bioresource Technology, 99(14), 6587-6601. [ Links ]
Zong, L., Ramanathan, S. & Chen, C. (2010a). Fragment-based approach for estimating thermophysical propierties of fats and vegetable oils for modeling biodiesel production processes. Ind. Eng. Chem. Res., 49(2), 876-886. [ Links ]