Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383
C.T.F Cienc. Tecnol. Futuro vol.5 no.1 Bucaramanga July/Dec. 2012
CHARACTERIZATION Chlorophytas MICROALGAE WITH POTENTIAL IN THE PRODUCTION OF LIPIDS FOR BIOFUELS
CARACTERIZACIÓN DE MICROALGAS Chlorophytas CON POTENCIAL EN LA PRODUCCIÓN DE LÍPIDOS PARA BIOCOMBUSTIBLES
Denis-Lorena Jaimes-Duarte1, Wilder Soler-Mendoza1, Josman Velasco-Mendoza1
Yaneth Muñoz-Peñaloza1 and Néstor-Andrés Urbina-Suárez1*
1Universidad Francisco de Paula Santander, Cucuta, Norte de Santander, Colombia
E-mail: sinbiufps@gmail.com andres130583@gmail.com
(Received Jul. 11, 2012; Accepted Nov. 01, 2012)
*To whom correspondence should be addressed
ABSTRACT
This work is part of a megaproject that seeks to isolate microalgae of the Chlorophyta division native to Norte de Santander and identify their potential applications such as lipid production to be used as biofuel. Here we present the isolation of 11 microalgae strains from the Chlorophyta division found in two different wastewater environments. The collected strains were cultivated in selective media and purified through serial dilutions, depletion culture, and application of penicillin and gentamicin. Biomass production was evaluated and two strains were selected: CHL1 (Chlorella sp.) and DES1 (Desmodesmus sp.). The strains were cultivated on wastewater and PCG media (control), and their biomass concentration and lipid content were measured. Both strains reached similar biomass concentrations compared to their respective controls (CHL1 PCG 1.5 mg/L ± 0.035 mg/L, CHL1 AR 1.68 mg/L ± 0.036, DES1 PCG 1.66 mg/L ± 0.007, DES1 AR 2 mg/L ± 0.03) and their lipid content was slightly higher compared to their controls. The results show that the isolated and evaluated strains may have potential to be lipid producers, since their environmental and nutritional conditions have not been modified yet and adaptation may improve the production yield of lipids.
Keywords: Biodiesel, Waste water, Microalgae, Lipids, Biomass, Biofuels.
RESUMEN
Este trabajo hace parte de un megaproyecto que busca aislar microalgas de la división Chlorophyta nativas del Norte de Santander (Colombia) e identificar sus potenciales aplicaciones, dentro de las cuales se encuentra la producción de lípidos para ser utilizados como biocombustibles. Se presenta el aislamiento de 11 cepas de microalgas de la división Chlorophyta de dos ambientes distintos caracterizados por ser efluentes de aguas residuales. Se sembraron en medio selectivos y se purificaron a través de diluciones seriadas, siembras por agotamiento y aplicación de penicilina y gentamicina. Se evaluaron la producción de biomasa y se escogieron dos cepas la CHL1 (Chlorella sp.) y DES1 (Desmodesmus sp.) y se sembraron en aguas residuales y medio PCG (control), midiendo la concentración de biomasa y el contenido lipídico de las mismas. Ambas cepas alcanzaron concentraciones de biomasa similares a sus respectivos controles (CHL1 PCG 1.5 mg/L ± 0.035 mg/L, CHL1 AR 1.68 mg/L ± 0.036, DES1 PCG 1.66 mg/L ± 0.007, DES1 AR 2 mg/L ± 0.03) y en cuanto al contenido de lípidos, este fue ligeramente mayor a los obtenidos en los controles. Los resultados obtenidos mostraron que las cepas aisladas y evaluadas prometen ser promisorias para la producción de lípidos, ya que aún no se han modificado condiciones ambientales y nutricionales que pueden aumentar el contenido de los mismos de estas cepas.
Palabras clave: Biodiesel, Aguas residuales, Microalgas, Lípidos, Biomasa, Biocommbustibles.
RESUMO
Este trabalho faz parte de um megaprojeto que busca isolar microalgas da divisão Chlorophyta nativas do Norte de Santander (Colombia) e identificar suas potenciais aplicações, dentro das quais se encontra a produção de lipídios para ser utilizados como biocombustíveis. Apresenta o isolamento de 11 cepas de microalgas da divisão Chlorophyta de dois ambientes distintos caracterizados por ser efluentes de águas residuais. Ambas as cepas alcançaram concentrações de biomassa similares aos seus respectivos controles (CHL1 PCG 1.5 mg/L ± 0.035 mg/L, CHL1 AR 1.68 mg/L ± 0.036, DES1 PCG 1.66 mg/L ± 0.007, DES1 AR 2 mg/L ± 0.03) e em quanto ao conteúdo de lipídios este foi ligeiramente maior aos obtidos nos controles. Os resultados obtidos mostraram que as cepas isoladas e avaliadas prometem ser promissórias para a produção de lipídios, já que ainda não se modificou condições ambientais e nutricionais que possam aumentar o conteúdo de lipídios destas cepas.
Palavras chave: Biodiesel, Águas residuais, Microalgas, Lipídios, Biomassa, Biocombustíveis.
1. INTRODUCTION
Currently, one of the highest priorities for public and private institutions as well as economic sectors of our country, is the search for new alternatives to satisfy the energetic demand; which contribute to sustainable development, thus solving environmental problems, such as CO2 concentrations, greenhouse effect gas and flora and fauna pollution. Due to the reduction in oil reserves, the production of alternative fuels has received important attention (Ma & Hanna 1999; Song, Fu & Shi, 2008; Meng et al., 2009; Kalia & Purohit, 2008). Biodiesel is one of the most important fuel due to its worldwide demand; it may be acquired from vegetable oils, recycled kitchen oil, waste oil, animal lard, and lipids from microalgae (Van Gerpen, 2005; Song et al., 2008). Microalgae have the ability to generate different secondary metabolites, as well as high concentrations and accumulation of compounds of economic interest such as proteins, lipids, starch, glycerol and pigments among others (Urbina, 2010). Currently, the use of lipids from microalgae has been used in biodiesel production, since it is an alternative that contributes in satisfying the global diesel demand. This is a promising technology given the advantages it offers, in contrast with oily plants, such as: higher photosynthetic efficiency; higher efficiency in nutrient absorption; and short sustained production periods throughout the year, due to brief duplication periods of microalgae (Hernández et al., 2009).
Some plants are the main input for the production of biodiesel, the use of plants with high oil content has been widely studied. The main oily plants used are palm, colza, soy, sunflower, coconut, peanuts, olive, and mustard among others (Ma & Hanna, 1999; Al-Zuhair, 2007; Li, Du & Liu, 2008; Meng et al., 2009; Song et al., 2008). The main issue posed by the use of these cultures is its indiscriminate use which has caused environmental problems such as deforestation of tropical regions (Dismukes et al., 2008; Schenk et al., 2008). However, the obstacle in the use of this technology not only lies in the environmental problems caused by it, but also, in the elevated costs of raw materials, the ample areas required, and the enormous volume of water necessary for irrigation (Li, Xu & Wu, 2007; Chisti, 2007; Chisti, 2008; Schenk et al., 2008). This topic is still under discussion, however, the biodiesel industry requires alternate raw materials that allow operating continuously and overcoming the limitations that have been pointed out (Liu & Zhao, 2007); a promising alternative is the production of oil from microalgae cultures (Hernández et al., 2009).
The selection of the type of microalgae is the first step on the development of a production process, since its success is based mainly upon it. The microalgae must have the proper characteristics for culture conditions which are very specific in order to obtain a particular product. Among the main desirable characteristics for big scale production are: fast growth, high content of added value products, development in extreme environments, big cells in colonies or filaments, great tolerance to high CO2 levels (15% or more), to pollutants and physical effects of agitation or turbulence. Additionally, it must not exciter auto-inhibitors (Griffiths & Harrison, 2009; Hernández et al., 2009). There is wide experience in the use of microalgae for wastewater treatment, since they are efficient in nutrient, organic matter, heavy metal and xenobiotic removal, among others (Hernández & Olguín, 2002; Olguín, 2003; Muñoz & Guieysse, 2006). However, there are few records concerning the use of wastewater in the culture of oily microalgae and simultaneous production of biodiesel. (Loera-Quezada & Olguín, 2010).
Microalgae technology presents diverse limitations, the most outstanding of them being the difficulty in keeping monocultures with high biomass yield, the selection of the strains of oily microalgae, the lack of information regarding scaling production systems and the high use of energy from pumping, gas transfer, mixing, collecting, and dehydration of biomass (Chisti, 2007; Chisti, 2008; Dismukes et al., 2008; Hu et al., 2008; Schenk et al., 2008; Song et al., 2008; Wijffels, 2008; Rodolfi et al., 2009).
Finally, to achieve what has been previously stated, it is necessary to isolate strains native to environments such as wastewater in order to obtain promising strains in the production of lipids to produce environmentally and economically feasible biodiesel. The Isolation and characterization of strains of native microalgae of the urban wastewater of Norte de Santander, on the one hand would resolve the pollution of the pamplonita river and use of them by the agencies of value-added production, either as biomass or chemicals likely to be used as biofuel. The objective of this study was to isolate and characterize native strains of the Norte de Santander of the division Chlorophyta, evaluating the production of biomass and the potential use of waste-water as a culture medium for the production of lipids in energy interest. It is important to point out that this technology has not been used in our region and it is still emerging in the country.
2. EXPERIMENTAL DEVELOPMENT
Studied Organism
Microalgae were isolated from samples collected in habitats that presented characteristics such as a high nitrogen content and organic matter (Point 1: wastewa-ter (WW) from the Bogotá channel; Point 2: WW from the unilibre channel). The microalgae isolated from the samples collected were analyzed and a selection was performed of the microalgae belonging to the Chlorophyta division thru microscopic morphology and physiology, and the use of selective culture media (PCG, BOLD BG11). For the lipid production phase using WW, two of the isolated strains were used- the CHL1 (Chlorella sp) and DES1 (Desmodesmus sp).
Culture Media
For the isolation stage, the PCG medium was used (Perales-Vela, González-Moreno, Montes-Horcasitas & Cañizares-Villanueva, 2007; Perales-Vela, 2004), which is a medium designed with a relatively smaller amount of nitrates than the traditional algal media which indirectly induces the production of metabolites, such as carotenoids and lipids, in the microalgae of the Chlorophyta division. As for the evaluation and lipid production stage, PCG was used as a control medium, using WW from the city of Cúcuta (final discharge of the WW of the city) without adding any other nutrient.
Isolation and Purifcation
The procured samples were submitted to serial dilutions of 100 - 10 -5 and planted into liquid and solid media with exhaustion and massive planting. Each planting was done in triplicate. Microscopic observation was performed and the genus was determined according to the observed morphology. Regarding the purification, streptomycin and penicillin were used as antibiotics, applied in four different concentrations (0.5, 1, 1.5 y 2 ppm), in the PCG + glucose medium (Urbina, 2010). Cultures were plated and incubated at 37 and 25°C to verify the presence or absence of bacteria and fungi. After the strains were purified, the production of the biomass in the PCG medium in batch cultures was evaluated in 500 ml photobioreactors with the following conditions: light intensity of 200 umoles/m2/s, temperature of 30° C, pH of 7-5 and 8-5, and aeration of 1 vvm. The procedural time was 15 to 20 days to reach the death stage, with a 12:12 photoperiod and periodic samplings every 24 hours.
Physicochemical Characterization of Wastewater
To characterize the wastewater, monitoring was done with samples from the selected points: Point 1 (WW of the Bogotá channel), Point 2 (WW of the unilibre channel). The parameters measured were: COD, nitrates, inorganic phosphorus, pH, temperature, acidity, alkalinity, and hardness - all according to the protocols established by the standard methods (APHA, AWWA, WPCF 1992).
Photobioreactor and Culture Conditions
Autotrophic cultures were performed in batch and using the PCG culture medium and WW medium. The WW used were not sterilized, but were sedimented and filtered to remove as many suspended solids and sediments as possible. An airlift type photobioreactor was used with a volume of 2 L, and the culture conditions were: light intensity of 200 umoles/m2/s, temperature of 30°C, pH of 7-5 to 8-5, agitation of 150 rpm, and aeration of 1 vvm. The procedural time was 15 to 20 days to reach the death stage, with a 12:12 h photoperiod and periodic samplings every 24 hours.
Monitoring and Analysis of Parameters
The biomass was quantified using dry weight (ash free) taking aliquots of 10 ml volume which were vacuum-filtered onto nitrocellulose membranes of 0.22 um pore diameter (Millipore ®). Previously, the membranes were brought to a constant weight, keeping them at 90 - 100° C for a period of 24 hours. Once filtered, the samples were dried for 24 hours at 90 - 100° C to obtain a constant weight and determine their concentration thru gravimetry. As to the nitrate quantification, this was performed by the brucine-sulfamic acid method (APHA, AWWA, WPCF 1992, modified by Urbina (2010)). The inorganic phosphorus quantification was performed thru the modified method of Taussky and Shorr (1953). For the extraction and quantification of lipids, a pre-treatment was applied for cell disruption which consisted in subjecting the microalgae to an agitation with pearls (Urbina, 2010). The extraction was performed using the method of Bligh and Dyer (1959).
Data Processing
All the experiments were repeated in triplicate. The results were verified statistically by Student's t-test (P <0.05) using the SigmaPlot stat program version 14.
3. RESULTS AND DISCUSSION
Isolation of the Strains of the Chlorophyta Division
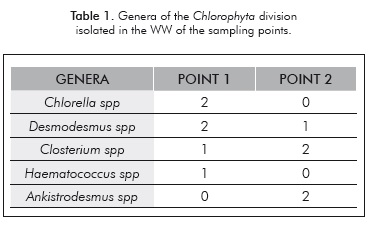
The samples taken from point 1 and point 2 were cultivated on solid and liquid media. From point 1, six strains of the Chlorophyta division were isolated; while from point 2 five strains were isolated, reaching a total of 11 isolated strains belonging to the Chlorophyta division. Table 1 shows the genera identified:
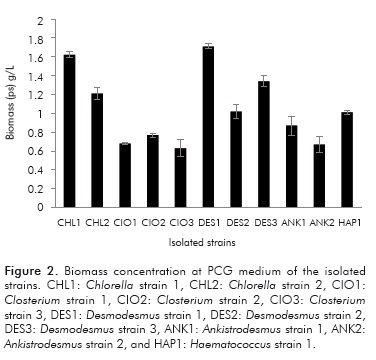
Within the genera found, it is worth noting that strains belonging to the Chlorella and Desmodesmus genera were isolated, microalgae that have been reported by several authors as lipids producers. (Hernández et al., 2009; Chisti, 2007; Chisti, 2008) The 11 strains were planted in the PCG medium and in 500 mL pho-tobioreactors, and the concentration of biomass was assessed during a time span of 15 days. Figure 1 shows the concentrations of biomass reached in the PCG medium for each of the isolated strains.
The growth results shown in Figure 1 illustrates that the highest concentration of biomass was present in the CHL1 strains (Chlorella sp) at a concentration of 1.62 g/L ± 0.032, and in the strain DES1 (Desmodesmus sp) at a concentration of 1.71 g/L ± 0.026. As for the remaining strains, the biomass values ranged between 0.63 and 1.34 g/L. Urbina (2010) found that for the genus Scenedesmus (Desmodesmus) the biomass concentrations in autotrophy in similar conditions and in the PCG medium were 1.4 g/L ± 0.039. Perales-Vela et al. (2007) reported biomass values for microalgae of this same genus below 1 g/L, while Quevedo Morales and Acosta (2008) obtained values between 0.16 - 0.45 g/L in cultures of up to 40 days. Comparing the results reported in the previously mentioned literature with those obtained in this work, the DES1 strain showed higher biomass values and a shorter culture time than those reported in the aforementioned works. In the case of Chlorella; Li, Du and Liu (2008) reported biomass concentrations for this microalga in autotrophy conditions of up to 2 g/L, while in heterotrophy conditions the concentrations increased to up to 16 g/L. Most reports of biomass for this genus of microalgae in autotrophy conditions range between 0.5 - 5 g/L. It is worth noting that the highest concentrations of biomass are caused because of additions of CO2, as it is key for maximizing the growth and the amount of lipids, and should be avoided that it inhibit or limit growth (Mazzuca et al., 2000). In this work, the maximum concentration achieved by the CHL1 strain was higher than in some aforementioned studies - but in others it was lower- and this is because, in this work, some nutritional conditions have not been improved with, for example, the addition of CO2 or the addition of nitrogen, and therefore in subsequent phases some nutritional and environmental conditions will be improved to allow further growth of the same.
Production of Biomass Strains CHL1 and DES1 in 2 L Photobioreactors
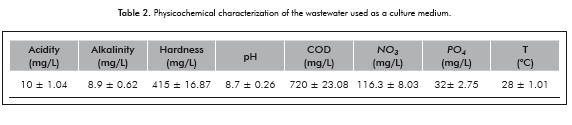
For this stage, the two strains which achieved the highest biomass concentrations in the previous stage were taken, DES1 and CHL1. These strains were planted in wastewater media to evaluate the production of bio-mass and lipids in this type of culture medium. The WW were evaluated as a culture medium - the WW were initially filtered and then passed thru a sedimentator to remove the greatest amount of solids present therein. Table 2 shows the physicochemical characterization performed to the selected residual water:
The physiochemical results show that the nitrate concentrations are within the ranges of the average WW: Pacheco et al. (2001) reported nitrate concentrations in domestic WW in ranges from 7 to 156 mg/L, just as Antón and Díaz (2000) reported that the nitrate concentrations in wastewater have, on average, a value between 60 to 90 mg/L of nitrate. As for the other parameters measured, their values are in the average range of those reported in the literature.
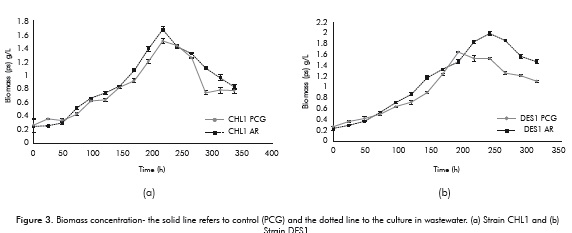
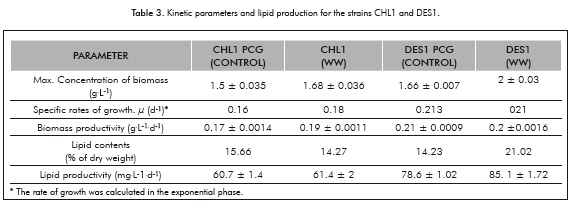
Figure 3 shows the biomass growth of the CHL1 and DES1 strains in the PCG control medium and in the medium with WW.
Figure 3a and 3b show that the behavior of both strains, both in the PCG as well as in the wastewater, was linear, with the highest rate of growth exhibited towards the 150th hour for both strains. One important trait of the obtained results is that the behavior of the CHL1 and DES1 strains were similar when cultured in the medium PCG and in the medium of WW; even the biomass concentrations achieved were higher in the WW than in the PCG medium for both strains (CHL1 PCG 1.5 ± 0.035, CHL1 WW 1.68 ± 0.036, DES1 PCG 1.66 ± 0.007, DES1 WW 2 ± 0.03) and it should be noted that the measurements were performed by ash-free dry weight. These results allowed the observation that the adaptation of the strains CHL1 and DES1 planted in wastewater was satisfactory, since they managed to grow and achieve higher biomass concentrations than the control, without having modified the medium to induce growth. The reports of microalgae grown in domestic wastewater to be used for lipids production are somewhat limited, since most work has focused on heavy metals removal or complex wastewater (Perales et al., 2007).
Kinetic Parameters and Lipid Production in Media with Wastewater
For the calculation of kinetic parameters, cultures were performed in 2L photobioreactors. Table 3 summarizes the calculated parameters:
The total content of lipids in the microalgae can range from 1 to 90% of the dry weight, depending on the species and culturing conditions (Spolaore et al. 2006; Chisti, 2007); it has been reported that when the microalgae are subjected to stress conditions imposed by chemical and physical environmental stimuli, singly or in combination, synthesis occurs and the accumulation of large quantities of triglycerides, accompanied by considerable alterations in the composition of lipids and fatty acids. Nitrogen deficiency is, with regard to the nutrients, the factor that most affects the metabolism of the lipids (De, Chaudhury & Bhattacharyya, 1999; Li et al., 2008; Hu et al., 2008; Mendoza et al., 2008; Solovchenko et al., 2008; Gouveia & Oliveira, 2009; Rodolfi et al., 2009). Yet, in the same manner, as it has been reported that under normal conditions without metabolic, nutritional, and environmental alterations, the lipid content of the microalgae of the Chlorophyta division can achieve between 5 - 30% of dry weight, depending on the type of microalgae and the age of the crop. (Spolaore et al., 2006; Hu et al., 2008; Gouveia & Oliveira, 2009). Similar results were found in this work where the lipid percentages of the two strains tested were between 15.66 to 21.02%; the highest percentages of lipids were recorded in the strains planted in the WW media. This can be explained because the availability of nitrogen in the WW is less than in the PCG medium, which implies a slight stress due to this nutrient, which may induce a higher accumulation of lipids. Regarding lipid productivity for the CHL1 strain, the values achieved were of 60.7 mg·L-1d-1 ± 1.4 in the PCG medium and 61.4 mg·L-1d-1 ± 2 in the WW medium. Francisco, Neves, Jacob-Lópes and Franco (2010) reported productivities for lipid production of 127.2 4 mg·L-1d-1 using Chlorella vulgaris in a culture with sufficient nutrients, while Widjaja, Chien and Hu (2009) reported a productivity of 12.77 mg·L-1d-1 in conditions of nutrient suppression. In this work, the productivities achieved were relatively high in comparison to those of other studies, even without implementing some actions that induce lipid production. Regarding the DES1 strain, the lipid productivities achieved were of 78.6 mg·L-1d-1 ± 1.02 in the PCG medium and 85.1 mg·L-1d-1 ± 1.72 for the WW medium, similar to the findings for the CHL1 strain where the lipid productivity was slightly higher in the medium with WW than in the PCG medium, which can also be attributed to the stress caused by nutrient availability in the WW that leads to the accumulation of metabolites, in which lipids can be found. As to the microalgae of the Des-modesmus or Scenedesmus genus¸ very little has been reported regarding the production of lipids in media such as wastewater. Some authors, such as Matsunaga et al., (2009), have worked with Scenedesmus genera in the absence of nutrients, reaching productivities of up to 80 mg·L-1d-1. Finally, the obtained results allow us to visualize a potential use of these two strains in lipid production to obtain biodiesel, and therefore it necessary to begin generating variables that allow the induction of lipid accumulation.
4. CONCLUSIONS
- This work allowed the isolation of 11 strains of the Chlorophyta division, evaluating the two strains CHL1 and DES1 belonging to the Chlorella sp and Desmodesmus sp genera, which adapted satisfactorily to the wastewater used as a culture medium, reaching biomass values similar to the controls (CHL1-PCG: 1.5 ± 0.035, CHL1-WW: 1.68 ± 0.036, DES1-PCG: 1.66 ± 0.007, DES1-WW: 2 ± 0.03).
- The isolation of these strains appears to be promising, since they allow an alternative solution to the problems created in the city of Cúcuta by the discharge of wastewater into the river Pamplonita, and additionally to start implementing the microalgae technology as a possible third generation biofuels generator, which -as of yet - has not been addressed in the region.
- The lipid results achieved using wastewater showed similar behaviors to the values reached by the PCG control medium, where the strain of the Desmodes-mus genus cultured in the WW medium reported the highest percentage (21.02%) of lipids. Regarding the Chlorella strains in the WW and PCG media and the Desmodesmus cultured in PCG, their lipid percentages varied within a range of 14 to 15%. It is noteworthy to highlight that in this work no variables have yet been used to induce lipid accumulation.
- Finally, more research is still required to overcome current obstacles for obtaining biodiesel thru these organisms and increase the productivity of micro-algae biomass of higher lipid content with the right profle. The working group is addressing the issue in order to improve this technology.
ACKNOWLEDGMENTS
We wish to acknowledge the FINU fund of the Universidad Francisco de Paula Santander for funding this project.
REFERENCES
Al-Zuhair, S. (2007). Production of biodiesel: possibilities and challenges. Biofuels Bioprod. Bioref., 1(1), 57-66. [ Links ]
Antón, D. J. & Díaz, D. C. (2000). Sequía en un mundo de agua. San José Toluca: Piriguazú Ediciones/ CIRA UAEM. [ Links ]
APHA, AWWA , WPCF (1992). Standard Methods for the Examination of Water and Wastewater. 18th ed. American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF). Washington DC, EEUU. 1085 pp. [ Links ]
Bligh, E. G. & Dyer, W. J. (1959). A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol., 37(8), 911-917. [ Links ]
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnol. Adv., 25: 294-306. [ Links ]
Chisti, Y. (2008). Biodiesel from microalgae beats bioethanol. Trends Biotechnol., 26: 126-131. [ Links ]
De, B.K., Chaudhury, S. & Bhattacharyya, D.K. (1999). Effect of nitrogen sources on y-linoleic acid accumulation in Spirulina platensis. JAOCS J. of the American Oil Chem. Soc, 76(1), 153-156. [ Links ]
Dismukes, G. C, Carrieri, D., Bennette, N., Ananyev, G M. & Posewitz, M. C. (2008). Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr Opin. Biotechnol., 19: 235-240. [ Links ]
Francisco, E. C, Neves, D. B., Jacob-Lopes, E. & Franco, T. T. (2010). Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality. J Chem. Technol. Biotechnol, 85(3), 395-403. [ Links ]
Gouveia, L. & Oliveira, A. C. (2009). Microalgae as a raw material for biodiesel production J Ind. Microbiol. Biotechnol, 36(2), 269-274. [ Links ]
Griffiths, M. J. & Harrison, S. T. L. (2009). Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol., 21(9), 493-507. [ Links ]
Hernández, A., Vázquez-Duhalt, R., Sánchez, M., Serrano, L. & Martínez, A. (2009). Biodiesel a partir de microalgas. BioTecnol., 13(3), 38-60. [ Links ]
Hernández, E. & Olguín, E. J. (2002). Biosorption of heavy metals influenced by the chemical composition of Spirulina sp. (Arthrospira) biomass. Environ. Technol, 23(12), 1369-1377. [ Links ]
Hu, Q., Sommerfeld, M., Jarvis, E., Ghirardi, M., Posewitz, M., Seibert & Darzins, A. I. (2008). Microalgal triacylg-lycerols as feedstock for biofuel production: perspectives and advances. Plant. J., 54(4), 621-639. [ Links ]
Kalia, V. C. & Purohit, H. J. (2008). Microbial diversity and genomics in aid of bioenergy. J. Ind. Microbiol. Biotech., 35(5), 403-419. [ Links ]
Li, Q., Du, W. & Liu, D. (2008). Perspectives of microbial oils for biodiesel production. Appl. Microbiol. Biotechnol., 80(5), 749-756. [ Links ]
Li, X., Xu, H. & Wu, Q. (2007). Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol. Bioeng., 98(4), 764-771. [ Links ]
Liu, B. & Zhao, Z. (2007). Biodiesel production by direct methanolysis of oleaginous microbial biomass. J. Chem. Technol. Biotechnol., 82(8), 775-780. [ Links ]
Loera-Quezada, M. & Olguín, E. (2010). Las microalgas oleaginosas como fuente de biodiesel:retos y oportunidades. Rev. Latinoam. Biotecnol. Amb. Algal., 1(1), 91-116. [ Links ]
Ma, F. R. & Hanna, M. A., (1999). Biodiesel production: a review. Bioresour. Technol., 70(1),1-15. [ Links ]
Matsunaga, T., Matsumoto, M., Maeda, Y. , Sugiyama, H., Sato, R. & Tanaka, T. (2009). Characterization of marine microalga, Scenedesmus sp. strain JPCC GA0024 toward biofuel production. Biotechnol. Lett., 31: 1367-1372. [ Links ]
Mazzuca, T., García, F., Camacho, F., Acién, F. & Molina, E. (2000). Carbon dioxide uptake efficiency by outdoor microalgal cultures in tubular airlift photobioreactors. Biotech. Bioeng., 67(4), 465-475. [ Links ]
Mendoza, H., Molina-Cedres, C., de la Jara, A., Nordström, L., Freijanes, K. & Carmona, L. (2008). Variación cuantitativa y cualitativa de la composición en ácidos grasos de Cryphtecodinium cohnii en condiciones de supresión de nitrógeno. Grasas y Aceites, 59(1), 27-32. [ Links ]
Meng, X., Yang, J., Xu, X., Zhang, L., Nie, Q. & Xian, M. (2009). Biodiesel production from oleaginous microorganisms. Renewable Energy, 34(1), 1-5. [ Links ]
Muñoz, R. & Guieysse, B. (2006). Algal-bacteria processes for the treatment of hazardous contaminants: a review. Water Res., 40(15), 2799-2815. [ Links ]
Olguín, E. J. (2003). Phycoremediation: key issues for cost-effective nutrient removal processes. Biotechnol. Adv., 22(1-2), 81-91. [ Links ]
Pacheco, J., Marín, L., Cabrera, A., Steinich, B. & Escolero, O. (2001). Nitrate temporal and spatial patterns in 12 water-supply wells, Yucatán, México. Environ. Geol. 40(6), 708-715 [ Links ]
Perales-Vela, H., González-Moreno, S., Montes-Horcasitas, C. & Cañizares-Villanueva, R. O. (2007). Growth photo-synthetic and respiratory responses to sub-lethal copper concentrations in Scenedesmus incrasatulus (Clorophy-ceae). Chemosphere. 67(11), 2274 - 2281 [ Links ]
Perales-Vela, H., (2004). Photosynthetic and respiratory characterization of Scenedesmus incrassatulus during biore-moval of Cu2+. Doctoral Thesis, Centro de Investigación y de Estudios Avanzados, Departamento de Biotecnología y Bioingeniería, Instituto Politécnico Nacional, México Distrito Federal, México. 210 pp. [ Links ]
Quevedo, C., Morales, S. & Acosta, A. (2008). Scenedesmus sp growth in different culture mediums for microalgal protein production. Vitae, 15(1), 25-31. [ Links ]
Rodolfi, L., Zittelli, G.C., Bassi, N., Padovani, G., Biondi, N., Bonini, G. & Tredici, M.R. (2009). Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng., 102(1), 100-112. [ Links ]
Schenk, P. M., Thomas-Hall, S. R., Stephens, E., Marx, U.C., Mussgnug, J. H., Posten, C., Kruse, O. & Hankamer, B. (2008). Second generation biofuels: high-efficiency micro-algae for biodiesel production. Bioenerg. Res., 1(1), 20-43. [ Links ]
Solovchenko, A. E., Khozin-Goldberg, I., Didi-Cohen, S., Cohen, Z. & Merzlyak, M.N. (2008). Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Pa-rietochloris incisa. J. Appl. Phycol., 20(3), 245-251. [ Links ]
Song, D., Fu, J. & Shi, D. (2008). Exploitation of oil-bearing microalgae for biodiesel. Chin. Jour. Biotech., 24(3), 341-348. [ Links ]
Spolaore, P., Joannis-Cassan, C., Duran, E. & Isambert, A. (2006). Commercial applications of microalgae. J. Biosci. Bioeng., 101(2), 87-96. [ Links ]
Taussky, H. & Shorr, E. (1953). A microcolorimetric method for determination of inorganic phosphorus. J.Biolg. Chem., 202: 675-685. [ Links ]
Urbina, N. A. (2010). Establecimiento del cultivo mixotrófico de Scenedesmus incrassatulus para la producción de carot-enoides en un fotobiorreactor multitubular. Tesis de maestría. Centro de Investigación y de Estudios Avanzados, Departamento de Biotecnología y Bioingeniería, Instituto Politécnico Nacional. Unidad Zacatenco. México. 104 pp. [ Links ]
Van Gerpen, J. (2005). Biodiesel processing and production. Fuel. Process. Technol., 86: 1097-1107. [ Links ]
Widjaja, A., Chien, C.C. & Ju, Y.H. 2009. Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J. Taiwan. Inst. Chem. E., 40(1), 13-20. [ Links ]
Wijffels, R.H. (2008). Potential of sponges and microalgae for marine biotechnology. Trends Biotechnol., 26(1), 26-31. [ Links ]