Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383
C.T.F Cienc. Tecnol. Futuro vol.5 no.2 Bucaramanga Jan./June 2013
ACID HYDROLYSIS OF WATER HYACINTH TO OBTAIN FERMENTABLE SUGARS
HIDRÓLISIS ÁCIDA DEL JACINTO DE AGUA PARA OBTENER AZÚCARES FERMENTABLES
Juan-Guillermo Reales-Alfaro1*, Lizeth-Tairina Trujillo-Daza1, Giselle Arzuaga-Lindado1, Hader-Iván Castaño-Peláez2 and Ángel-David Polo-Córdoba1
1Universidad Popular del Cesar, Valledupar, Cesar, Colombia 2Politécnico Colombiano Jaime Isaza Cadavid, Medellín, Antioquia, Colombia
e-mail: juanreales@unicesar.edu.co
(Received: Sep. 05, 2012; Accepted: May 27, 2013)
* To whom correspondence should be addressed
ABSTRACT
In order to take advantage of the aquatic weed water hyacinth (Eichhornia crassipes) as lignocellulosic feedstock for the production of reducing sugars, a bromatological characterization of the leaves and stems of the plant was performed. Then, the effects of the sulfuric acid concentration (1 -3% v/v), the concentration of solids (10 to 12.5% w/v) and the reaction time (15 -25 min) at 121°C/15 psi in the production reducing sugars were studied by employing a central composite design. It was found that the acid concentration was the most significant variable. The optimal conditions were solids 11.25% p/v, H2SO4 2% v/v and 20 minute reaction time. These conditions yielded a concentration of reducing sugars of 33.3 g/L.
Keywords: Eichhornia crassipes, Biofuel, Lignocellulose, Fermentable sugars, Sulfuric acid pretreatment.
RESUMEN
Con el propósito de aprovechar la maleza acuática Jacinto de Agua (Eichhornia crassipes) como materia prima lignocelulósica para la obtención de azúcares reductores, se realizó en primera instancia una caracterización bromatológica de las hojas y tallos de la planta. Luego se estudió el efecto de la concentración de ácido sulfúrico (1 -3% v/v), la concentración de sólidos (10 -12.5% p/v) y el tiempo de reacción (15 -25 min.) a 121°C/15 psi, en la producción de azúcares reductores, empleando para ello un diseño central compuesto. Se encontró que la concentración de ácido fue la variable más significativa. Las condiciones óptimas fueron 11.25% p/v de sólidos, 2% v/v H2SO4 y 20 minutos de tiempo de reacción. A estas condiciones se obtuvo una concentración de azúcares reductores de 33.3 g/L.
Palabras clave: Eichhornia crassipes, Lignocelulosa, Biocombustible, Azúcares reductores, Pretratamiento con ácido sulfúrico.
RESUMO
Com o propósito de aproveitar a erva aquática Aguapé (Eichhornia crassipes) como matéria-prima lignocelulósica para a obtenção de açúcares redutores, foi realizada em primeira instância uma caracterização bromatológica das folhas e talos da planta. Posteriormente, foi estudado o efeito da concentração de ácido sulfúrico (1 -3% v/v), a concentração de sólidos (10 -12.5% p/v) e o tempo de reação (15 -25 min.) a 121°C/15psi, na produção de açúcares redutores, utilizando para tal fim um desenho central composto. Encontrou-se que a concentração de ácido foi a variável mais significativa. As condições ótimas foram 11.25% p/v de sólidos, 2% v/v H2SO4 e 20 minutos de tempo de reação. Nestas condições foi obtida uma concentração de açúcares redutores de 33.3 g/L.
Palavras-chave: Eichhornia crassipes, Lignocelulose, Biocombustível, Açúcares redutores, Pré-tratamento com ácido sulfúrico.
1. INTRODUCTION
Water hyacinth (Eichhornia crassipes), also known as water Buchón, Taruya, Camalote, Mop, Water Violet, is an aquatic plant native to the rivers of the Amazon Basin in South America (Gunnarsson & Petersen, 2007). Eichhornia crassipes is a floating aquatic weed, pop-rooted with rhizomes or stolons. The plant is variable in size and may grow in poor nutrient concentration, up to 0.05 and 0.01 ppm of nitrogen and phosphate (Moran, 2006; Cronk & Fennessy, 2001; Reddy & D'Angelo, 1990). Water hyacinth reproduces sexually by seed propagation or vegetative regeneration. Sexual reproduction takes place by the development of tubers on the basis of the rosette; they can grow up to 30 cm in length before developing a rosette daughter; the intensity of spreading by these means can result in a doubling of the infested area from 6 to 15 days. Studies suggest that water hyacinth increases biomass up to 12% per day and biomass accumulation rate can range from 7.7 to 60 g/m2/day (Ramírez, 2005; Reddy & D'Angelo, 1990).
Water hyacinth is considered an important raw material for agricultural fertilizers, animal feed, biogas, paper, cardboard and building materials. It is also useful in the absorption of some heavy metals in water and, currently, there is scientific and technological interest in obtaining ethanol from it. (Ganguly, Chatterjee & Dey, 2012; Kumar, Singh & Ghosh, 2009a; Mishima et al., 2008).
The use of this biomass to obtain ethanol is an alternative to help relieving problems associated with this plant that because of its rapid growth and spread in the absence of controlling species has nowadays created an imbalance situation of one of the major wetlands in the country: Zapatosa Swamp. Zapatosa Swamp has an area of 36000 hectares in the dry season and 50000 hectares in winter time, where 30% is occupied by weeds, equivalent to 10800 and 15000 hectares respectively. This situation dramatically affects the urban area of Chimichagua and Saloa villages which belong to the Department of Cesar (Rangel et al., 2007). This aquatic weed invades in a short time the waterway, obstructing the generation of power, irrigation, navigation and fishing. It also restricts the passage of light to the underwater environment and depletes oxygen levels (Ganguly et al., 2012; Ma et al., 2010; Kumar et al., 2009a).
There are chemical characterization reports that indicate that water hyacinth contains 18 - 35% cellulose, 18 -49% hemicellulose and 3.5 -9% of lignin composition, able enough to obtain fermentable sugars through different physical pretreatments, physicochemical, chemical and biological processes (Kumar, Barret, Delwiche & Stroeve, 2009 b; Mishima et al., 2008; Gunnar-sson & Petersen, 2007; Nigam, 2002). Some research works show that acid pretreatment has the property of hydrolyzing hemicellulose of the xylose material and other degradative compounds of the hydrolysis of this polymer. It remains thereby an insoluble form called cellulignin, a fraction characterized by its high content of cellulose and lignin, and low content of hemicellulose (Peñuela, Da Silva, Bezerra De Sousa & Pereira, 2007).
The pretreatment with dilute sulfuric acid to 2% v/v of water hyacinth has been effective in the removal of sugars in the hemicellulose fraction, especially pentoses, after seven hours of reflux and stirring at 250 rpm, yielding 18.8 g of Reducing Sugars (RS)/100 g dry biomass of which pentoses totaled 13.3 g RS/100 g RS of dry biomass (Kumar et al., 2009b). Comparative studies have also been made of dilute acid hydrolysis with new pretreatment methods such as ionic liquids, raw glycerine, and pure glycerine on water hyacinth with the purpose of transforming the hydrolyzed cellulose into ethanol.
The performance of total RS in the pretreatment with both pure glycerine and raw glycerine, as compared to dilute acid hydrolysis, did not differ significantly, whereas pretreatment with ionic liquids was less effective (Guragain et al., 2011).
During the pretreatment of the lignocellulosic material with sulfuric acid (0.2 - 2%) not only can sugar be obtained from hydrolysis and solubilization of cellulose and hemicelluloses. Also, due to the high temperatures (121 -220°C) they degrade, especially sugars from hemicellulose, two compounds derived from furan are originated: furfural, formed from the degradation of pentoses (xylose and arabinose) and 5-hydroxymethylfurfural (HMF), formed as a result of degradation of hexoses (glucose, mannose and galactose) (Balat, 2011; Oliva, 2003). In order to mitigate this undesirable effect, some detoxication methods have been employed like heating at high temperature and alkalinazing with Ca(OH)2 (Isarankura et al., 2007). However, one of the biggest challenges to increase the performance of the transformation of lignocellulosic biomass into ethanol is the fermentation of all sugars present in biomass (C5and C6).This has been worked intensively to obtain genetically modified microorganisms that can transform xylose and arabinose into ethanol. Recently, DNA technology has been used to provide some micro-organisms with the feature of fermenting five-carbon sugars with high efficiency. An example is the bacterial strain of the species Escherichia coli, developed by the University of Florida (USA) which can ferment sugars of six and five carbons at the same time (Patrouilleau, Lacoste, Yapura & Casanovas, 2006).
Another example is the introduction of operons that encode enzymes for xylose assimilation and pentose phosphate pathway in Zymomona mobilis, (Zhang et al., 1995), recombinant strains of E. coli with genes from Z. mobilis for more efficient conversion of pyruvate to ethanol (Dien, Nichols, O'Bryan & Bothast, 2000), E. coli strains of recombinant material for the production of endoglucanase (Wood, Beall & Ingram, 1997) and, finally, plasmids with genes of xylose reductase and xylitol dehydrogenase of P. stipitis in Saccharomyces spp. for efficient co-fermentation of glucose and xylose (Ho, Chen & Brainard, 1998). Based on the significant contents of hemicellulose/cellulose and low lignin contents in water hyacinth, and the possibility that through acid hydrolysis a significant amount of RS can be obtained, this work evaluates the effect of the concentration of solids, acid concentration and reaction time on reducing sugar production, as a pre-processing stage of the acid hydrolyzate transformation to ethanol water hyacinth.
2. METHODOLOGY
Substrate Preparation
The water hyacinth was collected from the Zapatosa Swamp from Chimichagua Township, located in the Department of Cesar. The aquatic plant collected was washed with distilled water to remove dirt. Leaves and stems were processed separately, milled in fresh, dried at 105°C/6 hours and subjected to a mechanical spraying, taking them to a size of 1.18 mm, in order to increase the contact surface between the sample and the sulfuric acid solution. Biomass was prepared by mixing equal parts of ground leaves and stems. A smaller size increases the contact between the solid and the acid but leads to additional energy costs on the comminution of the same. Therefore, the size obtained not only establishes favorable processing conditions but also additional savings (Gutiérrez & Arias, 2009; Misson, Haron, Ahmad & Saidina, 2009).
Acid Hydrolysis
Hydrolysis experiments were carried out in 200 ml Erlenmeyer flasks to the solids concentration, acid concentration and reaction time according to experimental design. The heating was performed in autoclave at 121°C/15 psi. Two mL of samples were taken from the supernatant and centrifuged at 5000 rpm for analysis of RS.
Statistical Approach
An experimental method was designed to optimize the acid hydrolysis using a Central Composite Design and response surface methodology. The effects of solids concentration were evaluated, as well as the acid concentration, and reaction time on the release of RS. Table 1 shows the experimental matrix.
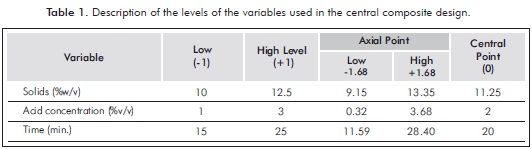
Regression analysis and analysis of variance (ANOVA) were performed using the software Statgraphics CenturionTM XV (StatPoint Inc., USA). Using response surface methodology, the concentration of RS released was analyzed with the following second-order polynomial equation:
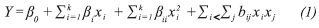
Where Y is the response variable, xi and xj are the independent variables in encoded units, and β0,βi, βii, bij settings are the coefficients of the model to the independent terms, linear, quadratic and interactive, respectively.
Analytical Methods
The physicochemical characterization of water hyacinth was performed using the following methods: moisture, gravimetric method 930.15/90 of the AOAC, ashes method 942.05/90 of AOAC, raw fiber, AOAC 962.09/90; proteins, Kjeldahl method, according to 955.04/90 of AOAC; fat, 920.39/90 technique of AOAC, pH, technique 10.041/84 of AOAC. The content of cellulose, hemicellulose and lignin were determined by the detergent extraction method (Robertson & Van Soest, 1981).
Determination of Reducing Sugars
Reducing sugars were determined by the method of 3.5 - dinitrosalicylic acid (DNS) (Miller, 1959). The performance of diluted acid hydrolysis was calculated as follows:
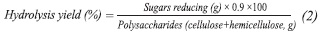
The 0.9 value corrects the contribution of a mole mass of water for each hydrolyzate glycosidic bond.
Severity Factor
To estimate the degree of severity of the acid hydrolysis process, an autoclave parameter developed by Silverstein et al. (2007) was used.
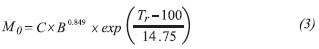
Where M0 is the severity parameter, C is the residence time, B is the concentration of acid, Tr is the reaction temperature in °C, 100 is the reference temperature in °C, and 14.75 is the conventional activation energy, assuming that the overall reaction is hydrolytic and first order.
Validation
The optimal conditions determined from the design analysis were validated experimentally. For this purpose, the evaluation of three replicates to these conditions was carried out.
3. RESULTS AND DISCUSSION
Characterization of Water Hyacinth
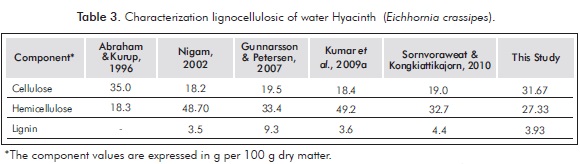
The results of the physicochemical characterization and lignocellulosic water hyacinth in a 1:1 ratio of leaves and stems are shown in Tables 2 and 3.
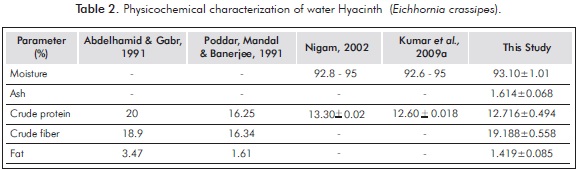
In analyzing the results obtained in the characterization of water hyacinth (Eichhornia crassipes) reported in Table 2, and making a parallel with other works, it is concluded that the moisture content calculated in this research (93.10%) is in the range reported by other authors. As for the results of the percentages of ash and fats obtained (1.614 and 1.419%), the percentage of fiber (19.188%) is found in the range reported by Abdelhamid and Gabr (1991) and Poddar et al. (1991). The differences found may be because the nutritional conditions of habitats affect the metabolic processes of the plant, resulting in different chemical characterizations. The evident raw protein content (12.716%) of water hyacinth makes it attractive as a source of nitrogen in bioconversion processes (Sornvoraweat & Kongkiattikajorn, 2010).
Table 3 shows that the cellulose and hemicellulose content of water hyacinth determined in this study has a composition similar to that reported by Abraham and Kurup (1996). In this study we can appreciate a slight difference of 4.34% between holocellulose fractions (cellulose + hemicellulose). Much of the research shows that the water hyacinth hemicellulose substantially predominates. However, in this study the cellulose fraction is predominant. The differences in the composition of water hyacinth lignocellulosic may lie in the use of different methods for the quantification of structural carbohydrates, because the methods of acid and neutral detergent fiber followed by Robertson and Van Soest (1981) were designed to characterize pasture and forage. The low content of lignin present in the water hyacinth allow better utilization of cellulose and hemicellulose in the hydrolysis process.
Acid Hydrolysis
Table 4 shows the experimental results under the various conditions according to the experimental de sign. The data shown in RS obtained vary from 4.9 to 34.3 g/L.
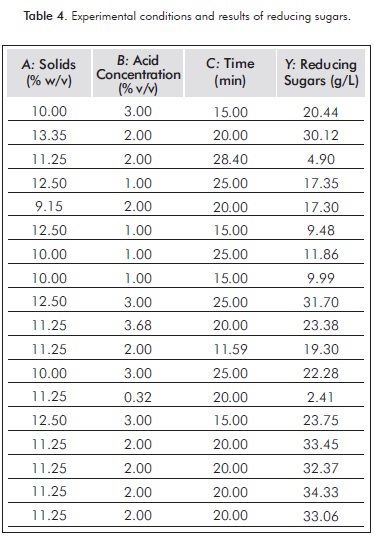
ANOVA (see Table 5) shows that the acid concentration and the quadratic terms of acid concentration and time have a P-value less than 0.05, indicating that they have a significant effect on the production of RS.
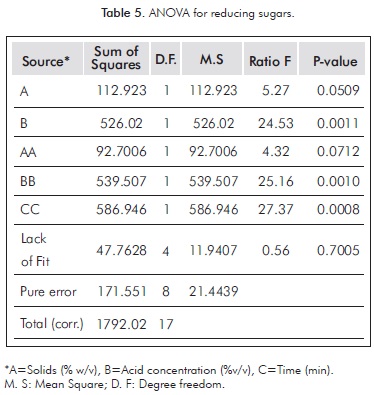
Figure 1 shows that the acid concentration (B) is one factor that has a significant positive effect on the production of RS.

Crossed factors (AA, CC and BB) have no physical meaning and are used to improve the mathematical fit of statistical model based on experimental data. The solid concentration, although it did not exert a significant effect on the conditions studied, makes a great contribution in obtaining fermentable sugars, a fact reflected in the p-value.
The statistical analysis yielded a mathematical model to predict outcomes within the range of each variable. The regression equation, with variables in encoded units and with the terms that have a significant and measurable effect on the release of RS, is as follows:
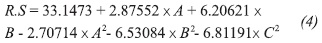
Where RS is the concentration of RS (g/L), A is the concentration of solids (% w/v), B is the concentration of sulfuric acid (% v/v) and C is the reaction time. The regression coefficient value for this model (R² = 0.88) indicates that the quadratic model fitted explains 88% of the variability in the production of RS. The effects of acid concentration and reaction time, keeping the concentration of solids constant, are shown in Figure 2.
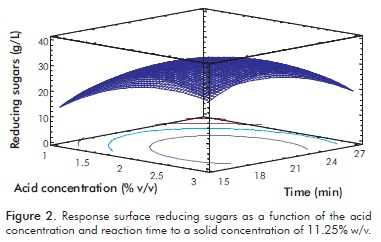
It can be observed that in the central region there is a maximum. As the acid concentration increases, there is a greater amount of sugars released, but there comes a point at which it begins to decrease. This may be explained by the greater presence of acid catalyst that produces transformations of hexose and pentose to 5HMF and furfural respectively, and the formation of organic acids such as lactic acid, propionic acid, levulinic acid and formic acid, among others. Figure 3 shows a slight increase in the concentration of RS to increase the solid concentration in both high and low concentrations of acid. However, in high acid concentrations more sugars are released than in low acid concentrations and, as Figure 2 shows, there is a maximum in the experimental region. This behavior is consistent with the reported by Ganguly et al. (2012), who claims that at high acid Harun, Dayang, Zainal and Yunus (2011), Kumar et al. concentrations a greater hydrolytic effect is obtained.
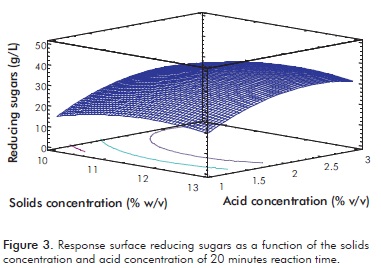
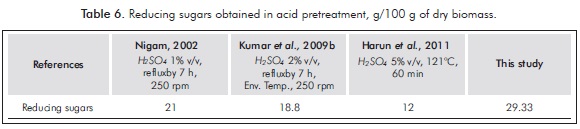
Upon estimating the optimal value, it was found that with a solid concentration of 12%, acid concentration 2.5% v/v and 20 minutes reaction, a 35.7 g/L of reducing sugar was obtained. By validating these conditions, and triplicating them, a reducing sugar concentration of 33.2±0.2 g/L was obtained, a value very close to that estimated by the model: 33.3±1.3 g/L. After performing a test to compare means, a P-value greater than 0.05 was obtained, indicating that there are no significant differences between them. A similar result is obtained under the conditions of the central design point, with a concentration of acid and a lower solids concentration, which is economically advantageous. Taking the economic factor as criterion, and without characterizing the inhibitory products generated in the pretreatment that conditions the selection depending on the destination of the pretreatment product, optimal conditions were chosen as 11.25% w/v solids, 2% H2SO4, and a reaction time of 20 minutes at 121°C/15 psi. Under these conditions a conversion of cellulose and hemicellulose of 45% in RS is achieved; that is, 29.33 g of RS/100 g of dry biomass, which exceeds the value obtained by Harun, Dayang, Zainal and Yunus (2011), Kumar et al. (2009b) and Nigam (2002a), as shown in Table 6.
This shows the greatest hydrolytic effect of the conditions found here. This effect can be attributed to the cleavage of hemicellulose in the sugars contained in it, such as xylose, and sugars such as mannose, arabinose also present in hemicellulose, and even glucose in minor proportions, given the hydrolytic characteristics of acid agent over structural carbohydrates of lignocellulosic biomass. This suggests that the solid fraction is rich in cellulose so it can further be enzymatically zaccharized into glucose. The liquid fraction can be fermented directly by a microorganism to ferment pentoses with prior detoxification, such as Pichia stipitis (Sánchez, Gutiérrez, Muñoz & Rivera, 2010).
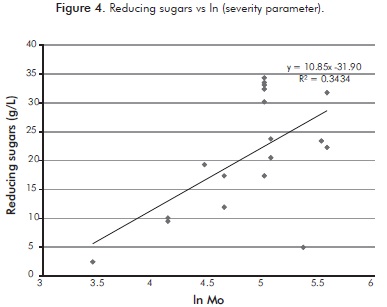
Figure 4 shows the poor relationship between the severity parameter and RS, indicating the inability of the severity parameter to predict the dependence of the response variable to the independent variables, in the absence of other variables such as the percentage of solids.
4. CONCLUSION
- The optimal conditions to obtain a significant yield of RS are in a ratio of 11.25% w/v of solids with a concentration of 2% v/v of sulfuric acid over 20 minutes at 121°C/15 psi. The variable that has a significant and positive effect on the studied conditions of acid hydrolysis of the water hyacinth is the acid concentration, the effect of which was favored by the high pressure at which the substrate was submitted. The central composite design optimizes sugar performance based on dry biomass, with 29.33 g of RS/100 g of dry biomass.
ACKNOWLEDGEMENTS
The authors thank the Centro de Investigación para el Desarrollo de la Ingeniería (CIDI) from Universidad Popular del Cesar and the Grupo de Investigación en Química Básica y Aplicada a procesos Bioquímicos, Biotecnológicos y Ambientes, from Politécnico Colombiano Jaime Isaza Cadavid, for their immense cooperation in the execution of this project.
REFERENCES
Abdelhamid, A. M. & Gabr, A. A. (1991). Evaluation of water hyacinth as feed for ruminants. Arch. Anim. Nutr., 41(7/8), 745-756. [ Links ]
Abraham, M. & Kurup, M. (1996). Bioconversion of tapioca (Manihot esculenta) waste and water hyacinth (Eichhornia Crassipes)-Influence of various physic-chemical factors. J. Ferm. Bioeng., 82(3), 259-263. [ Links ]
AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists. (1990). 15th ed. Arlington, VA. [ Links ]
Balat, M. (2011). Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review. Energy Conversion and Manag., 52(2), 858-875. [ Links ]
Cronk, J. & Fennessy, M. (2001). Wetland plants: Biology and ecology. Washington D. C.: Lewis Publishers. [ Links ]
Dien, B., Nichols, N., O'Bryan, P. & Bothast, R. (2000). Development of new ethanologenic Escherichia coli strains for fermentation of lignocellulosic biomass. Appl. Biochemistry Biotechnol., 84-86(1-9), 181-196. [ Links ]
Ganguly, A., Chatterjee, P. K. & Dey, A. (2012). Studies on ethanol production from water hyacinth. A review. Renew. Sust. Energ. Rev., 16(1), 966-972. [ Links ]
Gunnarsson, C. C. & Petersen, C. M. (2007). Water hyacinths as a resource in agriculture and energy production: A literature review. Waste Manag., 27(1), 117-129. [ Links ]
Guragain, Y., De Coninck, J., Husson, F., Durand, A. & Rakshit, S. (2011). Comparison of some new pretreatment methods for second generation bioethanol production from wheat straw and water hyacinth. Bioresour. Technol., 102(6), 4416-4424. [ Links ]
Gutiérrez, C. & Arias, J. (2009). Obtención de celulosa a partir de material lignocelulósico proveniente de la extracción de aceite de palma. Tesis de pregrado Facultad de Ingeniería, Universidad de Antioquia, Medellín, Colombia, 98pp. [ Links ]
Harun, M. Y., Dayang, A. B., Zainal, Z. & Yunus, R. (2011). Effect of physical pretreatment on dilute acid hydrolysis of water hyacinth (Eichhornia crassipes). Bioresour. Technol., 102(8), 5193-5199. [ Links ]
Ho, N., Chen, Z. & Brainard, A. (1998). Genetically engineered Saccharomyces yeast capable of effective co-fermentation of glucose and xylose. Appl. Environ. Microbiol., 64(5), 1852-1859. [ Links ]
Isarankura, C., Tantimongcolwat, T., Kongpanpee, T., Prabkate, P. & Prachayasittikul, V. (2007). Appropriate technology for the bioconversion of water hyacinth (Eichhornia crassipes) to liquid ethanol: future prospects for community strengthening and sustainable development. EXCLI J., 6: 167-176. [ Links ]
Kumar, A., Singh, L. K. & Ghosh, S. (2009a). Bioconversion of lignocellulosic fraction of water-hyacinth (Eichhornia crassipes) hemicellulose acid hydrolysate to ethanol by Pichiastipitis. Bioresour. Technol., 100(13), 3293-3297. [ Links ]
Kumar, P., Barret, D. M., Delwiche, M. J. & Stroeve, P. (2009b). Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res., 48(8), 3713-3729. [ Links ]
Ma, F., Yang, N., Xu, C., Yu, H., Wu, J. & Zhang, X. (2010). Combination of biological pretreatment with mild acid pretreatment for enzymatic hydrolysis and ethanol production from water hyacinth. Bioresour. Technol., 101(24), 9600-9604. [ Links ]
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal. Chem., 31(3), 426-428. [ Links ]
Mishima, D., Kuniki, M., Sei, K., Soda, S., Ike, M. & Fujita, M. (2008). Ethanol production from candidate energy crops: Water hyacinth (Eichhornia crassipes) and water lettuce (Pistia stratiotes L.). Bioresour. Technol., 99(7), 2495-2500. [ Links ]
Misson, M., Haron, R., Ahmad, M. & Saidina, M. (2009). Pretreatment of empty fruit bunch for production of chemicals via catalytic pyrolysis. Bioresour. Technol. 100(11), 2867-2873. [ Links ]
Moran, P. (2006). Water nutrients, plant nutrients, and indicators of biological control on waterhyacinth at Texas Field Sites. J. Aquat. Plant Manage. 44: 109-114. [ Links ]
Nigam, J. N. (2002). Bioconversion of water-hyacinth (Eichhornia crassipes) hemicellulose acid hydrolysate to motor fuel ethanol by xylose-fermenting yeast. J. Biotechnol. 97(2), 107-116. [ Links ]
Oliva, J. (2003). Efectos de los productos de degradación originados en la explosión por vapor de biomasa de chopo sobre Kluyveromyces marxianus. Tesis Doctoral Fac. Ciencias Biológicas, Universidad Complutense de Madrid, Madrid, España, 160pp. [ Links ]
Patrouilleau, R., Lacoste, C., Yapura, P. & Casanovas, M. (2006). Perspectivas de los biocombustibles en Argentina, con énfasis en el etanol de base celulósica. Informe Técnico, Unidad de Coyuntura y Prospectiva. Instituto Nacional de Tecnología Agropecuaria. [ Links ]
Peñuela, M. C., Da Silva, J., Bezerra De Sousa, M. & Pereira, N. (2007). Enzymatic hydrolysis optimization to ethanol production by simultaneous saccharification and fermentation. Appl. Biochemistry Biotechnol., 137-140(1-12), 141-153. [ Links ]
Poddar, K., Mandal, L. & Banerjee, G. C. (1991). Studies on water hyacinth (Eichhornia crassipes) -Chemical composition of the plant and water from different habitats. Indian Veterinary J., 68(9), 833-837. [ Links ]
Ramírez, S. (2005). Evaluación de la degradación natural de la especie Eichhornia crassipes (buchón) para el embalse del muña. Tesis de pregrado Fac. Ingeniería, Universidad de Los Andes, Bogotá, Colombia, 106pp. [ Links ]
Rangel, O., Rivera, O., Muñoz, Y., Medina, G., Ardila, M., Pulido, H.,Arellano, H., Carvajal, J., Martínez, M., Rocha, M., Romero, I., Moreno, M., Ávila, S., Estupiñan, S., Galvis, G., Gutiérrez, M., López, Y., Cruz, M., Galeano, G. & López, L. (2007). Estudio de inventario de fauna, flora, descripción biofísica y socioeconómica y línea base ambiental Ciénaga de Zapatosa. Informe Técnico, Corporación Autónoma Regional del Cesar. [ Links ]
Reddy, K. R. & D'Angelo, E. M. (1990). Biomass yield and nutrient removal by water hyacinth as influenced by water hyacinth (Eichhornia crassipes) as influenced by harvesting frequency. Biomass, 21(1), 27-42. [ Links ]
Robertson, J. B. & Van Soest, P. J. (1981). The detergent system of analysis and its application to human foods. The analysis of dietary fibers in food. New York: Marcel Dekker. [ Links ]
Sánchez, A. M., Gutiérrez, A., Muñoz, J. & Rivera, C. (2010). Producción de bioetanol a partir de subproductos agroindustriales lignocelulósicos. Revista Tumbaga, 5: 61-91. [ Links ]
Silverstein, R. A., Chen, Y., Sharma-Shivappa, R., Boyette, M. & Osborne, J. (2007). A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresour. Technol., 98(16), 3000-3011. [ Links ]
Sornvoraweat, B. & Kongkiattikajorn, J. (2010). Separated hydrolysis and fermentation of water hyacinth leaves for ethanol production. KKU Res. J., 15(9), 794-802. [ Links ]
Wood, B., Beall, D. & Ingram, L. (1997). Production of recombinant bacterial endoglucanase as a coproduct with ethanol during fermentation using derivates of Escherichia coli KO11. Biotechnol. Bioeng., 55(3), 547-555. [ Links ]
Zhang, M., Eddy, C., Deanda, K., Finkelstein, M. & Picataggio, S. (1995). Metabolic engineering of a pentose metabolism pathway in ethanologenic Zymomonas mobilis. Appl. Biol. Sci.., 267(5195), 240-243. [ Links ]













