Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383
C.T.F Cienc. Tecnol. Futuro vol.5 no.4 Bucaramanga Jan./June 2014
THE CORROSION PROCESS OF P-110 STEEL IN STIMULATION FLUIDS USED IN THE OIL INDUSTRY
EL PROCESO DE CORROSIÓN DEL ACERO P-110 EN FLUIDOS DE ESTIMULACIÓN UTILIZADOS EN LA INDUSTRIA DEL PETRÓLEO
Jorge A. Calderón*1, Gloria-Fernanda Bonilla1 and Javier-Alejandro Carreño2
1Universidad de Antioquia, Medellín, Colombia
2Instituto Nacional de Tecnología, Rio de Janeiro, Brasil
e-mail: jcaldergut@gmail.com
How to cite: Calderón, J. A., Bonilla, G. F. & Carreño, J. A. (2014). The corrosion process of P-110 steel in stimulation fluids used in the oil industry. CT&F - Ciencia, Tecnología y Futuro, 5(4), 35-48.
+XII Congreso Nacional de Corrosión y III Internacional de Integridad 2013, Universidad Tecnológica de Pereira and Asociación Colombiana de Corrosión y Protección (ASCOR), Colombia, 8 - 10, May 2013.
(Received: Sep. 24, 2013; Accepted: Jun. 05, 2014)
*To whom correspondence should be addressed
ABSTRACT
The use of chelating agents in the oil well stimulation processes has been proposed as an alternative to the acid treatment when the formations damage do not allow the usage of strong chemical attack. However, this procedure can arouse the corrosion of the steel used in the infrastructure of the oil production. In this study, the corrosion kinetics of the P-110 steel in ethylenediaminetetraacetic acid (EDTA) based fluids is assessed under different conditions of evaluation. Linear polarization was performed in different hydrodynamic regimes and at different temperatures, in order to evaluate the kinetic of steel corrosion. The working electrolyte solution comprises 10% disodium EDTA and 20% tetrasodium EDTA. The increase of temperature from 25 to 80°C in both electrolytes increments the corrosion rate of steel in at least one order of magnitude; particularly in EDTA-Na2 solutions. Corrosion rates of 0.29x101 and 1.67x102 mm·y-1 were measured at 25 and 80°C, respectively. The hydrodynamic regime plays an important role in the corrosion of steel only in the disodium solution, where corrosion rates were increased at higher rotation speeds of the electrode. The cathodic depolarization effect is more important in the EDTA-Na2 than in the EDTA-Na4 solution, making it more corrosive.
Keywords: EDTA electrolytes, Chelating agents, Electrochemical techniques, Metal dissolution.
RESUMEN
El uso de agentes quelantes en procesos de estimulación de pozos de petróleo ha sido propuesto como alternativa al tratamiento ácido cuando el daño de formaciones no permite un ataque químico fuerte. Sin embargo, este procedimiento puede incentivar la corrosión del acero utilizado en la infraestructura de producción de petróleo. En este estudio se evaluó la cinética de corrosión del acero P-110 en fluidos base ácido etildiaminotetraacético (EDTA) en diferentes condiciones. Medidas de polarización lineal fueron realizadas en diferentes regímenes hidrodinámicos y a diferentes temperaturas para evaluar la cinética de corrosión del acero. El electrolito de trabajo consistió en soluciones de EDTA disódico al 10%, y EDTA tetrasódico al 20%. El incremento de la temperatura de 25 a 80°C en ambos electrolitos aumenta la tasa de corrosión del acero en un orden de magnitud, particularmente en soluciones de EDTA-Na2. Las velocidades de corrosión fueron de 0.29x101 y 1.67x102 mm·y-1 a 25 y 80°C, respectivamente. El régimen hidrodinámico juega un papel importante solo en la solución disódica, en la cual las velocidades de corrosión se incrementaron a altas velocidades de rotación del electrodo. El efecto de despolarización catódica en la solución de EDTA-Na2 es más importante que en la solución de EDTA-Na4, haciéndola más corrosiva.
Palabras clave: Electrolitos de EDTA, Agentes quelantes, Técnicas electroquímicas, Disolución de metales.
RESUMO
O uso de agentes quelantes em processos de estimulação de poços de petróleo foi proposto como alternativa ao tratamento ácido quando o dano de formações não permite um ataque químico forte. Porém, este procedimento pode incentivar a corrosão do aço utilizado na infraestrutura da produção de petróleo. Neste estudo avaliou-se a cinética de corrosão do aço P-110 em fluídos base ácido etilenodiamino tetra-acético (EDTA) em diferentes condições. Medidas de polarização linear foram realizadas em diferentes regimes hidrodinâmicos e a diferentes temperaturas para avaliar a cinética de corrosão do aço. O eletrólito de trabalho consistiu em soluções de EDTA dissódico a 10%, e EDTA tetrassódico a 20%. O aumento da temperatura de 25 a 80°C em ambos os eletrólitos aumenta a taxa de corrosão do aço em uma ordem de magnitude, particularmente em soluções de EDTA-Na2. As velocidades de corrosão foram de 0.29x101 e 1.67x102 mm·y-1 a 25 e 80°C, respectivamente. O regime hidrodinâmico joga um papel importante só na solução dissódica, na qual as velocidades de corrosão aumentaram a altas velocidades de rotação do eletrodo. O efeito de despolarização catódica na solução de EDTA-Na2 é mais importante que na solução de EDTA-Na4, fazendo-a mais corrosiva.
Palavras-chave: Eletrólitos de EDTA, Agentes quelantes, Técnicas eletroquímicas, Dissolução de metais.
1. INTRODUCTION
Ducts or pipes are an important part of the infrastructure used in oil extraction well, accounting for about a third of the cost of this infrastructure (Lin et al., 2010). Production wells in oil and gas pipelines are prone to deterioration and failure under complex and adverse working conditions. In such processes, a large amount of particles of the rock are generated from the drilling and production of oil, which combined with the mixture of water saturated with CO2 of the oilfield may constitute a highly aggressive media, putting in risk the pipes and the installed infrastructure (Cai et al., 2010; Moreira et al., 2004; Carvalho, Joia & Mattos, 2005). In a simple cost-profit relationship, the use of low carbon steels for piping systems in oil and gas production has the advantage of being an inexpensive alternative, but the carbon steel tubes are easily oxidized making corrosion deterioration, corrosion-erosion and other wear damage come quickly.
One of the techniques used in oil well stimulation is the acidification. It seeks to increase well productivity by dissolving rocks, which limit, hinder or prevent the flow of oil during operation of the well (Nasr-El-Din, Al-Othman, Taylor & Al-Ghamdi, 2004). Different acids are used in the conventional acidification treatment, among them are: hydrochloric acid (HCl), hydrofluoric acid (HF), acetic acid (CH3COOH), formic acid (HCOOH), sulfamic acid (H2NSO3H) and chloroacetic acid (ClCH2COOH) (Migahed & Nassar, 2008).
An alternative to the acid treatment is the use of chelating agents such as ethylenediaminetetraacetic acid (EDTA) or nitrilotriacetic acid (NTA) (Fredd & Fogler, 1998; Portier et al., 2009). Chelating solutions are used in cleaning formations and well stimulation, particularly in the formations that can be damaged by strong acids (Frenier, Fredd & Chang, 2001). These substances act as a solvent, increasing the wetting process by water, and dissolving (totally or partially) the minerals or rocks containing Fe, Ca, Mg and Al. Particularly in calcareous deposits, chelating ions solvate the calcium from the rocks, allowing calcite dissolution and its transport either to the discharge surface of the well or into the formation (by injecting into the well). As a result, chelating ions act by a different mechanism respect to the acidification process: while acidification attacks the carbonate anion, the chelating ions act on cations, especially in calcium formations (Fredd & Fogler, 1998).
EDTA is a chelating or complexing compound used in various processes such as the recovery of heavy metals in catalytic processes because it is capable of solubilizing radioactive metals and increasing their mobility. It is used as a chemical agent that helps clean or remove iron oxide and some flakes or buildup on the steam plant systems and hot water circulation (Frey, 1981; Liu & Macdonald, 2001). In the stimulation of oil wells, it is used as a sequestering agent of cation present in the formations. The EDTA also increases the volume of the pores and inhibits the formation of calcareous compounds (Nasr-El-Din et al., 2004; Fredd & Fogler, 1998). When EDTA based fluids are working at low pH, the net effect is the stimulation of the well by dissolving the matrix, increasing the effect of hydrogen during the protonation and chelation processes (Fredd & Fogler, 1998). Furthermore, EDTA compounds found to be corrosive and can increase the rate of corrosion of steel (Palmer & Boden, 1992), generating an adverse situation in oil production facilities by increasing the steel deterioration of the installed infrastructure. The EDTA from the well stimulation fluid can act as a ferrous ion chelating agent (Frey, 1981; Liu & Macdonald, 2001) by stabilization of Fe2+ ions in solution. Additionally, EDTA can act as a cathode depolarizer (Palmer & Boden, 1992) in the electrochemical dissolution of the metal. Both mechanisms increase the steel corrosion.
The EDTA solutions exhibit different pH in equilibrium conditions, depending on the nature of the associated cation. Similarly, pH modification induces changes in the concentration of the species in equilibrium. According to this, the electrochemical behavior of steel in contact with EDTA solution will depend on the kind of EDTA compound present in the aqueous equilibria. The concentrations of the Fe2+ and Fe3+ will also depend on the nature of the EDTA compound. Increases in pH in turn increment the equilibrium dissociation constants for Fe–EDTA chelates, apparently due to the formation of mixed EDTA-hydroxy chelates [FeEDTA(OH)2-and FeEDTA(OH)23-] (Sunda & Huntsman, 2003). In order to obtain valid information of real concentrations of ferric and ferrous ions in the aqueous environment, which help understand the corrosion phenomena of steel in EDTA solution, models of hydrolysis of ferric ions must be considered. Knowledge of this hydrolysis is essential to nderstand control on ferric chelation kinetics, organic and inorganic chelation equilibria and the formation of colloidal systems.
A problem seen in the use of chelating agents EDTA base solutions is that these solutions may increase the corrosion of steel pipe production lines. Although it is known, there are very few studies in which the subject of study is the kinetics of dissolution of steel in EDTA solutions. Also, there is not enough knowledge on the effects of variables such as the hydrodynamic regime, the temperature, the concentration and type of EDTA in the corrosion of steel. Without such information, it is not possible to design effective activities to prevent or mitigate the corrosion of carbon steel.
The EDTA compounds can stimulate the corrosion of carbon steel in chloride solutions. In experiments of mass loss measurements and electrochemical tests, it was found that the increase in the EDTA concentration decreases the inhibition efficiency of some inhibitors (Umamathi et al., 2008). Nevertheless, some researchers have reported the use of EDTA compounds, like the EDTA-Hydroxylamine Sulfate-Fe2+ as corrosion inhibitors for stainless steel (Capobianco et al., 1994). This controversial situation is still unsolved.
In order to reduce or mitigate corrosion problems, control methods such as corrosion inhibitors, paint, surface treatment and cathodic protection are commonly designed. Those control methods must meet certain characteristics of stability, compatibility and reactivity to be effective. With the aim of designing corrosion inhibitors and other alternatives to control corrosion, it is necessary to understand the behavior of the system under operating conditions, as well as understand the kinetics of the electrochemical reactions occurring during the corrosion process. Those allow the characterization of the corrosion process, and the design and implementation of effective solutions for the corrosion problem at a reasonable cost. This paper provides the assessment of the behavior of P-110 steel in EDTA base aqueous fluids under different temperature and hydrodynamic conditions, in order to contribute to the knowledge of the kinetics of dissolution of steel in these fluids and to evaluate the influence of these variables on metal corrosion. Additionally, corrosion rates function with the nature of EDTA, when temperature and hydrodynamic conditions are present, as a contribution to the technology in the area of oil and gas production industry.
2. EXPERIMENTAL METHODOLOGY
Electrochemical measurements were conducted with electrodes manufactured from a P-110 steel bar with nominal composition (wt%): C 0.29, Mn 0.55, Si 0.3, P 0.016, Cr 0.002, Mo 1.01, Al 0.56, Nb 0.026, Cu 0.035, Fe balance. The electrodes were manufactured in order to obtain a Rotating Disc Electrode (RDE) with an area of 0.28 cm2. The lateral surface of the metal bar was insulated with epoxy resin. The RDE were manually polished with sandpaper grade 600. Then, they were washed and degreased in water and ethanol prior to the completion of the measures. Electrochemical measurements were performed in a three electrode cell, using the working electrode (RDE) steel P-110. A Saturated Calomel Electrode (SCE) was used as reference, and a platinum mesh of large area was used as auxiliary electrode. In this work, all potentials are referred to the SCE.
The electrolytes were prepared with ethylenediaminetetraacetic acid disodium salt (EDTA-Na2) 10% w/w or ethylenediaminetetraacetic acid tetrasodium salt (EDTA-Na4) at 20% w/w. The solutions were prepared separately with analytical grade reagents and distilled water. It was necessary to use a lower concentration of EDTA-Na2, due to its low solubility in water at room temperature. The natural pH values of the EDTA-Na2 and the EDTA-Na4 solutions were 4.5 and 11.5, respectively.
The electrochemical measurements such as potentiodynamic polarization curves were made at scan rate of 1mV·s-1, starting from a cathode overpotential of -300 mV to an anodic overpotential of +300 mV with respect to the Open Circuit Potential (OCP). All polarization curves were repeated at least three times in order to verify the reproducibility of the curves. It was verified that all corrosion systems (metal-electrolyte) were very reproducible in all evaluated conditions. Electrochemical measurements were made using an Autolab 302 potentiostat. Before starting the polarization measurements, the OCP was monitored for 45 min to reach its stationary state. Tafel plots were performed at different rotation rates of the electrode (100, 225, 400, 900 and 1600 rpm) and at different temperatures (25, 40 and 80°C). The Electrochemical Impedance Spectroscopy (EIS) measurements were carried out at OCP and at low anodic overpotentials in a frequency range of 40 to 5 mHz with a sinusoidal perturbation amplitude of 10 mV (RMS) and at 6 points per frequency decade. EIS measurements were carried out in separated experiments. EIS measurements at the OCP were made taking a fresh metal surface. Similarly, for each EIS measurement made at overpotentials different to the OCP, fresh metal surface was the initial state of the working electrode. After verification of the OCP, the corresponding overpotential was applied and the steady state of current was guaranteed before starting the EIS measurement. All electrochemical measurements were made using an Autolab PGSTAT 30 Potentiostat. At the most aggressive conditions, the effect of some additives such as a mutual solvent (butyleneglycol) and an emulsifier (Ultrawet®) were tested. From the electrochemical measurements, corrosion parameters were obtained, such as: corrosion potentials (EEcorr), corrosion current densities (jjcorr) and corrosion rates.
3. RESULTS AND DISCUSSION
Hydrodynamic Effects on the Corrosivity of EDTA Based Fluids
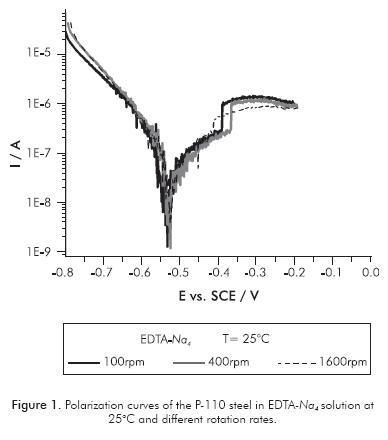
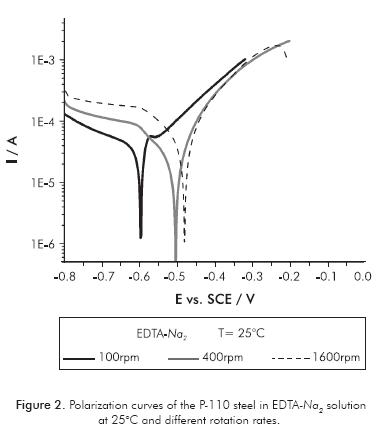
The effect of the hydrodynamic on the system under study was verified by plotting the polarization curves at different rotational rates of the RDE steel electrode P-110. Figures 1 and 2 show polarization curves performed on disodium and tetrasodium EDTA solutions, respectively. No significant effect of the hydrodynamic condition was observed on polarization curves in solution of EDTA-Na4. This means that the electrochemical parameters of metal corrosion as corrosion current density (jjcorr) and corrosion potential (EEcorr) do not change with variation of rotation rate of the electrode. The relationship between the shear stress on a rotating disk and its angular velocity defines the effect of hydrodynamic forces over the corrosion process. The shear stress ( ) is a function of radial distance r from the center of the disk, according to:
) is a function of radial distance r from the center of the disk, according to:

where ρ is the density of the fluid; v is the kinematic viscosity of the fluid, and Ω is the rate of rotation of the disk. As shown, the maximum shear stress occurs at the edge of the disk.
The average shear stress on the disk <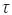 > is (Binyamin & Heller, 1999):
> is (Binyamin & Heller, 1999):
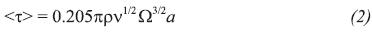
where α is the radius of the disk.
The average shear stress <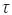 > calculated for the rotation rates used in this work (
> calculated for the rotation rates used in this work (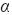 = 3x10-3 m) takes values of 0.06, 0.20, 0.5, 1.67 and 3.95 N·m-2; corresponding to rotation rates of 100, 225, 400, 900 and 1600 rpm, respectively. The calculi of (
= 3x10-3 m) takes values of 0.06, 0.20, 0.5, 1.67 and 3.95 N·m-2; corresponding to rotation rates of 100, 225, 400, 900 and 1600 rpm, respectively. The calculi of (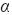 ) were made assuming values for ρ = 103 kg·m-3 and v = 8.9 x10-7 m2·s-1, which are typical values for a low concentration EDTA aqueous solution (Gambardella et al., 2006). It was verified that the steel P-110 in EDTA-Na4 solution at 25°C exhibits Ecorr = -0.54 V and jcorr = 2.3x10-7 A·cm-2 at all rotation rates. The hydrodynamic forces do not affect the corrosion process in this electrolyte because the kinetic of metal dissolution is controlled essentially by the electrochemical reaction.
) were made assuming values for ρ = 103 kg·m-3 and v = 8.9 x10-7 m2·s-1, which are typical values for a low concentration EDTA aqueous solution (Gambardella et al., 2006). It was verified that the steel P-110 in EDTA-Na4 solution at 25°C exhibits Ecorr = -0.54 V and jcorr = 2.3x10-7 A·cm-2 at all rotation rates. The hydrodynamic forces do not affect the corrosion process in this electrolyte because the kinetic of metal dissolution is controlled essentially by the electrochemical reaction.
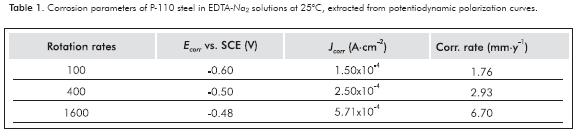
Moreover, in the solution of EDTA-Na2, a significant effect of the hydrodynamics was observed on the polarization curves and on the electrochemical parameters of the system. In EDTA-Na2 solution, the polarization curves are shifted to more anodic potentials; ie Ecorr is more positive as increasing the rotation rates of the electrode. Also, current densities and corrosion rates increase, as shown in Table 1.
A corrosion phenomenon is related with the behavior of P-110 steel immersed in disodium EDTA based solutions. It is worth noting that at very low anodic overpotentials, some processes of passivation and transpassivation appear at 30 and 50 mV, respectively. These processes are evident only at low rotation rates (100 and 225 rpm) and low temperatures (25 and 40°C). For high hydrodynamic conditions and high temperatures, such processes disappear and the anodic dissolution of metal concomitantly increases with polarization. The fact that the phenomenon of passivation of P-110 steel in the EDTA-Na2 solution is not manifest at high hydrodynamic conditions, indicates that the process of the passive film formation occurs through kinetics of formation-saturation-precipitation. In the EDTA-Na4 solution, no anodic passivation process was present in all evaluated conditions.
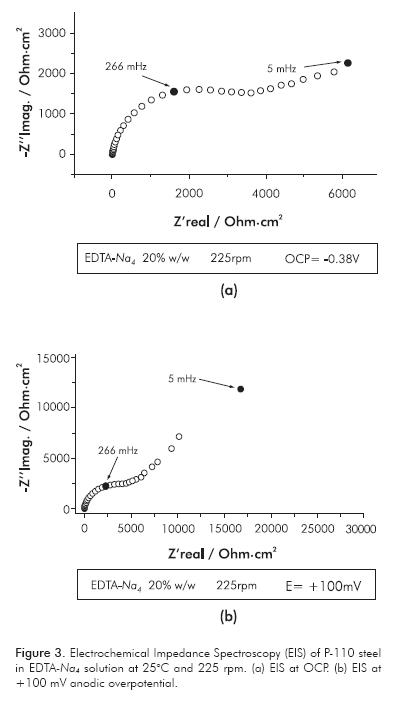
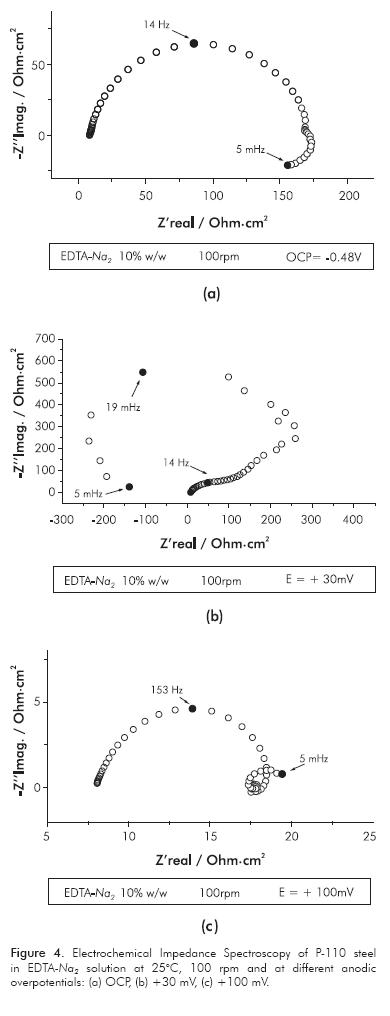
Figure 3 shows the EIS of P-110 steel in EDTA-Na4 solution at OCP and at +100 mV of anodic overpotential. The impedance diagrams are constituted by two capacitive loops, see Figure 3a. The first one is related with the double layer capacitance in parallel with charge transfer resistance of the anodic reaction. The charge transfer resistance and the capacitance of the steel at OCP have values of 2.8 kOhm·cm2 and 700 µF·cm-2, respectively. The capacitance value extracted from the first capacitive loop of the impedance is higher than those expected for a double layer capacitance of the free-accessible surface metal; it seems better to have values similar to the metal/oxide systems, which can develop values between 170-800 µF·cm-2 (Hiemstra & Van Riemsdijk, 1991). In our case of study we observed a capacitance value of 700 µF·cm-2. This value of capacitance is related to the first capacitive loop observed at high frequencies in the impedance diagram recorded at OCP and at low anodic overpotentials in the EDTA-Na4 electrolyte. The high value of capacitance of this loop indicates that there is a coupling between two relaxation processes, the charging of the electric double layer and the iron oxide film formation. The time constant of these processes is probably very similar, making not possible to observe the processes separately. According with those values, it is conceivable to have low corrosion rates and the existence of a resistive anodic film formed at the metal surface. When the anodic polarization is applied from the OCP, the capacitive loops increase in size, see Figure 3b. Consequently, the charge transfer resistance and the polarization resistance are increased.
Figure 4 shows the EIS of P-110 steel in EDTA-Na2 solution at OCP, +30 and +100 mV of anodic overpotential. Differently to that showed in EDTA tetrasodim salt, the impedance diagram at OCP is constituted by a capacitive loop at high frequencies and an inductive loop at low frequencies, Figure 4a. The existence of an inductive loop at OCP is normally related with the active metal dissolution in the corrosion phenomena (Keddam, Mattos & Takenouti, 1981). Additionally, the values of the charge transfer resistance and the capacitance, extracted from the capacitive loop at high frequencies, of the metal in EDTA disodium salt are lower than those exhibited in EDTA tetrasodium salt, being values of 160 Ohm·cm2 and 450 µF·cm-2, respectively. The impedance results confirm that increasing of metal dissolution and superior corrosion rates are expected in the disodium salt. At low anodic overpotential (+30 mV), the impedance ratifies the passivation of carbon steel, showing a negative resistance at the limit of the low frequencies, as showed by polarization curves in the EDTA-Na2 electrolyte, see Figure 4b. At high anodic polarization, the dissolution of steel occurs at high rates and through a more complicate dissolution mechanism, in which several intermediates species must be considered. This happens because impedance diagrams show at least two capacitive loops and two inductive loops related with faradaic processes during the anodic dissolution of the steel, as can be seen in Figure 4c.
There are also important differences in the kinetics of cathodic reactions occurring on the surface of P-110 steel when immersed in disodium or tetrasodium EDTA solutions. By observing the cathodic branch of polarization curves shown in Figures 1 and 2, it can be clearly seen that in the EDTA-Na4 solution, there is only one cathodic slope, most likely associated with the oxygen reduction reaction. Furthermore, in the cathodic branch of the polarization curves performed in EDTA-Na2 there are two cathodic slopes. The first one, being more accentuated, is probably associated to hydrogen reduction reaction, since this would be the preferred cathodic reaction at low cathodic overpotentials due to the low pH of the electrolyte (Flitt & Schweinsberg, 2005). A second cathodic slope is observed at higher cathodic overpotential, most likely associated with the reduction reaction of dissolved oxygen in the electrolyte. However, the EDTA specie can also be reduced in the cathodic branch of the polarization curve, since it has been found that EDTA may act differently to chelation of Fe2+ ions in the solution (Palmer & Boden, 1992). EDTA could be reduced from the acid (R-COOH) to an aldehyde (R-CHO), according to reaction 3. The reduction reaction of EDTA-Na2 , in this case, acts as a cathodic depolarizer increasing metal dissolution.
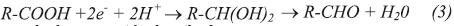
Temperature Effect on the Corrosivity of EDTA Fluids
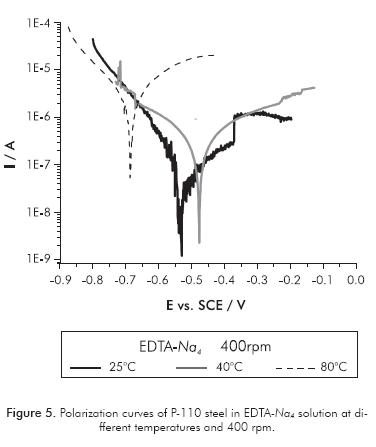
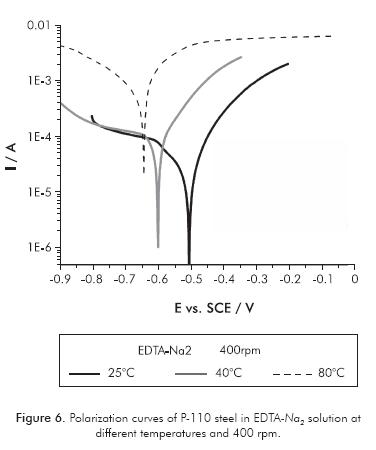
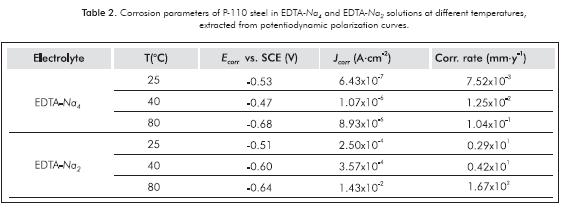
Figures 5 and 6 show the polarization curves of P-110 steel carried out in tetrasodium EDTA and disodium EDTA solutions, respectively. In both solutions there is a significant effect of temperature on the corrosivity of the electrolyte. Table 2 shows the parameters of steel corrosion in both solutions at different temperatures and defined hydrodynamic conditions for electrode rotation rate of 400 rpm.
The temperature increase accentuates the corrosion kinetics of P-110 steel, thereby significantly increasing the corrosion rate of the metal in both EDTA solutions. In general terms, it also highlights a shift of the corrosion potential to more negative values with increasing temperature, indicating higher activation of anodic and cathodic reactions occurring during the electrochemical dissolution. The increasing temperature of 80°C causes metal corrosion increase by at least 2 orders of magnitude in both electrolytes.
In the EDTA-Na2 solution, the two Tafel slopes in the cathodic branch gradually fade with the increasing of temperature, resulting in only one cathodic slope at high temperatures. Thus, it is possible that the cathodic reaction -which occurs at high cathodic overpotentials (oxygen reduction)- becomes less significant, probably due to the decrease of solubility of oxygen with increasing temperature. This makes the cathodic reduction reaction of EDTA more predominant at high temperatures, being this reaction responsible for the increase in the dissolution of metal; although, the proton reduction is still present at low cathodic overpotentials.
Effect of the Type of EDTA on the Corrosivity of the Fluids
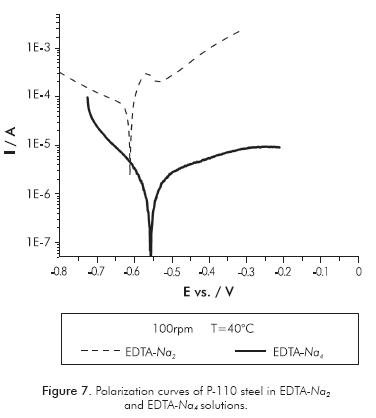
The base fluid EDTA-Na2 was much more corrosive than EDTA-Na4 in all temperatures and hydrodynamic conditions evaluated. Figure 7, for instance, presents the polarization curves carried on P-110 steel at 100 rpm and 40°C in both electrolytes. It can be seen that the polarization curve in EDTA-Na2 moves towards more negative potentials, indicating stronger activation of anodic and cathodic reactions occurring during the electrochemical dissolution. This is corroborated by comparing the corrosion rates values obtained at these conditions in the tetrasodium and disodium EDTA solutions, which are 7.5x10-3 and 2.9 mm·y-1, respectively. In solutions of disodium, EDTA was obtained at much higher corrosion rates, even when the EDTA disodium was in lower concentration than the tetrasodium EDTA solution, in all conditions tested. The difference in fluid corrosiveness of both EDTA electrolytes could be explained by the difference of chelation power of EDTA on the Fe2+ ions at different pH values (Barashev, Karelov, Anisimova & Manyachenkov, 2011). Conditional formation constant Kf' for EDTA solutions depends on pH value and are defined by the equation:
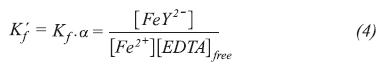
For reaction:

Where Kf is the formation constant of the complex [FeY2-] and has a value of 2.0x1014. 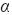 is the conditional constant, which is pH dependent and defines the fraction of EDTA remains as [Y4-]. [EDTA]free is the fraction of free EDTA in solution. For the pH values studied in this work, the conditional constants values for EDTA-Na2 and EDTA-Na4 are 3.67x10-8 and 0.95, respectively.
is the conditional constant, which is pH dependent and defines the fraction of EDTA remains as [Y4-]. [EDTA]free is the fraction of free EDTA in solution. For the pH values studied in this work, the conditional constants values for EDTA-Na2 and EDTA-Na4 are 3.67x10-8 and 0.95, respectively.
According to these values and considering molar concentration of 0.297 M EDTA-Na2 and 0.526 M EDTA-Na4, the ratio [FeY 2-]/[Fe 2+] would be 2.18x106 for EDTA-Na2 and 1.0x1014 for EDTA-Na4. According to these values, the concentration of free uncomplexed Fe2+ ion is much higher in the solution of disodium EDTA than in the tetrasodium EDTA, which could explain why disodium solution is more corrosive.
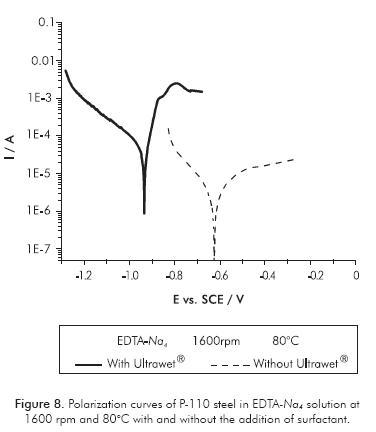
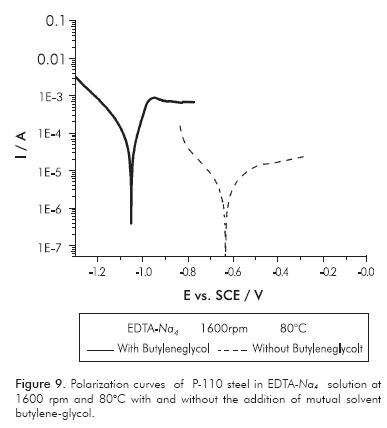
Effect of the Additives in the Corrosivity of EDTA Base Fluids
During the well stimulation operation, it is desirable in many cases to add some chemicals agents into the fluid in order to make the operation most efficient or prevent unwanted damage. The butylene-glycol is a mutual solvent which is added with the aim of removing heavy hydrocarbon deposits, and controlling the wettability of the surfaces during and after treatment. Sometimes surfactants are also added to stimulation fluids in order to prevent the formation of emulsions and improve the wettability. The Ultrawet® is a polyethoxylated nonionic surfactant used for such purposes. Potentiodynamic polarization show that additions of butylene-glycol and the Ultrawet® into EDTA-Na2 solution do not produce changes in the corrosivity of the solutions at the different hydrodynamic and temperature conditions evaluated. Furthermore, it was found that in the EDTANa4 solutions, corrosivity was altered. In Figures 8 and 9, the polarization curves of P-110 steel immersed in EDTA-Na4 solutions are shown, with and without the addition of additives Ultrawet® and butylene-glycol. It was observed that such additions increase the rate of steel corrosion in the tetrasodium solution at least at one order of magnitude.
4. CONCLUSIONS
-
Increasing the temperature from 25 to 80°C in both EDTA electrolytes increment the rate of corrosion of steel in at least one order of magnitude. In EDTA-Na4 solutions, corrosion rates of 7.52x10-3 and 1.04x10-1 mm·y-1 were obtained at 25 and 80°C, respectively; while in EDTA-Na2 solutions, corrosion rates were 0.29x101 and 1.67x102 mm·y-1 in the same temperatures. The hydrodynamic plays an important role in the corrosion of P-110 steel only in disodium EDTA solution. With the increasing of rotation rate of the electrode, current densities and corrosion rates also went up. In EDTA-Na2 solutions, corrosion rates of 1.76 and 6.70 mm·y-1 were obtained at 100 and 1600 rpm, respectively. The cathodic depolarization effect on the EDTA-Na2 is more important than in the EDTA-Na4 solution, making it more corrosive. Similarly, the concentration of the free uncomplexed Fe2+ ion is much higher in the disodium EDTA solution than in the tetrasodium electrolyte, which contributes to the corrosivity of the disodium electrolyte. Some additives, such as demulsifiers and solvents, increase the corrosion rate of the P-110 steel only tetrasodium electrolyte.
ACKNOWLEDGEMENTS
The authors thank Colciencias (Contract 0381-2013 Diáspora 2011) and Universidad de Antioquia (CODI Project N° MDC10-1-03) for the support received for this work.
REFERENCES
Barashev, A. R., Karelov, S. V., Anisimova, O. S. & Manyachenkov, S. V. (2011). Innovative technology for recycling the negative segments of alkaline batteries using recoverable solvent. Metallurgist, 55(5-6), 381-385. [ Links ]
Binyamin, G. & Heller, A. (1999). Stabilization of wired glucose oxidase anodes rotating at 1000 rpm at 37°C. J. Electrochem. Soc., 146(8), 2965-2967. [ Links ]
Cai, Y. D., Guo, P. C., Liu, D. M., Chen, S. Y. & Liu, J. L. (2010). Comparative study of CO2 corrosion behavior of N80, P110, X52 and 13Cr pipelines in simulated stratum water. Sci. China Technol. Sci., 53(9), 2342-2349. [ Links ]
Capobianco, G., Goatin, C., Moretti, G., Patron, S. & Toniolo, L. (1994). EDTA-hydroxylamine sulfate-Fe2+: An efficient corrosion inhibitor system for an industrial crystallization plant. Corros. Sci., 50(11), 886-897. [ Links ]
Carvalho, D. S., Joia, C. J. B. & Mattos, O. R. (2005). Corrosion rate of iron and iron-chromium alloys in CO2 medium. Corros. Sci., 47(12), 2974-2986. [ Links ]
Flitt, H. J. & Schweinsberg, D. P. (2005). A guide to polarisation curve interpretation: deconstruction of experimental curves typical of the Fe/H2O/H+/O2 corrosion system. Corrosion Science, 47(9), 2125-2156. [ Links ]
Fredd, C. N. & Fogler, H. S. (1998). The influence of chelating agents on the kinetics of calcite dissolution. J. Colloid Int. Sci., 204(1), 187-197. [ Links ]
Frenier, W. W., Fredd, C. N. & Chang, F. (2001). Hydroxyaminocarboxylic acids produce superior formulations for matrix stimulation of carbonates at high temperatures. SPE Annual Technical Conference and Exhibition, New Orleans. SPE-71696-MS. [ Links ]
Frey, D. A. (1981). Case histories of corrosion in industrial boilers. Mater. Performance, 20(2), 49-55. [ Links ]
Gambardella, F., Galán-Sánchez, L. M., Ganzeveld, K. J., Winkelman, J. G. M. & Heeres, H. J. (2006). Reactive NO absorption in aqueous FeII(EDTA) solutions in the presence of denitrifying micro-organisms. Chem. Eng. J., 116: 67-75. [ Links ]
Hiemstra, T. & Van Riemsdijk, W. H. (1991). Physical chemical interpretation of primary charging behaviour of metal hydroxides. Colloids Surf., 59(8), 7-25. [ Links ]
Keddam, M., Mattos, O. R. & Takenouti, H. (1981). Reaction model for iron dissolution studied by electrode impedance. I. Experimental Results and Reaction Model. J. Electrochem. Soc., 128(2), 257-266. [ Links ]
Lin, N. M., Xie, F. Q., Zhou, J., Zhong, T., Wu, X. Q. & Tian, W. J. (2010). Microstructures and wear resistance of chromium coating on P110 steel fabricated by pack cementation. J. Cent. South Univ. Technol., 17(6), 1155-1162. [ Links ]
Liu, J. & Macdonald, D. D. (2001). The passivity of iron in the presence of ethylenediaminetetraacetic acid. II. The defect and electronic structures of the barrier layer. J. Electroch. Soc., 148(11), B425-B430. [ Links ]
Migahed, M. A. & Nassar, I. F. (2008). Corrosion inhibition of Tubing steel during acidization of oil and gas wells. Electrochim. Acta, 53(6), 2877-2882. [ Links ]
Moreira, R. M., Franco, C. V., Joia, C. J. B., Giordana, S. & Mattos, O. R. (2004). The effects of temperature and hydrodynamics on the CO2 corrosion of 13Cr and 13Cr5Ni2Mo stainless steels in the presence of free acetic acid. Corros. Sci., 46(12), 2987-3003. [ Links ]
Nasr-El-Din, H. A., Al-Othman, A. M., Taylor, K. C. & Al-Ghamdi, A. H. (2004). Surface tension of HCl-based stimulation fluids at high temperatures. J. Petrol. Sci. Eng., 43(1-2), 57-73. [ Links ]
Palmer, J. W. & Boden, P. J. (1992). Corrosion of steel in EDTA. Br. Corros. J., 27(4), 305-309. [ Links ]
Portier, S., Vuataz, F. D., Nami, P., Sanjuan, B. & Gérard, A. (2009). Chemical stimulation techniques for geothermal wells: experiments on the three-well EGS system at Soultz-sous-Forêts, France. Geothermics, 38(4), 349-359. [ Links ]
Sunda, W. & Huntsman, S. (2003). Effect of pH, light, and temperature on Fe-EDTA chelation and Fe hydrolysis in seawater. Mar. Chem., 84(1-2), 35-47. [ Links ]
Umamathi, T., Selvi, J. A., Kanimozhi, S. A., Rajendran, S. & Amalraj, A. (2008). Effect of Na3PO4 on the corrosion inhibition efficiency of EDTA-Zn2+ system for carbon steel in aqueous solution. Indian J. Chem. Technol., 15(6), 560-565. [ Links ]
AUTHORS
Jorge Andrés Calderón Gutiérrez
Affiliation: Universidad de Antioquia
Metallurgical Engineer, Universidad de Antioquia
M. Sc. in Chemistry Science, Universidad de Antioquia
Ph. D. in Metallurgical and Materials Engineering, Universidade Federal de Rio de Janeiro
Post Ph. D. in Metallurgical and Materials Engineering, Universidade Federal de Rio de Janeiro
e-mail: jcaldergut@gmail.com
Gloria Fernanda Bonilla
Affiliation: Universidad de Antioquia
Chemistry Student, Universidad de Antioquia
e-mail: gfbonillam@gmail.com
Javier Alejandro Carreño
Affiliation: Instituto Nacional de Tecnología
Metallurgical Engineer, Universidad Industrial de Santander
M. Sc. in Metallurgical and Materials Engineering, Universidade Federal de Rio de Janeiro
Ph. D. in Metallurgical and Materials Engineering, Universidade Federal de Rio de Janeiro
e-mail: javier.alejandro@int.gov.br