Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
CT&F - Ciencia, Tecnología y Futuro
versão impressa ISSN 0122-5383
C.T.F Cienc. Tecnol. Futuro vol.5 no.4 Bucaramanga jan./jun. 2014
BEHAVIOR OF ALUMINUM COATING BY CVD-FBR IN STEAM OXIDATION AT 700°C
COMPORTAMIENTO DEL RECUBRIMIENTO DE ALUMINIO POR CVD-FBR EN LA OXIDACIÓN EN VAPOR A 700ºC
José-Luddey Marulanda-Arévalo1, 2*, Francisco-Javier Pérez-Trujillo2 and Saúl-Isaac Castañeda-Quintana2
1Universidad Tecnológica de Pereira, Pereira, Risaralda, Colombia
2Universidad Complutense de Madrid, Madrid, España
E-mail: jlmarulanda@utp.edu.co
How to cite: Marulanda-Arévalo, J. L., Pérez-Trujillo, F. J. & Castañeda-Quintana, S. I. (2014). Behavior of aluminum coating by CVD-FBR in steam oxidation at 700°C. CT&F - Ciencia, Tecnología y Futuro, 5(4), 75-84.
+XII Congreso Nacional de Corrosión y III Internacional de Integridad 2013, Universidad Tecnológica de Pereira and Asociación Colombiana de Corrosión y Protección (ASCOR), Colombia, 8 - 10, May 2013.
(Received: Nov. 07, 2013; Accepted: Jun. 03, 2014)
*To whom correspondence should be addressed
ABSTRACT
Aluminum coatings have been obtained using the Chemical Vapor Deposition of Fluidized Bed Reactor technique (CVD-FBR), on austenitic stainless steels (AISI 304, AISI 316 and AISI 317), in order to evaluate resistance to steam oxidation at 700ºC. The coatings were characterized in their morphology/composition and crystalline phases using Scanning Electron Microscopy (SEM), Energy Dispersive X-ray Analysis (EDAX) and X-ray Diffraction (XRD). In addition, thermodynamic simulation was performed using Thermo-Calc® software to make the deposition of the coating on said steels similar to optimal conditions. All specimens -with and without aluminum coating by CVD-FBR- were oxidized at 700ºC, in a steam atmosphere of nearly 100% for 1000 h. The aluminum coating prevented the formation of oxide islands or crust, and reduced steel mass gain. Coated AISI steel showed a 25% reduction in mass gain compared to the same steel uncoated and oxidized under the same conditions. AISI 316 and AISI 317 steels underwent loss of mass because the brittle oxide that was formed came loose.
Keywords: Aluminum coatings, Chemical Vapor Deposition in Fluidized Bed Reactors (CVD-FBR), High-temperature corrosion, Steam oxidation, Austenitic stainless steels.
RESUMEN
Se han obtenido recubrimientos de aluminio por la técnica de deposición química de vapor por lecho fluidizado (CVD-FBR de sus siglas en inglés), sobre aceros inoxidables austeníticos (AISI 304, AISI 316 y AISI 317), con el propósito de evaluar la resistencia a la oxidación en vapor de agua a 700ºC. Los recubrimientos fueron caracterizados en su morfología/composición y fases cristalinas mediante las técnicas de Microscopía Electrónica de Barrido (MEB), con análisis de rayos X por dispersión de energía (EDAX de sus siglas en ingles) y Difracción de Rayos X (DRX). Además, se realizó simulación termodinámica mediante el software comercial Thermo-Calc®, con la finalidad de aproximar a las condiciones óptimas la deposición del recubrimiento sobre los mencionados aceros. Todos los especímenes sin y con recubrimiento de aluminio por CVD-FBR, fueron oxidados a la temperatura de 700ºC, en una atmósfera próxima al 100% de vapor de agua por 1000 h. El recubrimiento de aluminio evitó la formación de islas o costra de óxidos y disminuyó la ganancia de masa de los aceros. El acero AISI 304 con recubrimiento presentó una reducción del 25% en la ganancia de masa en comparación con el mismo acero sin recubrimiento y oxidado en las mismas condiciones; los aceros AISI 316 y AISI 317 presentaron pérdida de masa, debido a que el óxido formado se desprende por su naturaleza quebradiza.
Palabras clave: Recubrimientos de aluminio, Deposición química por lecho fluidizado (CVD-FBR), Corrosión a alta temperatura, Oxidación en vapor de agua, Aceros inoxidables austeníticos.
RESUMO
Foram obtidos recobrimentos de alumínio pela técnica de deposição química de vapor por leito fluidizado (CVD-FBR por suas siglas em inglês), sobre aços inoxidáveis austeníticos (AISI 304, AISI 316 e AISI 317), com o propósito de avaliar a resistência à oxidação em vapor d’água a 700ºC. Os recobrimentos foram caracterizados em sua morfologia/composição e fases cristalinas mediante as técnicas de Microscopia Eletrônica de Varredura (MEB), com análise de raios X por dispersão de energia (EDAX por suas siglas em inglês) e Difração de Raios X (DRX). Além disso, foi realizada simulação termodinâmica mediante o software comercial Thermo-Calc®, com a finalidade de aproximar às condições ótimas a deposição do recobrimento sobre os mencionados aços. Todos os espécimes, sem e com recobrimento de alumínio por CVD-FBR, foram oxidados à temperatura de 700ºC, em uma atmosfera próxima a 100% de vapor d’água por 1000 h. O recobrimento de alumínio evitou a formação de ilhas ou camada de óxidos e diminuiu o ganho de massa dos aços. O aço AISI 304 com recobrimento apresentou uma redução de 25% no ganho de massa em comparação com o mesmo aço sem recobrimento e oxidado nas mesmas condições; os aços AISI 316 e AISI 317 apresentaram perda de massa, devido a que o óxido formado se solta por sua natureza quebradiça.
Palavras-chave: Recobrimentos de alumínio, Deposição química por letio fluidizado (CVD-FBR), Corrosão a alta temperatura, Oxidação em vapor d’água, Aços inoxidáveis austeníticos.
1. INTRODUCTION
Chemical Vapor Deposition by Fluidized Bed Reactor (CVD-FBR) is a process that combines the main advantages of fluidized beds, which are: temperature uniformity, mass and heat transfer, and a high degree of gas mixing with fluidized particles. The aforementioned lead to the reaction of all the active species in the bed, combined with the benefits of chemical vapor deposition, such as the ability to control with relative ease the composition of the deposited material, obtaining single, multiple or compound layers, and in general, structured nano materials with dimensions smaller than 1 µm (Lau, Sanjurjo & Wood, 1992; Anthymidis, Stergioudis & Tsipas, 2001). The CVD-FBR process enables the deposition of coatings in the temperature range from 300 to 900ºC, obtaining a variety of wear resistant layers of C, N, CN, Ti, V, Cr, Nb, Ta, Mn, W and Mo on several types of metals such as Fe, Ni, Co and the alloys thereof (Pierson, 1999; Arai, Takeda & Endo, 1989). In addition, by means of CVD-FBR at one atmosphere of pressure, coatings have been obtained in a temperature range from 300 to 1200ºC, of Al, Cr, Si, B and Ti, in order to improve the behavior of ferritic and austenitic steels against oxidation, corrosion at high temperatures and wear (Stoloff, Liu & Deevi, 2000; Lau et al., 1992; Bolívar, 2007).
In recent years, research has been done on obtaining coatings that will make it possible to reduce oxidation problems and contribute to increasing operating temperatures of thermal power plants, without the materials losing their mechanical, structural and morphological properties. That is why studies have been conducted on Al, Mn, Si, Ti-N, Cr-N, Al-Mn, Al-Si and other coatings applied by CVD, deposited on different steels (ferritic, martensitic, austenitic, etc.) These coatings reached a thickness of 10 µm on average and were evaluated in extreme steam oxidation conditions at temperatures from 500 to 800ºC (Maitra & Gupta, 2003; Ehlers et al., 2006; Agüero et al., 2007; Bolívar, 2007). The increase in operating temperature would improve the thermal efficiency of power generation plants, thus reducing the quantity of fossil fuel used and the emissions of greenhouse gases such as CO2, SO2 and NOx. These are known for being harmful to the atmosphere, the climate and the future of the planet (Ennis & Czyrska-Filemonowicz, 2003). The aluminum coating deposited by CVD has oxygen affinity, thus allowing the formation of the layer of alumina (Al2O3), which increases protection against steam oxidation (Sánchez, Bolívar, Hierro & Pérez, 2009; Marulanda, Castañeda & Remolina, 2013; Bolívar, 2007).
Lau et al. (1992), studied the reactions of the Al bed and activator gases HCl (g)+H2 in the temperature range from 127 to 627°C, using a mass spectrometer to obtain information on the growth process of the Al coating on Cu by CVD-FBR. Based on the results, it was verified that the halogenated precursors formed were aluminum chlorides Al3Cl(g) and AlCl(g). It was observed that AlCl(g) was the most important gas precursor in the mechanism to form the layer of Al. The authors managed to deposit a fine layer of Al on copper wire using a mixture of Ar with 0.12% HCl and 7% of H2 by volume.
Pérez et al. (2001) researched the obtainment of the Al coating at temperatures below 600°C by CVD-FBR (Sánchez et al., 2009). In order to do so, they used a bed formed by Al dust, which was fluidized with Ar using the mixture of HCl/H2 gases as an activator. The experiments were conducted in the temperature range from 500 to 600°C on different types of substrates, such as ferritic stainless steels, austenitic stainless steels and Ni-based super alloys. By using this system, they managed to obtain homogeneous coatings formed by the intermetallic compounds Fe2Al5 and FeAl3, for the Fe alloys. For the Ni alloys, it was determined that the layers were formed mainly by Ni2Al3 and NiAl.
This paper evaluates the advantages of the aluminum coating deposited using the CVD-FBR technique on austenitic stainless steels, in order to improve resistance to steam oxidation of the substrate. The coatings were treated thermally to form layers or iron aluminides (intermetallic compounds) on the respective steel, responsible for protection against steam oxidation, because they act as a source of aluminum for the formation of alumina (Al2O3), upon contact with steam during oxidation. Due to the high melting point of alumina, this protective layer prevents the volatilization of chromium during steel oxidation, in the form of oxides, hydroxides and chromium oxyhydroxides, which are volatile species that have been reported in other papers (Ebbinghaus, 1993; Asteman, Svensson & Johansson, 2002; Pérez & Castañeda, 2007). In austenitic steel without aluminum coating, oxidation accelerates at 700ºC due to the presence of steam, forming major crusts or islands of iron oxides, among which wustite (FeO) is the most harmful. There is also a considerable increase in the thickness of the oxide in the order of millimeters, creating internal strain with the substrate due to the size. This results in the oxide breaking and detaching from the substrate, which is in line with Asteman et al. (2002).
2. EXPERIMENTAL PROCEDURE
Preparation of Substrates
The tubes of uncoated austenitic stainless steel (AISI 304, AISI 316 and AISI 317) were machined in dimensions of 20 mm long x 6 mm wide x 2 mm thick, sanded using SiC emery paper from No. 100 to No. 600, and finally cleaned in an acetone bath by ultrasound for 10 minutes. The dimensions of all the test tubes were measured using a Vernier gauge with an accuracy of 0.05 mm.
Thermodynamic Simulation
Thermodynamic simulations were conducted using the Thermo-Calc program (Thermo-Calc®, 2003) to estimate the deposition parameters of the Al coating by CVD-FBR. Theoretic calculations are an initial approximation of the coating deposition conditions on the metal substrates, taking into account substrate temperature, pressure (1 atm), percentages of reactant gases (H2 and HCl), inert fluidizing gas (Ar), aluminum as filler material and fluidized bed material (Al2O3), among others. The estimated parameters were tested using experimental deposition, and those that demonstrated the best behavior were selected. Thermodynamic simulation consisted of analyzing the precursors that could be formed upon passage of the Ar, HCl and H2 gases. In addition, to achieve better aluminum coating, bed mass was estimated based on the proportions of the gases mentioned above. The simulation was carried out in the 500 to 660ºC temperature range, which was chosen based on previous papers (Pérez et al., 2001), in order to refine and improve coating deposition parameters. Furthermore, thermodynamic simulations of vapor oxidation were conducted on steel with and without Al coating, and in conditions similar to pilot tests. This was done to estimate the possible solid phases of oxides and gaseous species that could be formed during the respective testing. Thermo-Calc®, is a thermodynamic simulation program based on the Gibbs free energy minimization method, which calculates the thermodynamic balance of each simulated process.
Coating Deposition
The aluminum coatings were deposited by CVD-FBR at atmospheric pressure. The beds used were composed of aluminum dust of a purity by weight of 99.55%, with an average particle size of 400 μm, being alumina (Al2O3) used as a fluidized bed. The dust mixture was fluidized by inert argon gas (Ar) of a purity by weight of 99.999%. In addition, hydrochloric acid (HCl) –of a purity by weight of 99.999%– was used as a reactive gas, and hydrogen (H2) –of a purity by weight of 99.999%– was used as a reductive gas. The coatings deposited were heat-treated in the same reactor, in an argon atmosphere at 700ºC for 4 hours, in order to form the desired iron aluminides on the surface. All the substrates –with and without coating– were weighed on an analytical balance with an accuracy of 10-5g.
Steam Oxidation
Samples were oxidized in a steam atmosphere at 700ºC in a closed, cyclic oxidation reactor "Oxidation Loop" (Commercial instrument of Iberlabo S. A., Madrid, Spain). The system is made up of a steam generator with two furnaces at a constant temperature, one to generate steam and the other where the test samples are placed: type K thermocouples (chromel/alumel), gas controllers, pressure gauges, a water pump, teflon hoses, a quartz container to store water and steam condensers, among others (Marulanda, 2013). The steam is transported to the loop by a continuous flow of 2.4 ml/min of water and an N2 gas flow of 1700 ml/min. All the samples in this paper were oxidized in an atmosphere of approximately 100% steam during 1000 h of exposure. 16 samples were presented in each oxidation test, all of which were of the same material (austenitic stainless steel with or without coating). In order to observe the respective oxidation kinetics (mass vs. time gradient) of each sample, two samples were removed systematically at oxidation time intervals of 24, 50, 100, 200, 400, 600, 800 and 1000 h. They were all previously cooled in dry air to be weighed on the analytical balance. The morphology of the oxidized samples was characterized by Scanned Electron Microscopy (SEM). The crystalline phases present in the coatings were identified by X-ray Diffraction (XRD), in modes of (θ-2θ) and grazing angle, composition by Energy Dispersive X-ray Analysis (EDAX), and, in the surface, concentration profile and volume analysis.
3. RESULTS AND ANALYSIS
Simulation results indicated that aluminum halides such as AlCl3, AlCl2H, AlCl, Al2Cl6 could be generated, along with others in smaller proportions, such as: AlCl2 and AlClH2. In order to verify the theoretic results of thermodynamic simulation, experimental Al deposition tests were conducted systematically on austenitic AISI 304, AISI 316 and AISI 317 steels by CVD-FBR, until the best Al coating was found. That is to say, the thickest one with the most compact surface, best crystallinity and best thickness uniformity, among others. Results indicated that the best conditions occurred when the coating was deposited at a temperature of approximately 580ºC for 1.5 h, in a bed consisting of 10% by weight of aluminum dust and 90% by weight of inert bed (alumina), with a 1/18.5 HCl/H2 activator gas ratio. The active and neutral gas rate is 38% by volume of active gases and 62% by volume of neutral Ar gas.
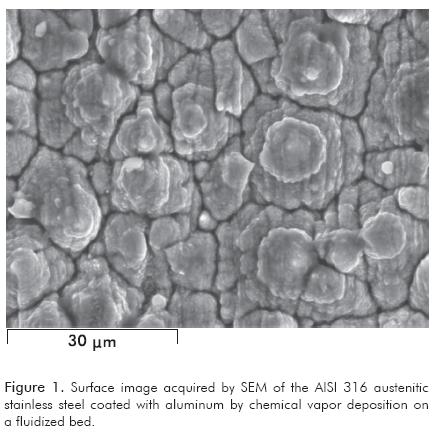
Figure 1 illustrates the image acquired by SEM at 1800 x of the surface of a sample of Al coating deposited on AISI 316 steel. It shows a surface with a rough morphology and the growth of globular grains of circular nucleation, and the formation of apparent cones with a porous constitution in the form of flakes. The chemical composition analysis by EDAX of the sample surface indicated a content in percentage by weight of: 50% of Al, 26% of Fe, 10% of Cr, 11% of Ni, 1% of Mn and a minor diffusion of Mo of the substrate of 0.88%.
The coating of Al by CVD-FBR on coated austenitic steels proved to be homogeneous, compact, crystalline and had a minimal variation in thickness of 8% (+/- 2.5 µm). Figure 2 illustrates two cross sections with the respective line analysis there of, showing the concentration profile of the aluminum coating on the AISI 304 stainless steel substrate, with and without thermal treatment in Ar at 700ºC. Results showed an atomic 75% Al on the surface of the sample without treatment (Figure 2a), which decreases to nearly atomic 40% Al in composition near the substrate-coating interface. They also showed Fe concentrations between atomic 20 and 30%, Cr between atomic 10 and 20%, and a minor amount of Ni up to atomic 10% dissolved in the coating. The largest Ni concentration on the surface corresponds to the metallization of the sample to prevent the coating from breaking when subjected to grinding or polishing for observations by SEM. The line analysis by EDAX in Figure 2b shows the chemical concentration profile of the sample with thermal treatment, clearly showing the diffusion of Al near the inside of the steel and Fe near the surface, which according to the stoichiometry thereof may be approximated to certain iron aluminides such as FeAl.
XRD analysis of the samples determined the presence of phases of: Al13Fe4, Fe2Al5, FeAl2 and Al5FeNi in the sample without thermal treatment. The sample with treatment showed the phases of FeAl, Al0.99Fe0.99Ni0.02, AlNi and Fe2AlCr. The results confirmed the composition analysis by EDAX illustrated in Figure 2, which validates the effect of thermal treatment in relation to the crystallinity of the sample, indicating the presence of the FeAl phase, considered stable and acting as a source of Al for the formation of the alumina layer, and helping to protect the sample from steam oxidation at 700°C.
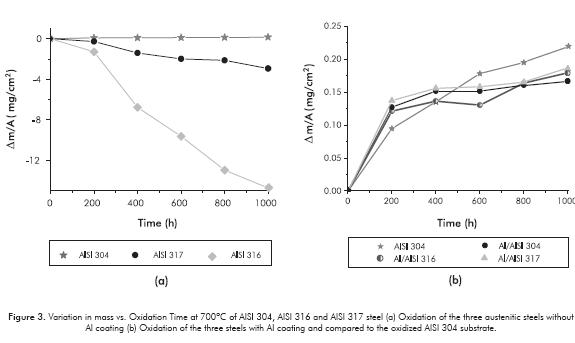
The results of thermogravimetry of the samples are illustrated in Figure 3. According to uncoated oxidized steels (Figure 3a), it can be confirmed that AISI 304 steel showed the least mass gain because it was best protected by the formation of a layer of chromium rich oxide. Furthermore, AISI 316 steel lost the most mass, forming a layer of iron-rich oxide, and AISI 317 steel lost the least amount of mass forming a layer with iron and chromium oxides. It is important to point out that the oxides formed on the latter two uncoated steels proved to be very fragile, to the extent that at the end of each test -when the oxide husk became thicker than 50 µm-, and when they were cooled and weighed, they broke easily. Therefore, the mass variation curves of the oxidized samples of AISI 316 and AISI 317 steels, without Al coating, underwent an apparent mass loss, not considering the amount of oxide that was fractured or detached from the sample. The oxidized sample of AISI 304 steel retained its oxide, and its mass variation curve was used as reference for comparison with the mass variation curves of steels with aluminum coating.
The thermogravimetry of the steels with Al coating is shown in Figure 3b. It is found that Al coating plays an important role, as it helps to decrease the oxidation rate of the steel and increases protection in these aggressive conditions of steam oxidation. For instance, the Al coating deposited on AISI 304 steel underwent a reduction of nearly 25% in mass gain during oxidation, compared to the same oxidized substrate in equal conditions, Figure 3b.
The results show that the aluminum in the coating and the iron of the substrate form iron aluminide or intermetallic phases, which wind up becoming the base material for the formation of the protective layer of Al2O3 during oxidation. This layer has a high thermal expansion coefficient and major chemical stability, thus preventing or delaying the steel's loss of chromium by volatilization. In this phenomenon, the gaseous species appear in the form of oxyhydroxides, hydroxides or chromium oxides, CrO2(OH)2(g), CrO2(OH)(g), Cr(OH)(g), Cr2O(g), Cr2O3(g), formed by contact with steam. These species were not studied herein, but they have been reported in other papers (Ebbinghaus, 1993; Castañeda, Bolívar & Pérez, 2010). The layer of alumina (Al2O3) and the chromium oxide (Cr2O3) are responsible for the protection of steel against oxidation in steam. When the uncoated steels are oxidized, they lose the critical percentage of chromium by reaction and/or volatilization, thus decreasing the protection against oxidation and accelerating the oxidation rate, causing a larger amount of iron oxides (hematite, wustite and magnetite) to form on the surface, which is why these steels undergo mass gain.
Of the three austenitic steels, AISI 304 steel was the one with the best behavior toward steam oxidation because it had a higher concentration of Cr, Mn and Si. During oxidation, this steel may form other protective oxides such as silica (SiO2), and/or mixed spinels or mixed Cr, Si and Mn oxides. AISI 316 steel formed the most oxides and had the lowest protection against steam oxidation, because it formed an outer layer of iron-rich oxides, which are porous. On the other hand, the AISI 317 steel showed an intermediate behavior, forming a layer with a mixture of chromium oxides and iron oxides. The loss of mass observed in AISI 316 steel, Figure 3a, is possibly related to the low adherence of the oxides formed or due to the microstrain caused by thermal changes when extracted from the oxidation loop, which break and detach from the sample evaluated, showing less protection.
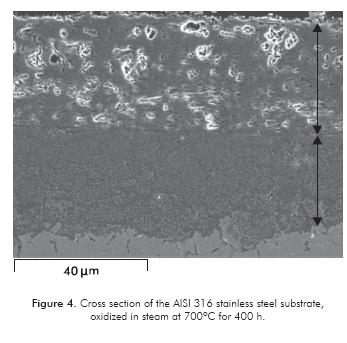
Figure 4 shows a SEM micrograph by detection of backscattered electrons from the cross section of the AISI 316 steel, oxidized by steam at 700ºC during 4 h of exposure. The image shows the presence of oxide husks from two areas: one outer area formed by a porous layer that is 30 µm thick (gray area with light areas), rich in iron and oxygen detected by EDAX analysis, with possible iron oxides: wustite (FeO), hematite (Fe2O3) and magnetite (Fe3O4). This area has a fragile appearance and/or low adherence, considering that it detaches when the sample is weighed. Its internal transport of oxygen from the dissociated gas is facilitated and, together with the disseminated iron from the steel, form the respective oxides based on their valence. Inside the oxide husk, there is another area (light gray area) formed by a compact, uniform layer of approximately 25 µm thick. This area is rich in Cr, Fe and O, where the chromium oxide is formed, along with protective mixed oxides and spinels that were identified by XRD.
The degradation and loss of protection to the oxidation of the steel is the result of the critical loss of chromium by volatilization, possibly due to the interaction between chromium oxide (Cr2O3) and steam, forming volatile species of chromium, such as: Cr2O(OH)2(g) or CrO2(OH)(g) (Ehlers et al., 2006; Cheng, Kuan & Tsai, 2006; Nasution, Velasco & Kim, 2009).
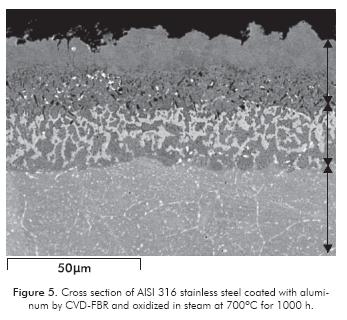
Figure 5 shows Micrograph by SEM at 1200 x, using backscattered electron detection of the cross section of the AISI 316 steel sample and coated with Al oxidized at 700ºC for 1000 h. There is a coating thickness of approximately 45 µm. In this figure, three areas are apparently observed; first of all, an outer porous layer (light gray area), which -according to EDAX composition analysis- is made up on the surface of atomic 70% aluminum and approximate atomic 15% oxygen, where the Al2O3 is possibly formed. The aluminum and oxygen diffuse toward the substrate, finding an atomic 10% of aluminum and an atomic 3% of oxygen at 50 µm of the substrate. This diffusion causes the empty spaces -or pores- observed during oxidation. In addition, a nickel content of approximately atomic 10% is observed, along with iron between atomic 30 and 70% and chromium between atomic 20 and 30%, which diffuse from the substrate.
In the intermediate area, which corresponds to a mixture of light and dark gray, small qualities of Al, Ni, Fe, Cr and Mo are observed. XRD analyses confirm that the outer layer is Al2O3 and the other layers correspond to phases of iron aluminides, such as FeAl, Fe3Al, Fe0.52Cr1.36, AlFe0.23Ni0.77, and intermediate phases of AlNi and (Al1-xCrx)2O3.The last area, according to the composition, is the AISI 316 steel substrate. In conformity to these results, it can be verified that the aluminum coating protects the steel from oxidation in steam at 700ºC, since the coating forms a protective of 700ºC, to the extent that the application of this layer of alumina (Al2O3) upon contact with the steam. coating could increase the operating temperature of In addition, intermetallic or iron aluminides are formed, the thermal plants, thus increasing efficiency and which work as a thermal barrier of these critical decreasing gas emissions. conditions of oxidation.
4. CONCLUSIONS
- The aluminum coating contributes to resistance to steam oxidation of AISI 304, 316 and 317 austenitic stainless steels, due to the formation of iron aluminides (intermetallic), which provide a source of aluminum during oxidation. This results in the formation of a protective layer of alumina (Al2O3), which is compact, adherent and, due to its high melting point, prevents loss of Cr by volatilization. In addition, it favors the formation of protective phases, such as nickel and chromium-rich spinels, underneath the coating.
- The Al coating applied by CVD-FBR achieved a reduction of approximately 25% in mass gain in AISI 304 steel, compared to the results obtained in the same steel without coating, oxidized in steam at 700ºC.
- The aluminum coating deposited by CVD-FBR on the AISI 304, 316 and 317 austenitic stainless steels protects and/or delays the oxidation rate in supercritical steam conditions at a temperature of 700°C, to the extent that the application of this coating could increase the operating temperature of the thermal plants, thus increasing efficiency and decreasing gas emissions.
ACKNOWLEDGEMENTS
The authors would like to thank Departamento Administrativo de Ciencia, Tecnología e Innovación -COLCIENCIAS for his doctoral fellowship and Universidad Tecnológica de Pereira for the study commission. The research group would like to thank the Spanish Ministry of Science for its contribution to this paper: Project MICINN ENE 2008-06755-C02-02 and CONSOLIDER CSD 2008-00023.
REFERENCES
Agüero, A., Muelas, R., Gutiérrez, M., Van Vulpen, R., Osgerby, S. & Banks, J. (2007). Cyclic oxidation and mechanical behavior of slurry aluminide coatings for steam turbine components. Surf. Coat. Technol., 201(14), 6253-6260. [ Links ]
Anthymidis, K. G., Stergioudis, E. & Tsipas, D. N. (2001). Boriding in a fluidized bed reactor. Mater. Lett., 51(2), 156-160. [ Links ]
Arai, T., Takeda, H. & Endo, J. (1989). US Patent No. 4871401A. Low temperature heat treatment; chromium, vanadium, titanium. Japan. [ Links ]
Asteman, H., Svensson, J. & Johansson, L. (2002). Oxidation of 310 steel in H2O/O2 mixtures at 600°C: The effect of water-vapour-enhanced chromium evaporation. Corros. Sci., 44(11), 2635-2649. [ Links ]
Bolívar, F. (2007). Evaluación del comportamiento a elevadas temperaturas de recubrimientos de Al, Si y de A1 modificados con Si y Hf depositados mediante CVDFBR sobre aceros ferritico-martensiticos (9-12Cr). Tesis Doctoral Facultad de Ciencias Físicas, Universidad Complutense de Madrid, España, 365pp. [ Links ]
Castañeda, S., Bolívar, F. & Pérez, F. (2010). Study of oxyhydroxides formation on P91 ferritic steel and CVD-FBR coated by Al in contact with Ar + 40% H2O at 650°C by TG-Mass spectrometry. Oxid. Met., 74(1-2), 61-78. [ Links ]
Cheng, S., Kuan, S. & Tsai, W. (2006). Effect of water vapor on annealing scale formation on 316 SS. Corros. Sci., 48(3), 634-649. [ Links ]
Ebbinghaus, B. (1993). Thermodynamics of gas phase chromium species: The chromium oxides, the chromium oxyhydroxides, and volatility calculations in waste incineration processes. Combust. Flame, 93(1-2), 119-137. [ Links ]
Ehlers, J., Young, D., Smaardijk, E., Tyagi, A., Penkalla, H., Singheiser. L. & Quadakkers, W. (2006). Enhanced oxidation of the 9% Cr steel P91 in water vapour containing environments. Corros. Sci., 48(11), 3428-3454. [ Links ]
Ennis, P. & Czyrska-Filemonowicz, A. (2003). Recent advances in creep-resistant steels for power plant applications. Sadhana, 28(3-4), 709-730. [ Links ]
Lau, K. H., Sanjurjo, A. & Wood, B. J. (1992). Aluminum and alumina coatings on copper by chemical vapor deposition in fluidized bed reactors. Surf. Coat. Technol., 54-55(1), 234-240. [ Links ]
Maitra, T. & Gupta, S. (2003). Intermetallic compound formation in Fe-Al-Si ternary system: Part II. Mater. Charact., 49(4), 293-311. [ Links ]
Marulanda, J. L. (2013). Estudio de la resistencia a la oxidación en vapor de aceros inoxidables austeníticos recubiertos con aluminio y silicio mediante deposición química de vapor en lecho fluidizado. Tesis Doctoral Facultad de Ciencias Químicas, Universidad Complutense de Madrid. España, 256pp. [ Links ]
Marulanda, J. L, Castañeda, S. I. & Remolina, A. (2013). Recubrimientos depositados por CVD-FBR para protección a alta temperatura. Dyna, 80(181), 181-191. [ Links ]
Nasution, I., Velasco, A. & Kim, H. (2009). Atmospheric pressure chemical vapor deposition mechanism of Al2O3 film from AlCl3 and O2. J. Crystal Growth, 311(2), 429-434. [ Links ]
Pérez, F. J. & Castañeda, S. (2007). Study of oxyhydroxides formation on P91 ferritic steel and slurry coated by Al in contact with Ar + 80% H2O at 650°C by TG-Mass spectrometry. Surf. Coat. Technol., 201(14), 6239-6246. [ Links ]
Pérez, F. J., Hierro, M. P., Pedraza, F., Carpintero, M. C., Gómez, C. & Tarin, R. (2001). Effect of fluidized bed CVD aluminide coatings on the cyclic oxidation of austenitic AISI 304 stainless steel. Surf. Coat. Technol., 145(1-3), 1-7. [ Links ]
Pierson, H. O. (1999). Handbook of Chemical Vapor Deposition (CVD). Principles, technology and applications. 2ª ed. Norwich: William Andrew Publishing. Noyes. [ Links ]
Sánchez, L., Bolívar, F., Hierro, M. & Pérez, F. (2009). Temperature dependence of the oxide growth on aluminized 9-12% Cr ferritic-martensitic steels exposed to water vapour oxidation. Thin Solid Films, 517(11), 3292-3298. [ Links ]
Stoloff, N. S., Liu, C. T. & Deevi, S. C. (2000). Emerging applications of intermetallics. Intermetallics, 8(9-11), 1313-1320. [ Links ]
Thermo-Calc Software. (2003). AB. Version P. foundation of computational thermodynamics. Stockholm, Sweden. [ Links ]
AUTHORS
José Luddey Marulanda Arévalo
Affiliation: Universidad Tecnológica de Pereira - Universidad Complutense de Madrid
Metallurgical Engineer, Universidad Industrial de Santander
M. Sc. in Metallurgical Engineering, Universidad Industrial de Santander
Ph. D. in Advanced Chemistry, Universidad Complutense de Madrid
e-mail: jlmarulanda@utp.edu.co
Francisco Javier Pérez Trujillo
Affiliation: Universidad Complutense de Madrid
Degree in Chemistry, Universidad Complutense de Madrid
Industrial Engineer, Universidad Complutense de Madrid
Ph. D. in Chemical Sciences, University of California
e-mail: fjperez@quim.ucm.es
Saúl Isaac Castañeda Quintana
Affiliation: Universidad Complutense de Madrid
Degree in Physics, Universidad Nacional Mayor de San Marcos
M. Sc. in Physical Sciences, Universidad Nacional Mayor de San Marcos
Ph. D. in Physical Sciences, Universidad Autónoma de Madrid
e-mail: sicastan@quim.ucm.es