Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
CT&F - Ciencia, Tecnología y Futuro
versión impresa ISSN 0122-5383
C.T.F Cienc. Tecnol. Futuro vol.5 no.5 Bucaramanga jul./dic. 2014
Journal of oil, gas and alternative energy sources
REVIEW OF STUDIES ON ASPHALTENE - WAX INTERACTION AND THE EFFECT THEREOF ON CRYSTALLIZATION
REVISIÓN DE LOS ESTUDIOS SOBRE INTERACCIÓN ASFALTENOS Y PARAFÍNAS E INCIDENCIA EN SU CRISTALIZACIÓN
REVISÃO DOS ESTUDOS SOBRE INTERAÇÃO ASFALTENOS E PARAFINAS E INCIDÊNCIA EM SUA CRISTALIZAÇÃO
Emiliano Ariza-León*1, Daniel-Ricardo Molina-Velasco1 and Arlex Chaves-Guerrero1
1 Universidad Industrial de Santander, Bucaramanga, Santander, Colombia
e-mail: earizal@uis.edu.co
* To whom correspondence should be addressed
How to cite: Ariza-León, E., Molina-Velasco, D. R. & Chaves-Guerrero, A. (2014). Review of studies on asphaltene - wax interaction and the effect thereof on crystallization. CT&F - Ciencia, Tecnología y Futuro, 5(5), 39-53.
(Received: May 15, 2014; Accepted: Nov. 14, 2014)
ABSTRACT
This paper reviews the impact of asphaltenes on wax crystallization and on the properties of crude oils, and the consequences thereof on the flow assurance of waxy crude oil. It initially includes studies of the crystallization of both pure n-alkanes as well as the mixtures thereof, because they have led to an understanding of how their precipitation occurs along with their transitions of crystalline phases, particularly, by studies of X-ray diffraction (XRD). This is followed by the studies and results of crystallization in crude oils and/or the fractions thereof and how their properties are affected in the presence of asphaltenes.
The results on the influence of asphaltenes are inconclusive and sometimes contradictory: some confirm that their presence decreases pour point, others affirm that it increases it and yet others say there is no effect. Similar results have been reported for properties such as crystallization point, phase transition and rheological properties. The influence of the chemical structure of asphaltenes on the change in crude oil properties has not been generalized either. It has been inferred that is possibly due to the interaction between aliphatic chains of asphaltenes and waxes, and it has been established that there is synergy in coprecipitation due to evidence in the analysis of organic deposits.
Keywords: Waxy crude oils, Asphaltene, Waxes, Crystallization, X-ray diffraction, Pour point, Rheological properties.
RESUMEN
Se presenta una revisión del efecto que tienen los asfaltenos en la cristalización de parafinas, en las propiedades de crudos parafínicos y las consecuencias en el aseguramiento de flujo. Inicialmente se incluyen los estudios de cristalización de n-alcanos puros y de sus mezclas donde se ha podido entender como ocurre su precipitación y transiciones de fases cristalinas, especialmente, por estudios de difracción de rayos-X (DRX). Se analizan los resultados de estudios de cristalización en crudos y/o sus fracciones y cómo la presencia de los asfaltenos afecta sus propiedades.
Sobre la influencia de los asfaltenos, los resultados no son concluyentes y en ocasiones contradictorios: algunos confirman su contribución a disminuir el punto de fluidez, otros que lo aumenta y en algunos casos que no hay efecto. Resultados semejantes se han reportado para el punto de cristalización, la transición de fases y propiedades reológicas. Tampoco se ha podido generalizar cuál es la influencia de la estructura química de los asfaltenos sobre las propiedades de los crudos. Se infiere que posiblemente se deba a la interacción entre sus cadenas alifáticas con las parafinas y se ha establecido que existe sinergia en la coprecipitación por la evidencia en los análisis de los depósitos orgánicos.
Palabras clave: Crudos parafínicos, Asfaltenos, Parafinas, Cristalización, Difracción de rayos X, Punto de fluidez, Propiedades reológicas.
RESUMO
É apresentada uma revisão do efeito que os asfaltenos têm na cristalização de parafinas, nas propriedades de crus parafínicos e nas consequências na garantia de escoamento. Inicialmente são incluídos os estudos de cristalização de n-alcanos puros e de suas misturas onde foi possível entender como ocorre sua precipitação e transições de fases cristalinas, especialmente, por estudos de difração de raios-X (DRX). São analisados os resultados de estudos de cristalização em crus e/ou suas frações e como a presença dos asfaltenos afeta suas propriedades.
Sobre a influência dos asfaltenos, os resultados não são conclusivos e em ocasiões são contraditórios: alguns confirmam sua contribuição para diminuir o ponto de fluidez, outros que o aumenta e em alguns casos que não o afeta. Resultados semelhantes são relatados para o ponto de cristalização, a transição de fases e as propriedades reológicas. Também não é possível generalizar qual é a influência da estrutura química dos asfaltenos sobre as propriedades dos crus. Infere-se que possivelmente é devida à interação entre suas cadeias alifáticas com as parafinas e estabelece-se que existe sinergia na coprecipitação pela evidência nas análises dos depósitos orgânicos.
Palavras-chave: Crus parafínicos, Asfaltenos, Parafinas, Cristalização, Difração de raios X, Ponto de fluidez, Propriedades reológicas.
1. INTRODUCTION
In Colombia, mature fields have major oil reserves to be recovered. However, many of them have problems with ensuring flow such as silting and the precipitation of organic and inorganic solids, whose severity can increase after many years of production. In extreme cases, if a solution is not found, it can lead to the closing of the field. In waxy crude oil production fields, precipitation and wax deposition issues have been attributed mainly to a decrease in fluid temperature without the appropriate characterization of the reservoir fluids, and without a clear diagnosis of the causes of the problem. This situation has led to the implementation of prevention and control methods with poor results, many of which are potentially adverse. Therefore, the purpose of this article is to review the state of the art on studies aimed at determining the effect or connection between asphaltenes and precipitation and deposition of waxes and finding out the relevance of this factor. In particular, in flow assurance problems in waxy crude oils.
This research focused on understanding the influence of asphaltenes on wax precipitation. Most researchers have assessed their impact on synthetic fluids, in which they have added asphaltenes extracted from deposits and from crude oils to commercial waxes dissolved in solvents, concluding that asphaltenes may have an impact on the crystallization of waxes by altering the behavior of properties such as crystallization point, pour point and rheological properties. In this article, we review the progress and possible direction of research related to the effect of asphaltenes on the wax crystallization process.
2. FLOW ASSURANCE PROBLEMS IN WAXY CRUDE OILS
More than 70% of the current fields on the global level are considered mature because they have reached their production peak or are in a stage of decline (Babadagli, 2007). In Colombia, this percentage is higher considering that, in the last two decades, only deposits with low reserves have been found. Over time, a field's production declines and problems arise, such as sand production problem, the formation of wax, asphaltene and scale deposits, increase in water and difficult to treat emulsions. This makes the primary recovery factor in the world an average of 34% (Satter, Iqbal & Buchwalter, 2007), while in Colombia it is less than 20% (Vargas, 2009), and in the Colorado Field, barely 7% (Ordoñez et al., 2003).
It is important to mention that one of the main factors influencing flow assurance (Irmann, 2011) during the primary production of deposits with major economic losses is precipitation and the deposition of organic solids (waxes and asphaltenes) and inorganic solids (sand, sulfate and carbonate scales, corrosion solids).
Fluid Phase Behavior in Oil Fields
Depending on the composition of the crude oil and its conditions of thermodynamic stability, this phenomenon can occur into reservoir, on the well face, inside the tubing on the way up from the bottom hole to the surface, in the surface lines and facilities or in the pipelines. The presence of wax crystals in the crude oil causes special rheological behavior, including yield stress, shear stress and dependence on time under a state of flow and the formation of a strong thermo-reversible gel. This behavior will depend on the shear stress and thermal history of the sample (Zhao et al., 2012; Da Silva & Coutinho, 2004). Some research has indicated that crude oils at temperatures higher than the crystallization point show a Newtonian behavior and, at lower temperatures, have a non-Newtonian behavior (Wardhaugh, Boger & Tonner, 1988; Dimitriou, 2013). In fields with wax precipitation problems, an increase in the water cut may help reduce deposition because the steel pipe is hydrophilic.
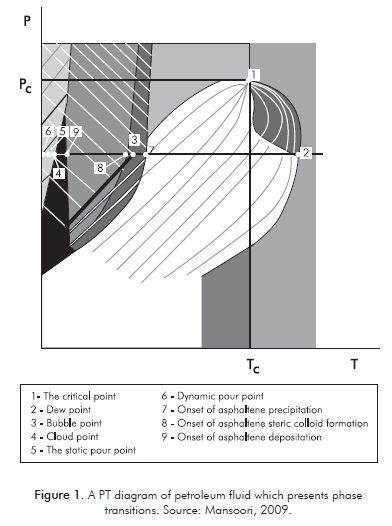
Oil fields are classified according to the fluids that can be found in them, such as: gas, gas and condensate, light, intermediate or heavy crude oil, and oil sands or oil shales (Danesh, 2003). Phase changes can occur during the production, transport and processing of crude oil such as gas separation, condensation and the formation of solids (Figure 1). The complexity of phase behavior is due to the variety and polydispersity of the hydrocarbon molecules in the presence of others of a different nature (Mansoori, 2009). In phase transition, an abrupt change takes place in one or more physical properties and a small or null change occurs in the intensive thermodynamic variables, such as temperature and pressure. First-order transitions (Mansoori, 2009) are those that are associated with significant latent heat, i.e. compound exchanges with its surroundings, and a fixed and elevated amount of energy in the form of heat at a constant pressure and temperature, as is the case of the change in phase from liquid to solid during the crystallization of oil waxes.
Waxy crude oil is composed of complex mixtures of hydrocarbons, particularly alkanes, which in normal conditions are liquid from C5 to C15 and solid thereafter, which can crystallize due to changes in temperature, pressure and/or composition. It is considered that molar concentration of alkanes (xn), in accordance with the number of carbons, is greater for those of a lower number and have an exponential distribution type that decreases as the alkanes of a higher molecular weight appear, with the melting point ranging from 20°C for C16 and 117°C for C100. To analyze the liquid-solid and solid-solid phase shift, researchers have worked with binary and ternary mixtures of odd and even alkanes and some multicomponents, which facilitates the understanding of the behavior in order to be able to move on to more complex studies in the future with crude oil.
3. EFFECT OF ASPHALTENES ON WAX PRECIPITATION
This section discusses the subject of the wax precipitation in crude oil, by first presenting one of the several ways of understanding the chemical composition of a crude oil: Saturates, Aromatics, Resins and Asphaltenes (SARA). Since the saturate fraction is the largest in waxy crude oils, the following is a review of the precipitation of pure n-alkanes and the studies on their crystallization, followed by a review of the influence of asphaltenes on the precipitation of pure waxes, wax fractions and waxes in crude oil.
Chemical Composition of Petroleum
Petroleum is a complex mixture of hydrocarbons and molecules and other elements (Speight, 2007) such as nitrogen, oxygen, sulfur and metals, as well as water and solids (fines). The simplified model of petroleum suggested by Pfeiffer and Saal (1940) is based on four fractions (Hammami & Ratulowski, 2007): First, SARA, which are defined based on solubility and polarity (Aske, 2002). Two fractions are a problem for crude oil extraction and transport: waxes containing heavy hydrocarbons (C16+) belonging to the saturates (linear, branched and cyclic alkanes) and asphaltenes, whose changes in pressure, temperature and composition can precipitate and become Organic Deposits (OD). These deposits may vary in composition from white waxes to black asphalts with or without the presence of other organic and/or inorganic materials (Sotomayor, 2000).
There are two theories on the form as the asphaltenes are present in the crude oil: molecular and colloidal; the first considers that the asphaltenes are dissolved in the crude oil (Fussell, 1979) in a liquid-liquid balance of the two pseudocomponents (crude oil and asphaltenes), which is altered when the fluid undergoes changes in its thermodynamic state such as pressure, temperature or composition, thus generating the precipitation of asphaltenes (Hirschberg, deJong, Schipper, & Meijer, 1984). Other researchers consider asphaltenes as a solid phase (Mansoori, 1997). The molecular theory sustains that the asphaltene flocculation process is reversible.
According to the latter, known as the colloidal theory (Leontaritis & Mansoori, 1987), asphaltene molecules are surrounded by resins that act as peptizing agents that keep them in colloidal dispersion in the crude oil. However, since the asphaltenes do not have a sole aromatic ring, the resins may have adverse effects on the stability thereof (Murgich & Strausz, 2001). Aromatic compounds serve as solvents for both asphaltenes and paraffin waxes (Lira-Galeana & Hammami, 2000).
Wax Precipitation
Wax precipitation during production has been attributed mainly to the cooling of the crude oil traveling from the production formation to its final destination. The temperature at which the first wax crystal appears in the crude oil is called the crystallization point - Wax Appearance Temperature (WAT). However, since it is difficult to detect this appearance, some researchers describe it as the temperature at which the formation of wax is less than 0.1% in the crude oil, at a given pressure (Ball & Jones, 2009).
Once the first seeds or nucleation centers of the crystals appear, they begin to grow and, depending on the hydrodynamic flow conditions, the deposition of solid material may occur. When the crude oil continues on its path and the cooling occurs, it can reach a temperature at which mobility is lost (pour point) causing a reduction in flow and operational problems.
It is important to distinguish between the precipitation and deposition of waxes (Zhu, Walker & Liang, 2008). When temperature drops, the solubility of the wax in the crude oil decreases (Han et al., 2010), and other factors, such as pressure (Kutcherov & Chernoutsan, 2006), flow rate and the physical-chemical composition of the crude also vary, and the phenomenon of precipitation, also known as crystallization, takes place. Deposition is the formation of a layer or solid phase mainly comprised of wax and crude oil trapped on a surface in contact with the crude oil, such as rock pores and the inner surface of production pipes, valves or tanks. In the case of pipes, the deposit tends to grow or increase in thickness in accordance with hydrodynamic factors, diffusive processes of mass and energy as well as the physicalchemical characteristics of the crude oil (Aiyejina, Chakrabarti, Pilgrim & Sastry, 2011; Huang, Lee, Senra & Fogler, 2011).
In addition to temperature, there are other factors that influence wax precipitation, such as: the molecular weight and melting point of the waxes, the solvent -solute ratio, the pressure and nature of the solution or the physical-chemical composition thereof (Sadeghazad, Christiansen, Sobhi & Edalat, 2000). The content in the crude oil and the chemical structure of the waxes, resins and asphaltenes play an important role in the effectiveness of pour point depressants (Yi & Zhang, 2011).
Structural and Thermodynamic Properties of n-alkane
The geometric conformation of alkanes is simple, yet crystalline structures and their polymorphism are complex and exhibit a wide variety of the behavior of the solid phase (Wentzel & Milner, 2010). A substance exhibits polymorphism if it is able to crystallize in different crystalline forms that are chemically identical. When a solute crystallizes, it releases heat (heat of crystallization), the molecules reach a lower level of energy with the lattice and solution temperature increases (Mullin, 2001).
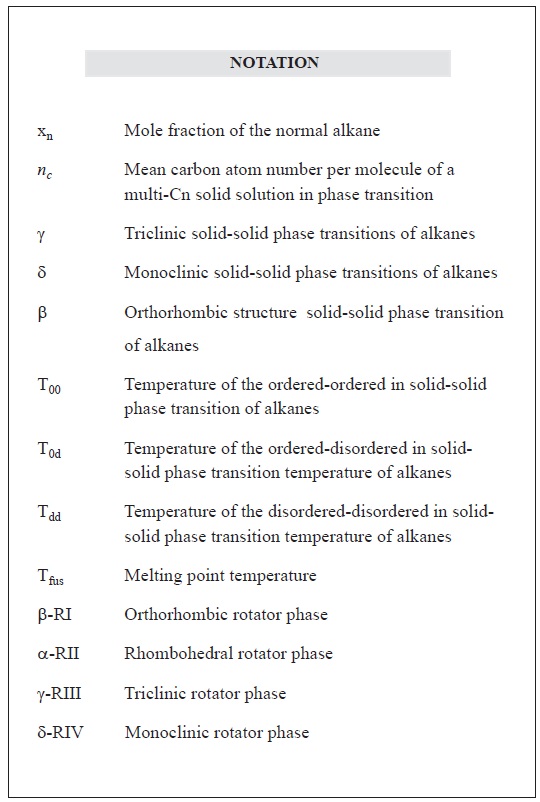
The n-alkanes at temperatures much lower than the melting point have different ordered phases: triclinic (γ) for even alkanes (8 <n< 26), monoclinic (δ) for n ≥ 26 and orthorhombic (β) for odd alkanes and n ≥ 36. When temperature is raised, they go through solid-solid transitions and ordered phases become disordered phases called "rotator" or meta-stable stages before the compound is melted. For longer chains, there may be other transitions from one ordered phase to another ordered phase, but always at temperatures below that of the transition from ordered to rotator phase (Rajabalee et al., 1999). "Rotator", also known as plastic phases (Dirand et al., 2002), are considered disordered because they have delocalized conformation defects that can affect any atomic position of the chain and can take place in two situations due to an increase in the temperature at a given concentration or an increase in the concentration at a defined temperature. In the case of the organized IR orthorhombic phase, the orientation of molecules are rotated at random by about 90° (Wentzel & Milner, 2010).
There are three types of phase transition: ordered-ordered whose temperature is T00, ordered-disordered with T0d and disordered-disordered with Tdd, up to the melting point of Tfus. The transition of crystalline phases from the ordered to disordered rotator type are: β-RI, α-RII, γ-RIII and δ-RIV. These phases have broader bands on the X-ray diffractogram, and in a greater proportion for long strings of alkane mixtures than for pure alkanes (Sirota, King, Shao & Singer, 1995).
The main phases of transition in the crystallization of alkanes are: disordered orthorhombic phase that goes to the rotator state called β-RI-β (Fmmm); rhombohedral rotator phase (α-RII-R 3 m); disordered triclinic phase (γ-RIII); disordered monoclinic phase (δ-RIV); disordered orthorhombic phase of odd alkanes β (Pbnm); ordered orthorhombic phase of odd alkanes (Pcnm) and ordered monoclinic phase of odd alkanes B (Aa) and C (A2).
Techniques such as Differential Thermal Analysis (DTA), Differential Scanning Calorimetry (DSC) (Wang et al., 2006), X-ray (Espeau & Ceolin, 2008) and Nuclear Magnetic Resonance (NMR) (Kuwabara & Horii, 1999) are among the most commonly used to detect the existence of new phases in the polymorphism of alkanes.
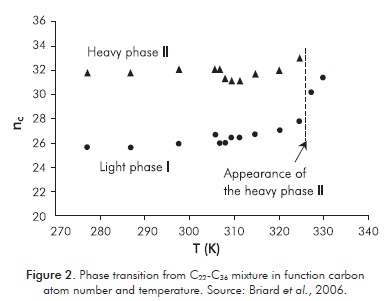
Most studies of this type have been conducted for binary and ternary mixtures. In the case of multicomponent samples of n-alkanes with a distribution of the number of carbons similar to those that have oil fractions, it is important to mention those of Briard et al. (2005; 2006) where structural and thermodynamic analysis by XRD were conducted, along with differential thermal analysis based on temperature. These researchers used nine samples from C23-C36 (consecutive mixture of 15 carbons) to C14-C36 (23 carbons), which were prepared using pure alkanes that were melted, mixed and then cooled to room temperature. They carried out DTA and X-ray diffraction analysis of samples between 70.15°C and -9.85°C, confirming the presence of different multicomponent crystalline solutions and the average carbon atoms per molecule (nc) where they occurred. Three groups were defined in accordance with the results and the behavior: 1 corresponding to the mixtures C22-C36, C21-C36 and C20-C36; group 2 from C19-C36 to C15-C36 and group 3 with the mixture of C14-C36. The mixtures from group 1 (Figure 2) showed two phases, a light disorderly α-RII and a heavy ordered rhombohedral phase II (β'). The mixtures from group 2 showed 3 phases and group 3 reached 4 phases. This means that the greater the difference in the number of carbons in the mixture, the more phases in the solid-solid transition.
Influence of Asphaltenes on Wax Precipitation
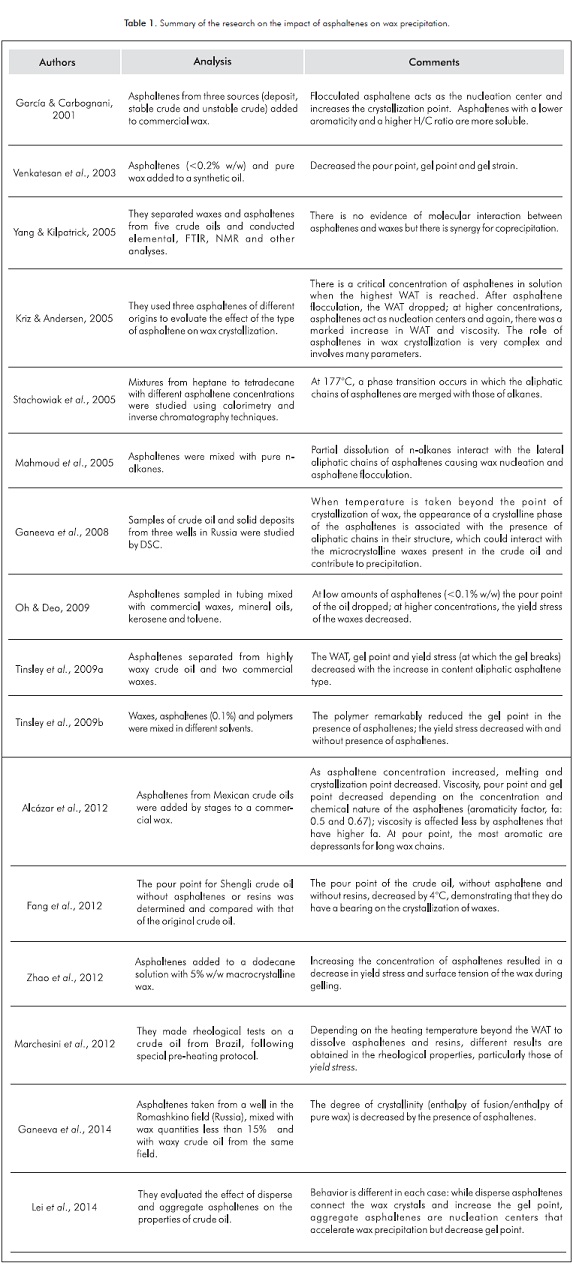
Research on the influence of asphaltenes on wax precipitation have been conducted mainly on fluids and mixtures of synthetic hydrocarbons with inconclusive and sometimes contradictory results. Some have confirmed that the presence of asphaltenes decreases pour point (Venkatesan et al., 2003), others report that they increase pour point (García & Carbognani, 2001) and yet others say that they have no effect (Yang & Kilpatrick, 2005).
García and Carbognani (2001), evaluated the effect of asphaltenes in solution and flocculated asphaltenes on the interaction with wax, for which they took asphaltenes with different aromaticity and stability: from operationally unstable crude oil, stable crude oil and deposit crude oil. The test oil was a highly waxy crude oil to which commercial wax was added. They concluded that the flocculated asphaltenes serve as the nucleation center and increase the crystallization point. Asphaltenes from the crude stable (lower aromaticity and higher H/C ratio) are more easily solubilized that those extracted from the unstable crude oil and from the deposit.
Lei et al. (2014) also evaluated the effect of the aggregate and dispersed asphaltenes on crude oil properties. Two crude oils were used (2.3 and 1.1% w/w of asphaltenes, respectively) and studied with optical microscopy, DSC and the viscosity. Other rheological properties were measured with a rheometer. The asphaltenes were precipitated with n-heptane, separated by centrifugation and again added to the crude oil in order to measure the properties and compare them with those determined for asphaltenes dispersed in the crude oil. They found that the aggregate asphaltenes serve as the nucleation center of the wax molecules, thus contributing to the precipitation thereof, by weakening the crystalline networks, delaying gelling and increasing the viscosity of the crude oil at temperatures below the crystallization point. In the meantime, the dispersed asphaltenes act as connectors between the wax crystals, accelerating the gelling of the crude oil and increasing gel strain.
Venkatesan et al. (2003) studied the effect of asphaltene polarity on the rheological properties of waxes. Three fractions extracted from the crude oil of the Zuata field in Venezuela were added to a synthetic oil with previously dissolved waxes at 5% w/w. They found that the asphaltene fraction with the lowest polarity causes the largest decrease in gel point at 4°C and yield point by up to 110 kPa compared to the sample of oil without asphaltenes.
Yang and Kilpatrick (2005) evaluated the chemical composition and molecular properties of five crude oils and their corresponding organic deposits using techniques such as Fourier Transform Infrared Spectroscopy (FTIR), NMR and elemental analysis. Three of the deposits had a high wax content (more than 44% w/w) and it was determined that the precipitation and deposition thereof was due to the heavier waxy component, and the asphaltenes (less than 6.7% w/w) extracted from the crude oil, as well as the respective deposit had the same chemical composition. The other two deposits were asphaltene (asphaltene content > 27%) and they found that these asphaltenes are less soluble than those corresponding to the crude oil. They concluded that the asphaltenes are an occluded component in the wax deposit and they do not affect wax precipitation.
Kriz and Andersen (2005) used a deasphalted waxy crude oil from the North Sea and three asphaltenes: from an organic deposit, a stable crude oil and an unstable crude oil. They initially prepared samples with the crude oil by adding the same amount of original asphaltene of the crude oil (0.01% w/w), achieving a 10ºC increase in crystallization point. At a higher concentration (0.05 %), there was a decrease in this temperature and then a slight increase with concentrations higher than 5%. The authors of that paper argue that the fragile balance between the fractions of crude oil with high molecular weight (asphaltenes-resins-waxes) is an important factor in the stability of the crude oil. The effect of asphaltenes on the crystallization of waxes depends more on the degree of dispersion or flocculation of the asphaltenes than the type or origin thereof. At a very low concentration, asphaltenes are either dispersed or dissolved in the crude oil matrix and can interact with the waxes. When the crude oil is over-cooled, wax crystals are formed and grow, in which the asphaltenes can be incorporated until a critical concentration thereof is reached, where the crystal structure is the strongest, and WAT is the highest. At concentrations slightly higher than the critical concentration, where the complete dispersion cannot be maintained for the rest of the oil, the fragile balance is broken and the asphaltene molecules tend to flocculate with each other and adhere weakly to the wax crystal networks. Therefore, when there is shear stress, the structure is broken at the weak points and the WAT drops. At higher concentrations of asphaltenes, nucleation seeds are created and the WAT increases once again. Dynamic viscosity is increased by the presence of a high solid content (asphaltenes and wax crystals) in the system.
With asphaltenes obtained from a crude oil, and pure alkanes C20, C28 and C36, Mahmoud, Gierycz, Solimando and Rogalski (2005) prepared mixtures at concentrations from 10% and 90% molar of waxes to study the interactions between them. In calorimetric studies by DSC, at a cooling rate of 0.5°C/min, they noted exothermic effects in all the mixtures, both with precipitated and dissolved asphaltenes. This phenomenon is explained by the partial dissolution of n-alkanes upon interacting with the lateral aliphatic chains of the asphaltenes. The almost solid nature of the partially immobilized alkanes causes the effect of wax nucleation and induces asphaltene flocculation.
Oh and Deo (2009) studied the influence of asphaltenes at temperatures below pour point. They used two types of waxes in their testing, along with different mineral oils, kerosene and toluene. The asphaltenes were taken from the tubing of a well in Rangely field (Colorado-USA). They found that asphaltenes fat 0.1% w/w cause a reduction in pour point of up to 4°C. At each concentration of asphaltenes, as temperature decreases beyond the pour point, yield stress increases.
Tinsley, Jahnke, Dettman and Prud’home (2009a), used a solvent made up of a mixture of alkanes (C10-C14) and methylnaphthalene, two commercial waxes and asphaltenes (H/C at 1.41) separated from a highly waxy crude oil. The mixtures prepared with asphaltenes at concentrations lower than 0.2% w/w and wax concentrations up to 10% w/w showed that the precipitation temperature of the crude oil decreases by nearly 2°C. They also noted that with high concentrations of asphaltenes, gel point drops by up to 3°C and the yield stress by 1200 Pa, because the asphaltenes act as an additive that modifies wax crystals and alters their morphology.
Tinsley et al. (2009b) tested different polymers for wax control, used pure waxes with multicomponent systems C20 to C47 and solvents, and conducted tests with and without asphaltenes. They prepared mixtures of waxes at 8% w/w with 0.1% of asphaltenes extracted from Shengly crude and each polymer was added at 0.1%. They noted that one of the polymers decreased gel point by 37°C in the presence of asphaltenes. The yield stress of the samples -with and without asphaltenes-decreased, although it was more effective in the presence thereof. The study showed that the type of asphaltenes used act as natural depressants of the gel point.
Alcázar and Buenrostro (2011), with three Mexican crude oils with organic precipitation problems during production and transport, observed that the presence of asphaltenes has a significant impact on the solid-liquid balance and rheological behavior of crude oils. They found that the pour point and gel point decrease as the amount of asphaltenes increases, considering that the composition of the crude oils is very similar. Although the crude oil with the highest crystallization point was the one with highest asphaltene content, it has the lowest pour point and gel point, which is because the asphaltenes disrupt the growth of wax crystals thus retarding the gelling thereof. In another research by the same researchers (Alcázar, García & Buenrostro, 2012), asphaltenes dissolved in o-xylene and extracted from two Mexican crude oils (with severe precipitation) were added to a commercial wax dissolved in n-decane at different concentrations (0 and 0.05% w/w). They concluded that asphaltenes contribute with a slight decrease in crystallization point and a major decrease in pour point. Also, viscosity decreases due the presence of asphaltenes with a greater aromaticity factor. Gel point decreases with waxes of short chains in the presence of asphaltenes that have a lower aromaticity factor; the opposite occurs with the pour point. On the other hand, asphaltenes with a greater aromaticity factor inhibit and disrupt the formation of wax crystals and promote a less stable gel structure.
Fang Zhang, Ma and Zhang (2012) determined the influence of asphaltenes and resins on the pour point of Sengli crude (°API of 30.8 with a content of 2.63, 24.59 and 18.25% w/w of asphaltenes, resins and waxes respectively). They found that the pour point of the crude oil without asphaltenes is reduced by 2°C, and without asphaltenes or resins by 4°C, which means that these fractions, particularly asphaltenes, have the effect of increasing pour point, contrary to other research where they found that they act as natural depressants. They explained that these crude oil fractions generate nucleation centers contributing to the formation of crystalline networks accelerating the loss of fluidity.
Zhao et al. (2012) evaluated the various factors that affect the gelling process of waxes such as thermal history, fraction history, asphaltene content and chemical additives. They used dodecane and xylene as solvents, pure macro and microcrystalline wax and asphaltenes extracted from crudes Se-7-E06 and Se-7-E07. They found that the yield stress decreases when asphaltene concentration increases, possibly due to heterogeneous nucleation, adsorption or steric impediment. They also found that the increase in asphaltenes decreases the surface tension of a microcrystalline wax dissolved in dodecane.
Marchesini, Alicke, De Souza and Ziglio (2012), in a study on the preparation of samples for the rheological characterization of waxy crude oils, determined that the samples must first be heated to temperatures above the WAT (without exceeding the limits of the temperature applied to the crude in industrial processes) for enough time to clear the thermal history of the crude oil. This is done in order to dissolve asphaltenes and resins and, thus, prevent them from interacting during the cooling process in the formation of wax crystal networks that affect the crystallization process, by decreasing yield stress and acting as natural pour point depressants. They used samples of a crude oil from a Brazilian field, which were stored in 12 bottles, each one homogenized before undergoing rheological tests, which were conducted in two rheometers with different geometries.
Russian researchers have contributed with studies about the coprecipitation of waxes and asphaltenes, of which three of the most outstanding are described below. Stachowiak, Viguié, Grolier and Rogalski (2005), by reverse calorimetry and chromatography, determined the infinite dilution coefficients of n-alkanes (C7, C8, C9, C10, C12, C13) dissolved in solvents containing asphaltenes as follows: pure asphaltenes, a mixture of 70% asphaltenes and 30% of C36 and a mixture of 30% asphaltenes and 70% of C36. The activity coefficients of n-alkanes dissolved in asphaltenes increases up to a temperature of 177°C, and tend to stabilize at higher temperatures, which implies that the partial enthalpy of excess alkane mixture is zero at that temperature and negative at lower temperatures. A phase transition occurs at this temperature, involving changes in the structure of the aggregates, because the lateral aliphatic chains of the asphaltenes melt. The n-alkanes incorporated in the asphaltene aggregates could exist in a frozen state and the immobility of their aliphatic chains would be responsible for the loss of entropy and enthalpy compared to the liquid state.
Ganeeva et al. (2008) studied the crude oils and solid deposits of three fields in Russia and found that the deposits were mainly made up of resins, asphaltenes and waxes. The crude oils revealed the presence of a crystalline phase of hydrocarbons with a melting point greater than 55°C. The asphaltenes were carefully extracted from the crude oils and the deposits, following the standard techniques and subject to the calorimetric study, which detected a crystalline phase with a melting point greater than 90°C. This melting point would correspond to waxy hydrocarbons with a high-molecular-weight (microcrystalline waxes). The question is why does this change take place if theoretically asphaltenes have no melting point? This occurs because asphaltenes have alkyl chains, which may interact with the waxes, thus contributing to the occurrence of precipitation.
Ganeeva, Yusupova, Romanov and Bashkitseva (2014) analyzed -between 30°C and 200°C and using calorimetry and polarization microscopy- the asphaltene mixtures of crude oils from five wells of the Romashkino field (Russia), and mixtures of waxes (C24)-asphaltenes (3, 5, 7, 10 and 15% w/w) dissolved in benzene and crude oil. They found that in the asphaltenes (“archipielago” type), which for most researchers are continental type, there are ordered amorphous phases that are identified by thermal changes in the heat curves between 70°C and 130°C, and between 130°C and 170°C; and a liquid crystal phase between 180°C and 200°C, which means that the crystalline phase of asphaltenes can precipitate with waxes that have high molecular weight. The degree of crystallinity (enthalpy of fusion/enthalpy of pure wax) for the concentration of wax at 15% w/w would be 15%, but by mixing it with asphaltenes, it is 4.9%. That is to say, it causes a decrease in the degree of crystallinity of 67%. When this mixture was added to a test crude, its crystallinity dropped by 16%. They also found that only one fraction of the flow of heat released takes part in the crystallization, and the remaining heat is used -at high temperatures- in the formation of the molecular structure of ordered phases of the asphaltenes.
Table 1 provides the summary of the research discussed above.
4. CONCLUSIONS
- Most research has been conducted with pure commercial alkanes or mixtures thereof to which asphaltenes obtained from crude oil have been added, in order to study the phenomenon of crystallization which is understood but does not represent the behavior of the crude oils. Therefore, further study on the subject is required.
- The phenomenon of wax crystallization is a complex process, because although the geometric conformation of alkanes is simple, crystalline structures and their polymorphism are complex and exhibit a wide variety of the behavior in the solid phase. However, as the number of alkanes in the mixtures increases, both ordered and disordered phase transition are found, which has been demonstrated using the x-ray diffraction technique.
- It is important to note that despite the broad spectrum of scientific publications and articles on the subject, there is no consensus or agreement regarding the results that can be generalized on the effect of asphaltenes on wax precipitation. Some research has found increases and others, decreases in the properties of the fluid, such as point of crystallization, pour point and rheology. Whereas, in other studies no changes have been noticed.
- The complex and still indefinite chemical structure of asphaltenes makes it difficult to generalize their effect on wax crystallization, so it is necessary to perform a rheological and compositional study for each crude oil, taking into account the following, among others: the concentration of this fraction, its nature of relative polarity, its aliphatic nature and aromaticity factor. Finally, although interaction between asphaltene molecules and those of waxes has not been confirmed, it is always stated that the changes in properties are due to the possible link between the aliphatic chains of asphaltenes and wax chains.
- A better understanding of the behavior and interaction of waxes and asphaltenes will enable the application of timely preventive and corrective methods in mature fields that have organic precipitation problems, thus helping to improve their productivity.
ACKNOWLEDGEMENTS
The authors would like to thank Universidad Industrial de Santander (UIS) and the Escuela Colorado field, for supporting their research in the area of organic precipitation and hydrocarbon flow assurance.
REFERENCES
Aiyejina, A., Chakrabarti, D., Pilgrim, A. & Sastry, M. (2011). Wax formation in oil pipelines: A critical review. Int. J. Multiphase Flow, 37(7), 671-694. [ Links ]
Alcázar, L. & Buenrostro, E. (2011). Characterization of the wax precipitation in Mexican crude oils. Fuel Process. Technol., 92(12), 2366-2374. [ Links ]
Alcázar, L., García, L. & Buenrostro, E. (2012). Effect of asphaltenes on equilibrium and rheological properties of waxy model systems. Fuel, 93: 200-212. [ Links ]
Aske, N. (2002). Characterization of crude oil components, asphaltene aggregation and emulsion stability by means of near infrared spectroscopy and multivariate analysis. Ph. D. thesis, Department of Chemical Engineering, Norwegian University of Science and Technology, Norway, 51pp. [ Links ]
Babadagli, T. (2007). Development of mature field - A review. J. Petrol. Sci. Eng., 57(3-4), 221-246. [ Links ]
Ball, R. & Jones, J. (2009). Thermodynamics of the deposition of complex waxes and asphaltenes in crude oil. Open Thermodyn. J., 3: 34-37. [ Links ]
Briard, A., Bouroukba, M., Petitjean, D., Hubert, N., Moise, J. & Dirand, M. (2005). Thermodynamic and structural analyses of the solid phases in multi-alkane mixtures similar to petroleum cuts at ambient temperature. Fuel, 84(9), 1066-1073. [ Links ]
Briard, A., Bouroukba, M., Petitjean, D., Hubert, N., Moise, J. & Dirand, M. (2006). Thermodynamic and structural analyses and mechanisms of the crystallisation of multi-alkane model mixtures similar to petroleum cuts. Fuel, 85(5-6), 764-777. [ Links ]
Danesh, A. (2003). PVT and phase behaviour of petroleum reservoir fluids. Netherlands: Elsevier. [ Links ]
Da Silva, J. & Coutinho, J. (2004). Dynamic rheological analysis of the gelation behaviour of waxy crude oils. Rheol. Acta, 43(5), 433-441. [ Links ]
Dimitriou, C. (2013). The rheological complexity of waxy crude oils: Yielding, thixotropy and shear heterogeneities. Ph. D. thesis, Department of Mechanical Engineering, Massachusetts Institute of Technology, USA, 320pp. [ Links ]
Dirand, M., Chevallier, V., Bouroukba, M., Petitjean, D., Behar E. & Ruffier-Meray, V. (2002). Normal alkanes, multialkane synthethic model mixtures, and real petroleum waxes: Crystallographic structures, thermodynamic properties, and crystallization. J. Chem. Eng. Data, 47(2), 115-143. [ Links ]
Espeau, P. & Céolin, R. (2008). Polymorphism of even-numbered carbon atom n-alkanes revisited through topological P-T diagrams. J. Phys. Chem. B, 112(7), 2063-2069. [ Links ]
Fang, L., Zhang, X., Ma, J. & Zhang, B. (2012). Investigation into a pour point depressant for Shengli crude oil. Ind. Eng. Chem. Res., 51(36), 11605-11612. [ Links ]
Fussell, L. (1979). A technique for calculating multi-phase equilibria. SPE J., 19(4), 203-208. [ Links ]
Ganeeva, Y., Foss, T., Khalikova, D., Yusupova, T. & Romanov, G. (2008). Calorimetric study of the crystalline phase of solid petroleum hydrocarbons and asphaltene-resin-wax deposits. Petrol. Chem., 48(6), 428-433. [ Links ]
Ganeeva, Y., Yusupova, T., Romanov, G. & Bashkitseva, N. (2014). Phase composition of asphaltenes. J. Therm. Anal. Calorim., 115(2), 1593-1600. [ Links ]
García, M. & Carbognani, L. (2001). Asphaltene-paraffin structural interactions. Effect on crude oil stability. Energy & Fuels, 15(5), 1021-1027. [ Links ]
Hammami, A. & Ratulowski, J. (2007). Precipitation and deposition of asphaltenes in production systems: A flow assurance overview. In: Mullins, O., Sheu, E., Hammami, A. & Marshall, A. Asphaltenes, heavy oils and petroleomics, New York: Springer, 617-660. [ Links ]
Han, S., Huang, Z., Senra, M., Hoffmann, R. & Fogler, H. S. (2010). Method to determine the wax solubility curve in crude oil from centrifugation and high temperature gas chromatography measurements. Energy & Fuels, 24(3), 1753-1761. [ Links ]
Hirschberg, A., deJong, L., Schipper, B. & Meijer, J. (1984). Influence of temperature and pressure on asphaltene flocculation. SPE J., 24(3), 283-293. [ Links ]
Huang, Z., Lee, H., Senra, M. & Fogler, H. (2011). A fundamental model of wax deposition in subsea oil pipelines. AIChE J., 57(11), 2955-2964. [ Links ]
Irmann. T. (2011). Flow assurance - A system perspective. [Website]. [Consulted: December 2013]. Available at: <http://www.uio.no/studier/emner/matnat/math/MEK4450/h11/undervisningsmateriale/modul-5/MEK4450_FlowAssurance_pensum-2.pdf> [ Links ].
Kriz, P. & Andersen, S. (2005). Effect of asphaltenes on crude oil wax crystallization. Energy & Fuels, 19(3), 948-953. [ Links ]
Kutcherov, V. & Chernoutsan, A. (2006). Crystallization and glass transition in crude oils and their fractions at high pressure. Int. J. Thermophys., 27(2), 474-485. [ Links ]
Kuwabara, K. & Horii, F. (1999). Solid-state 13C NMR analyses of the orthorhombic-to-hexagonal phase transition for constrained ultradrawn polyethylene fibers. Macromolecules, 32(17), 5600-5605. [ Links ]
Lei, Y., Han, S., Zhang, J., Bao, Y., Yao, Z. & Xu, Y. (2014). Study on the effect of dispersed and aggregated asphaltene on wax crystallization, gelation, and flow behavior of crude oil. Energy & Fuels, 28(4), 2314-2321. [ Links ]
Leontaritis, K. & Mansoori, G. (1987). Asphaltene flocculation during oil production and processing: A thermodynamic colloidal model. SPE International Symposium on Oilfield Chemistry, San Antonio, USA. SPE 16258. [ Links ]
Lira-Galeana, C. & Hammami, A. (2000). Wax precipitation from petroleum fluids: A review. In: Yen, T. & Chilingarian, G. Asphaltenes and asphalts, 2. Amsterdam: Elsevier Science. 40: B, 557-608. [ Links ]
Mahmoud, R., Gierycz, P., Solimando, R. & Rogalski, M. (2005). Calorimetric probing of n-alkane-petroleum asphaltene interactions. Energy & Fuels, 19(6), 2474-2479. [ Links ]
Mansoori, G. (1997). Modeling of asphaltene and other heavy organic depositions. J. Petrol. Sci. Eng., 17(1-2), 101-111. [ Links ]
Mansoori, G. (2009). A unified perspective on the phase behaviour of petroleum fluids. Int. J. Oil, Gas and Coal Technology, 2(2), 141-167. [ Links ]
Marchesini, F., Alicke, A., de Souza, P. & Ziglio, C. (2012). Rheological characterization of waxy crude oils: Sample preparation. Energy & Fuels, 26(5), 2566-2577. [ Links ]
Mullin, J. (2001). Crystallization. Fourth edition. Oxford: Butterworth Heinemann. [ Links ]
Murgich, J. & Strausz, O. (2001). Molecular mechanics of aggregates of asphaltenes and resins of the athabasca oil. Petrol. Sci. Technol., 19(1-2), 231-243. [ Links ]
Oh, K. & Deo, M. (2009). Characteristics of wax gel formation in the presence of asphaltenes. Energy & Fuels, 23(3), 1289-1293. [ Links ]
Ordóñez, A., Guarín, F., Parra, J., Vargas, J., Suárez, H., Castillo, G. & Castro, J. (2003). Diagnósticos y estrategias de recobro. Informe Final Campo Colorado. El Centro. Ecopetrol S.A. [ Links ]
Pfeiffer, J. & Saal, R. (1940). Asphaltene bitumen as colloid system. J. Phys. Chem., 44(22), 139-149. [ Links ]
Rajabalee, F., Métivaud, V., Mondieig, D., Haget, Y. & Cuevas-Diarte, M. (1999). New insights on the crystalline forms in binary systems of n-alkanes: Characterization of the solid ordered phases in the phase diagram tricosane + pentacosane. J. Mater. Res., 14(6), 2644-2654. [ Links ]
Sadeghazad, A., Christiansen, R., Sobhi, G. & Edalat, M. (2000). The prediction of cloud point temperature: In wax deposition. SPE Asia Pacific Oil & Gas Conference and Exhibition, Brisbane, Australia. SPE-64519. [ Links ]
Satter, A., Iqbal, G. & Buchwalter, J. (2007). Practical enhanced reservoir engineering: Assisted with simulation software. Tulsa: PennWell. [ Links ]
Sirota, E., King, H., Shao, H. & Singer, D. (1995). Rotator phases in mixtures of n-alkanes. J. Phys. Chem., 99(2), 798-804. [ Links ]
Sotomayor, J. (2000). Las fases sólidas orgánicas en la explotación de petróleo y gas - una contribución al análisis de sus causas. GPA Estudios y Servicios Petroleros SRL. Nota técnica No 26. [Website]. [Consulted: December 2013]. Available at: <http://www.oilproduction.net/cms/files/gpa/26.pdf> [ Links ].
Speight, J. (2007). The chemistry and technology of petroleum. Fourth edition. New York: CRC press. [ Links ]
Stachowiak, C., Viguié, J., Grolier, J. & Rogalski, M. (2005). Effect of n-alkanes on asphaltene structuring in petroleum oils. Langmuir, 21(11), 4824-4829. [ Links ]
Tinsley, J., Jahnke, J., Dettman, H. & Prud'home, R. (2009a). Waxy gels with asphaltenes 1: Characterization of precipitation, gelation, yield stress, and morphology. Energy & Fuels, 23(4), 2056-2064. [ Links ]
Tinsley, J., Jahnke, J., Adamson, D., Guo, X., Amin, D., Kriegel, R., Saini, R., Dettman, H. & Prud'home, R. (2009b). Waxy gels with asphaltenes 2: Use of wax control polymers. Energy & Fuels, 23(4), 2065-2074. [ Links ]
Vargas, C. (2009). Nuevos aportes a la estimación del potencial de hidrocarburos en Colombia. Rev. Acad. Colomb. Cienc., 33(126), 17-43. [ Links ]
Venkatesan, R., Östlund, J., Chawla, H., Wattana, P., Nydén, M. & Fogler, H. (2003). The effect of asphaltenes on the gelation of waxy oils. Energy & Fuels, 17(6), 1630-1640. [ Links ]
Wang, S., Tozaki, K., Hayashi, H., Inaba, H. & Yamamoto, H. (2006). Observation of multiple phase transitions in some even n-alkanes using a high resolution and super-sensitive DSC. Thermochimica Acta, 448(2), 73-81. [ Links ]
Wardhaugh, L., Boger, D. & Tonner, S. (1988). Rheology of waxy crude oils. International Meeting on Petroleum Engineering, Tianjin, China. SPE 17625. [ Links ]
Wentzel, N. & Milner, S. (2010). Crystal and rotator phases of n-alkanes: A molecular dynamics study. J. Chem. Phys., 132(4), 1-10. [ Links ]
Yang, Z. & Kilpatrick, P. (2005). Asphaltenes and waxes do not interact synergistically and coprecipitate in solid organic deposits. Energy & Fuels, 19(4), 1360-1375. [ Links ]
Yi, S. & Zhang, J. (2011). Relationship between waxy crude oil composition and change in the morphology and structure of wax crystals induced by pour-point-depressant beneficiation. Energy & Fuels, 25(4), 1686-1696. [ Links ]
Zhao, Y., Kumar, L., Paso, K., Safieva, J., Sariman, M. Z. & Sjöblom, J. (2012). Gelation behavior of model wax-oil and crude oil systems and yield stress model development. Energy & Fuels, 26(10), 6323-6331. [ Links ]
Zhu, T., Walker, J. & Liang, J. (2008). Evaluation of wax deposition and its control during production of Alaska north slope oils. Final report. Department of Energy National Energy Technology Laboratory. USA. [ Links ]
AUTHORS
Emiliano Ariza-León
Affiliation: Universidad Industrial de Santander
Petroleum Engineering, Universidad Industrial de Santander
M.Sc. in Hydrocarbons Engineering, Universidad Industrial de Santander
Ph. D. Student in Chemical Engineering, Universidad Industrial de Santander
e-mail: earizal@uis.edu.co
Daniel-Ricardo Molina-Velasco
Affiliation: Universidad Industrial de Santander
Chemist, Universidad Industrial de Santander
M.Sc., Ph.D. in Chemistry, Universidad Industrial de Santander
e-mail: dmolina@uis.edu.co
Arlex Chaves-Guerrero
Affiliation: Universidad Industrial de Santander
Chemical Engineering, Universidad del Valle
Spe. in Thermal Sciences, Universidad del Valle
M.Sc. in Mechanical Engineering, Universidad de Puerto Rico
Ph. D. in Chemical Engineering, Universidad de Puerto Rico
e-mail: achavesg@uis.edu.co