Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383
C.T.F Cienc. Tecnol. Futuro vol.6 no.1 Bucaramanga Jan./June 2015
ANALYSIS OF MIXING PARAMETERS FOR POLYMER GELS USED FOR THE CORRECTION OF WATER INJECTION PROFILES
ANÁLISIS DE LOS PARÁMETROS DE MEZCLA PARA GELES POLIMÉRICOS USADOS PARA CORRECCIÓN DE PERFILES DE INYECCIÓN
ANÁLISE DOS PARÂMETROS DE MISTURAS DE GELES POLIMÉRICOS USADAS NA CORRECÇÃO DOS PERFIS DE INJECÇÃO
Gustavo-Adolfo Maya-Toro1*, Rubén-Hernán Castro-García1, Robinson Jiménez-Díaz1 and Samuel-Fernando Muñoz-Navarro2
1Ecopetrol S. A. - Instituto Colombiano del Petróleo (ICP), A. A. 4185 Piedecuesta, Santander, Colombia, e-mail: gustavo.maya@ecopetrol.com.co
2Escuela de Ingeniería de Petróleos, Universidad Industrial de Santander, Bucaramanga, Santander, Colombia
How to cite: Maya-Toro, G. A., Castro-García, R. H., Jiménez-Díaz, R. & Muñoz-Navarro, S. F. (2015). Analysis of mixing parameters for polymer gels used for the correction of water injection profiles. . CT&F - Ciencia, Tecnología y Futuro, 6(1), 43-68.
*To whom correspondence should be addressed
(Received: Feb. 28, 2015; Accepted: Jun. 02, 2015)
ABSTRACT
Polymer gels as modifiers of the injection profile in secondary recovery processes are a widely used technic in the industry, however, its use is based on the success of their applications, without existing a detailed knowledge of the mechanisms of the phenomenon, leaving open big doubts on design factors, risk variables, prediction of results to be obtained, etc.
Design of applications in the field is performed following the concepts formulated since the creation of the technology, involving only fluid-fluid tests, with a qualitative approach that does not allow a detailed analysis of the phenomena.
Fluid-fluid tests, commonly used in this technology, are the starting point of this research to further involve quantitative analysis such as ultraviolet rays and rheological tests for better understanding of the process. From this research were obtained new ways to assess mixing parameters needed prior to field application of gels for plugging of water thief zones.
Keywords: Chemical enhanced oil recovery, Polymer injection, Water injection.
RESUMEN
Los geles poliméricos como modificadores del perfil de inyección en procesos de recobro secundario son una técnica ampliamente utilizada en la industria, sin embargo, su uso se fundamenta en el éxito de sus aplicaciones, sin existir un conocimiento detallado de los mecanismos del fenómeno, y dejando abiertas grandes dudas sobre factores de diseño, variables de riesgo, predicción de los resultados a obtener, etc.
El diseño de las aplicaciones en campo es realizado siguiendo los conceptos formulados desde la creación de la tecnología, involucrando solamente pruebas fluido-fluido, con enfoque cualitativo que no permite analizar en detalle los fenómenos presentes.
Esta investigación parte de las pruebas fluido-fluido comúnmente usadas en esta tecnología para posteriormente involucrar análisis cuantitativos como rayos ultravioleta y pruebas reológicas, obteniendo resultados que permiten entender mejor el proceso bajo estudio. Se obtiene de la investigación realizada nuevas formas de evaluar los parámetros de mezcla necesarios previa a la aplicación en campo de geles para taponamiento de zonas ladronas de agua.
Palabras clave: Recobro químico, Inyección de polímeros, Inyección de agua.
RESUMO
O gel de polímero como modificador do perfil de injeção em processos de recuperação secundária é uma técnica amplamente utilizada na indústria, no entanto, seu uso está baseado no sucesso de suas aplicações, sem que ainda exista um conhecimento detalhado sobre os mecanismos do fenómeno, deixando abertas grandes dúvidas sobre fatores de desenho, variáveis de risco, predição de resultados a ser obtidos, etc.
O desenho de aplicações no campo se faz segundo os conceitos formulados desde a criação da tecnologia, envolvendo apenas testes fluído-fluído, com uma abordagem qualitativa que não possibilita uma análise detalhada do fenómeno.
Os testes fluído-fluído, comumente utilizados nesta tecnologia, são os pontos de partida da pesquisa para integrar ainda mais a análise qualitativa como raios ultravioletas e testes reológicos para uma melhor compreensão do processo. Com base nesta pesquisa, conseguimos novas formas de avaliar os parâmetros de mistura necessários antes da aplicação em campo de geles para a obturação de zonas water thief.
Palavras-chave: Recuperação química, Injeção de polímeros, Injeção de água.
1. INTRODUCTION
One of the most important families within enhanced recovery is the family known as chemical recovery. This has focused on mobility control and reduction of interfacial tension, however, the concept of chemical recovery has evolved mainly due to the appearance of techniques such as injection of polymer gels, colloidal dispersion gels, foams and their mixtures.
This study is focused on the use of polymer gels to correct injection profiles in secondary processes and, in many cases, as a preparation for recovery process by injection of polymers and/or surfactants. Since its first use in the 80s (Sydansk & Moore, 1990), commercial use of polymer gels for the correction of injection profiles has not stopped, becoming a common practice in many countries.
Despite the fast expansion of the polymer gels application, there are a number of questions about this technology. Some doubts not addressed include the time the gel needs to form, the duration of the gel, the potential damage to zones with saturation of unsweep oil and uncertainty on the final result of any design.
Known literature demonstrates in a practical way the benefits of the polymer gel (Norman et al., 2006; Al-Muntasheri, Nasr-El-Din & Zitha, 2008); however, beyond the qualitative analysis proposed by the creators, and other few studies, the matter is still a research subject. In this paper fluid-fluid analysis with quantitative techniques to shed light on the mechanisms of operation of the subject are proposed.
Some of the findings of this research allow having a better design of field applications. Traditionally it has been thought that to produce a plugging gel in a reservoir polymer concentration must be as high as possible, being limited only by the formation injectivity. Results presented herein suggest that a high concentration of polymer is more related to duration of gel than to its formation, otherwise the concentration of the x-linker, which being the limit reagent of the mixture, governs the formation of the same.
Non-traditional studies on polymer gels, such as ultraviolet analysis or rheological tests, show that formation of gel corresponds to an inter-molecular reorganization rather than to a generation of a new product.
2. THEORETICAL FRAMEWORK
In the current scenario “polymers, gels for controlling injection profile in depth, surfactants, alkali, emulsions, foams and their combinations” (Sheng, 2011) are accepted as chemical recovery methods, however, it is important to take into account that given the diversity of methods and mechanisms “one definition could not always be satisfactory” (Sheng, 2011).
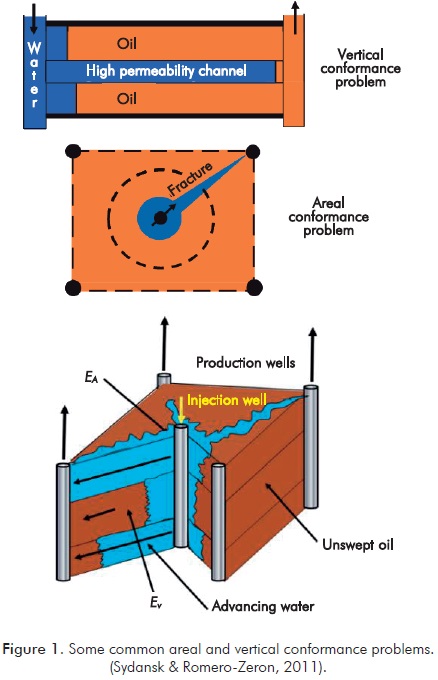
The term conformance, understanding polymer gels as a possibility within chemical recovery, is defined as the measure of volumetric sweep efficiency during the recovery process (Sydansk & Romero-Zeron, 2011). Figure 1 shows examples of conformance problems, both vertical and horizontal.
To improve conformance, the industry has posed all types of approaches, among them: use of cements, polymer flooding, treatments for permeability reduction (gels, resins, relative permeability modifiers, etc.), foams, solid particles, mechanical methods in well, completion and drilling methods, location of wells, spacing reduction, selective abandonment and closure of wells, balancing patterns, detailed description of reservoir, etc. Of all these methods, gels, and, in particular, polymer gels, have become the conformance technology more widely applied (Sydansk & Romero-Zerón, 2011).
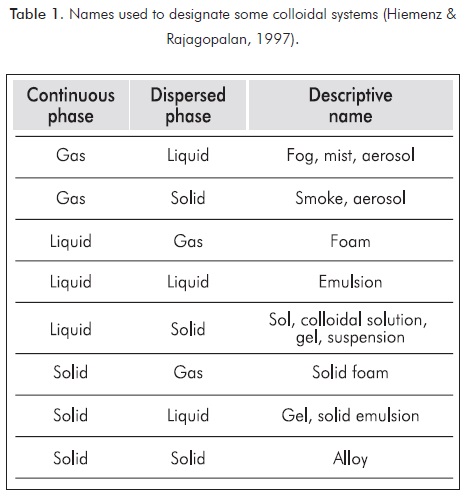
Colloids are defined as those particles with a linear dimension between 10-9 and 10-6 meters (Hiemenz & Rajagopalan, 1997). This interval is generated due to the relationship between the colloidal particle and the medium containing it. In general, the terms dispersed phase and continuous phase are used to refer to particles and medium containing them, respectively. Other authors defined colloids based on the weight of the particles or its atomic number. Table 1 shows a summary of some names used to designate colloidal systems.
Solutions for chemical conformance, mainly composed of a polymer solution plus a x-linker, usually consist of a dispersed solid phase in another continuous liquid phase, remaining within the colloids science.
Technology of polymer gels in hydrocarbon industry had its beginning in the 60s. In 1968 the first patent was granted (Goetz, 1968), which explains formation of polymer gels and pose its use for both replacing water in a displacement process (polymer flood) and for temporary blocking (chemical conformance). In 1973 a new patent (Gall, 1973) delved into underground permeability correction, focusing on the location of polymer gels in producing formations. The patent posed for the first time the possibility to correct permeability of a formation by obtaining a gel formed by binding a polymer with a metal ion, called x-linker. This patent details the need to inject into the formation a first batch of water-polymer solution with a specific concentration of polymer, then the metal ion was injected, waiting that both react in the formation to obtain a gel. Field applications, however, do not yield positive results on the decrease of water production associated with the production of oil (Sydansk & Argabright, 1987).
In 1987 in the United States a new patent was granted for the application of polymer gels in hydrocarbon reservoirs (Sydansk & Argabright, 1987). In this new case the polymer and the x-linker are mixed from the surface, injecting into the formation a single batch of solution (water + polymer + x-linker) which is subject to a stand time to wait the formation of the polymer gel. This last procedure showed its efficiency when used in 29 treatments in Wyoming, United States (Sydansk & Moore, 1990). The greatest benefit was obtained when polymer gels were used in injection wells, so that water associated with oil production in production wells influenced by the process decreased significantly. Average cost per incremental barrel generated by treatments was USD 0.34.
Despite of their commercial application (Wouterlood, Falcigno & Norman, 2002), operation mechanisms of polymer gel technology for its application in hydrocarbon reservoirs have not been explained in depth. Different studies have addressed the particular subject of the formation of polymer gels even before commercial success of the technology. In 1981, formation time of gel for various metal ions was studied by rheology (Terry et al., 1981). In 1994 possible binding mechanisms between Polyacrylamide and Cr+3 were studied using analytical chemistry methods (Lockhart, 1994). Also in 1994, a study on possible crossing mechanisms between Polyacrylamide and Cr+3, focusing on reaction rate and using ultraviolet ray measurements was presented (Klaveness & Ruoff, 1994). In 1995 the previous study was deepened including rheological measurements (Klaveness, Ruoff & Kolnes, 1995).
Despite of the above, no study has been conclusive on the formation of the gel, its structure or similar characteristics that can provide a better understanding on the operation of the technology.
3. EXPERIMENTAL DEVELOPMENT
The code of Sydansk presented in US Patent 4.683.949 creator of current technology (Sydansk & Argabright, 1987), in addition to some evidence taken from the practice recommended for assessing polymers used in improved recovery operations (API, 1990), add up the total of experimental tests prior to a treatment with polymer gels. Considering the work presented by Sydansk corresponds to a qualitative scale (bottle tests), which is shown in Annex Table A1, and that API RP 63 is designed “mainly for qualitative comparison of performance and general selection of water soluble polymers”, the existing gap in understanding phenomena involved is clear.
Bottle tests needed for Sydansk type qualitative interpretation include preparing the mixture of polymer and x-linker to desired proportions under absence of oxygen. Bottles are keep at a specific temperature. Each measurement over time involves removing the bottle from the oven and flip it to determine the qualitative Sydansk value. Given the necessary process, these tests can fail over time, especially at high temperatures.
The experimental approach proposed seeks to obtain more solid tools (not qualitative) for the design of polymer gels. The experimental design applies for the same system (polymer + x-linker) the traditional analysis described and quantitative techniques that have been used in the literature (ultraviolet and rheology) to study these types of mixtures. The analysis of results allows drawing parallels between the different techniques and poses a more accurate way to design polymer gels.
Sydansk Type Test
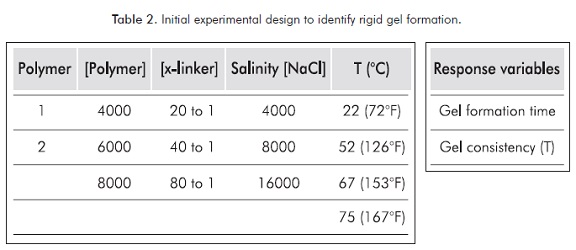
With the aim to identify such mixtures that form a rigid gel, traditional Sydansk tests are performed using two partially hydrolyzed polyacrylamides (HPAM) of different molecular weights. Table 2 details the experimental design for this case.
When considering five (5) controllable variables, each with levels selected within ranges used in commercial activity, the result is 216 mixtures observed during one month to determine which of them produces a rigid gel.
Short Sydansk Type, Ultraviolet (UV) and Rheology Tests
Taking the results from the previous step, three (3) controllable variables are added to study the effect of different ions concentrations (Ca++, Mg++ and Fe++) common in water used for preparing rigid gels. With this, the new experimental design is analyzed under the code of Sydansk, this time adding to simple observation ultraviolet rays and rheology analysis (G´and G’’) that allow identifying what happened in the mixture.
An important approach posed here is to reduce observation time in Sydansk type tests (1 month) to more acceptable values for commercial needs (hours-days).
4. RESULTS AND ANALYSIS
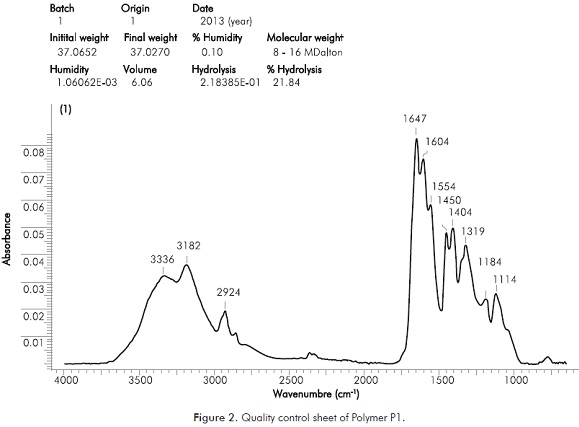
Two commercial polymers commonly used in deep processes with plugging gels inside the reservoir were selected (P1 and P2). It was ensured that polymers were polyacrylamides with degrees of hydrolysis between 20 and 25% and molecular weight of 8 and 16 MDalton. Figure 2 shows a control sheet of parameters, including infrared spectrum, of one of the polymers. In this figure it can be observed in that the profile obtained moves in waves common to HPAM. All results presented below correspond to polymer P1.
Sydansk Type Tests
Result tables of Sydansk type tests are presented in the Annex Tables A2, A3 and A4. Results obtained for the gel consistency (t) at the end of the tests do not allow identifying a clear trend for that parameter. Annex Figure B1 shows the dispersion of all values obtained at the end of the test.
Figures 3 to 8 show the effect of modifying in different ways the variables of the system (T, [x-linker], [NaCl] and [poly]) with the aim to identify its behavior.
Figure 3 shows the effect of temperature (T) on three specific mixtures (Figure 3a. Reaction [4000], [80:1], [4000]. Figure 3b. Reaction [6000], [80:1], [4000]. Figure 3c. Reaction [8000], [80:1], [4000]). It is clear that t does not depends directly of T.
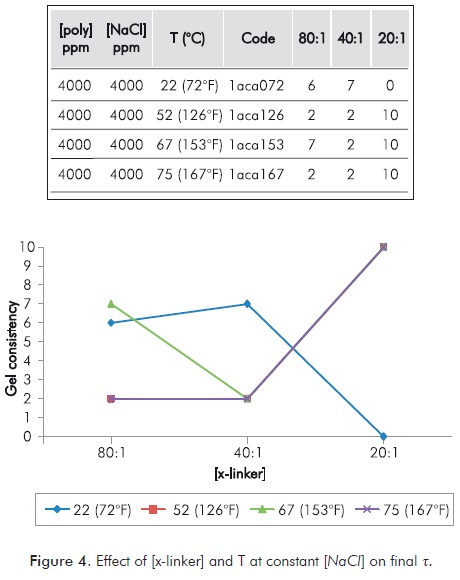
Figure 4 shows the effect on t of varying the concentration of the [x-linker] and T on a mixture with constant salinity [NaCl]. It can be noted how a higher [x-linker] does not ensure a higher t. Likewise, at specific T values high [x-linker] is not needed.
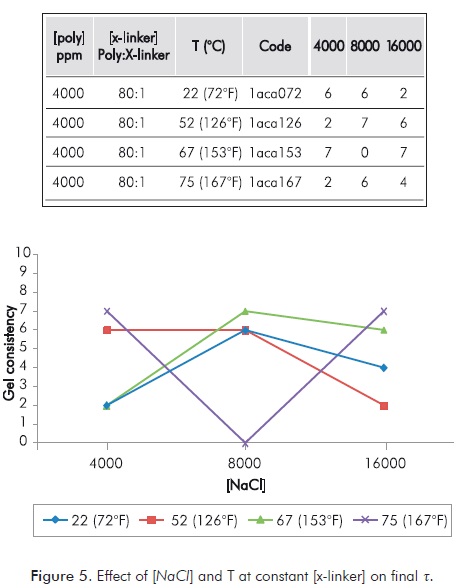
Figure 5 shows the changes in t caused by varying conditions of [NaCl] and T on a mixture with constant [x-linker]. Again, the effect observed does not allow demonstrating direct relations between the behavior of the variables.
Figure 6 shows the effect of varying both [NaCl] and [x-linker] at constant T on final t (Figure 6a shows [NaCl] vs. [x-linker]. Figure 6b shows [x-linker] vs. [NaCl]). Observations do not allow identifying clear trends.
Figure 7 details the effect of varying concentration of polymer [poly] and [x-linker] at constant T and [NaCl]. It is clear how the variation of properties does not show definitive trends on t.
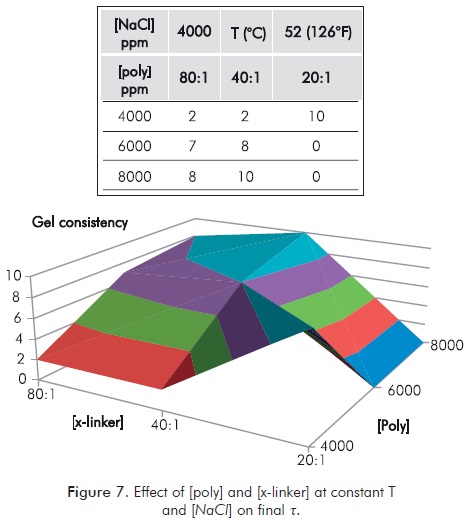
Figures above, 3 to 7, in all cases show that when properties under study are modified, t does not tend to a stabilization at which it maintains its value, or becomes stronger; on the contrary, variation of properties outside an undetermined range, always result in a decrease of t. All the foregoing indicates that once a gel is formed interactions that can destabilize it continue, making operation mechanisms unclear. It is worth mentioning that potential measurement and/or interpretation errors can play an important role in the results obtained. The above is inherent to the qualitative nature of the method used and justifies the need to have quantitative methods.
Sydansk Type Short Tests (12 hours)
Since conclusions to be drawn from classical analysis are inconclusive, additional tests that allow a better understanding of the operation mechanism of polymer gels are presented herein. Existing literature identifies measurements of ultraviolet spectrum as the best option to monitor phenomena taking place between a HPAM and an x-linker (Klaveness & Ruoff, 1994), therefore a series of specific mixtures are analyzed using this technique. Those reactions that have both a quick formation (24 hours) of gel and the highest value of t (10) at the end of test time (672 hours) were selected for tests with ultraviolet spectrum. Annex Table A5 shows the reactions that meet these characteristics.
Reactions shown in Annex Table A5 are performed again, being this time monitored every hour for 12 hours. Four of these reactions were performed again up to 672 hours (1 month) with the aim to have a better basis for analysis. Table 3 shows the results obtained for code of Sydansk 12 hours-tests.
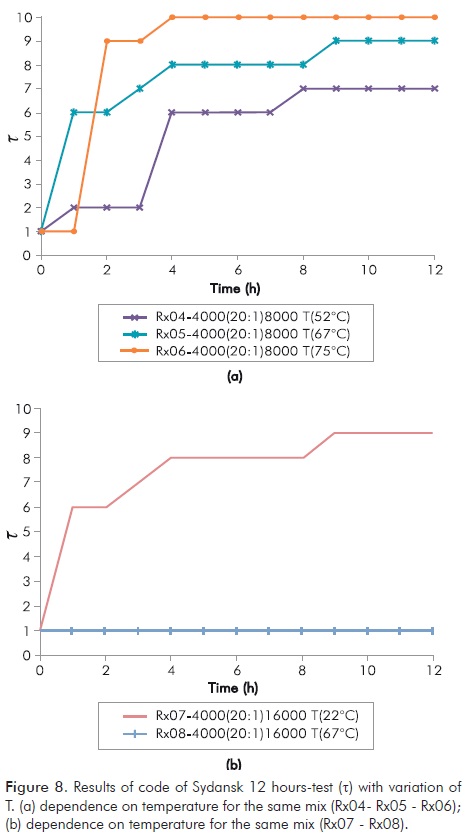
In Table 3 is clear that increasing [poly] at low temperature does not provide a higher rate to the formation of gel. The above suggests that high concentrations of polymer are important for duration of gel over time, but not for its formation rate. Also it is observed that after 12 hours of being prepared the mixture, only at temperatures greater than 22°C (72°F) formation of gel takes place, with a rapid increase in t of the same. Both findings demonstrate that the technology is susceptible to failure for both, not forming gel at low temperatures and forming it very quickly when temperature increases.
The above is shown in Figure 8, being clear for the same reaction its dependence on temperature (Figure 8a Rx04 - Rx05 - Rx06 and Figure 8b Rx07 - Rx08).
It can be observed that at short periods of time [NaCl] is not important for formation of gel, [NaCl] becomes important for its stability at longer periods of time and therefore to know whether a treatment will last over time.
Ultraviolet (UV) Spectrum Tests
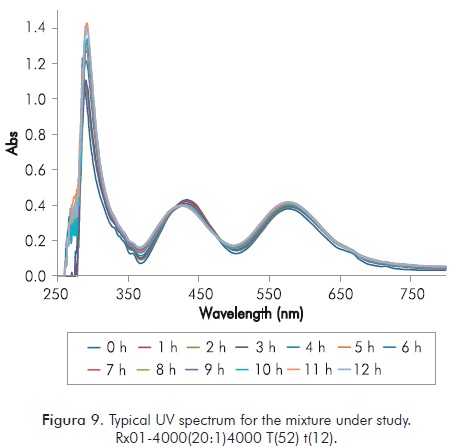
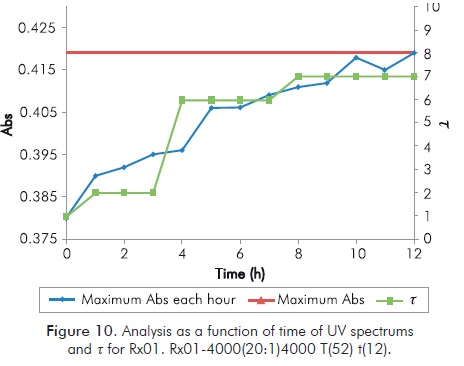
Figure 9 shows a characteristic image of UV spectrum for the mixture under study. In it the result of UV for Rx01 of Table 3 is taken each hour for 12 hours.
Klaveness studies (1994; 1995) set forth that for this reaction the formed gel must be monitored close to 570 nanometers, so that while t increases, absorbance in said wavelength must increase. When formation of gel stops, absorbance must maintain the same value and if t decreases absorbance should also decrease. For an overview of progress of the reaction, Figure 10 shows the maximum of each UV spectrum in the interval of interest as a function of time. The image shows the value of t at the same time.
Annex Figures B2 to B5 show for mixtures 7, 12, 13 and 16 of Table 3 the same analysis of Figure 10 for Rx01, including behavior at 672 hours. Annex Figures B2 to B5 show a clear correspondence between Sydansk type qualitative tests and UV quantitative tests, regardless of the test duration. The traditional test provides a response on the duration of the gel for an specified period of time (typically 1 month), but its qualitative scale is not very sensitive to potential losses of t. It is clear that at low temperatures it may take over 100 hours to the mixture to reach high t values, and taking into account that in field applications injection is immediate, dynamic conditions of the process may become the treatment inefficient. Typical temperatures of reservoir lead to high values of t in few hours, being this ideal for field applications, however, high temperatures do not guarantee duration of gel, therefore, extensive tests are still needed.
A method for designing gels for deep application inside the reservoir that combines UV analysis with observations in Sydansk scale, initially every hour during the first 12 hours and then every day until the 30th day, constitute a more appropriate procedure for deciding on the convenience of this technology for a group of specific conditions.
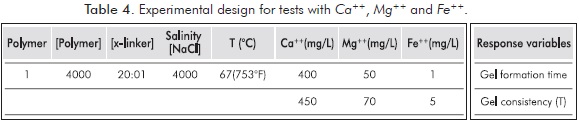
Effect of Ca++, Mg++ and Fe++ ions on the Formation of Polymer Gels
Available water in production fields frequently contains ions that influence any process in which water is used; therefore it is important to study their specific effects in the polymer gels formation.
It is a known fact that presence of Ca++, Mg++ and Fe++ ions negatively affects polyacrylamides; therefore these ions are involved in the analysis as controllable variables. Ions concentrations in the used solution are taken from regular injection Colombian waters.
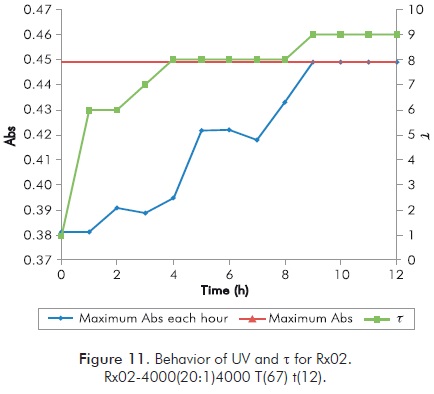
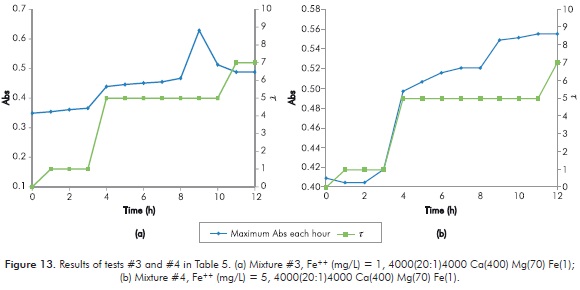
Mixture selected to study the effect of ions in polymeric gels formations corresponds to Rx02 of Table 3, mainly because it reaches a high value of t in a period of 12 hours, and a behavior of UV spectrum that reflects the variation of t; in addition, reaction temperature is not so high to allow a sudden formation of gel. Figure 11 shows the behavior of Rx02. Table 4 shows the experimental design for short Sydansk tests and UV tests with Ca++, Mg++ and Fe++ ions.
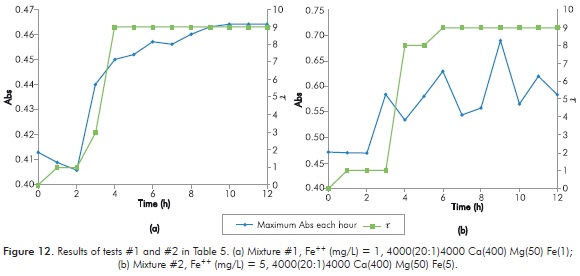
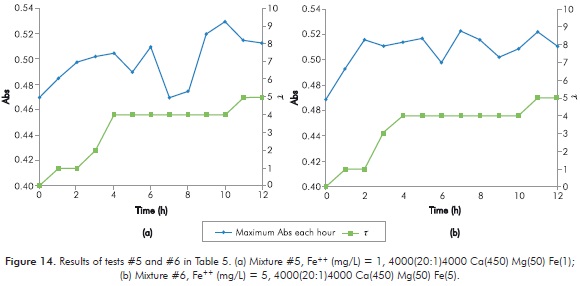
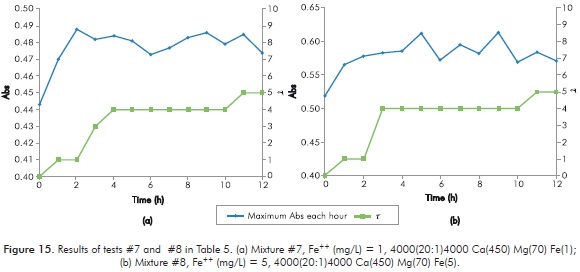
Table 5 shows the results obtained in every new mixture for short duration Sydansk type tests. Figures 12 to 15 show the results obtained with UV tests with its corresponding value of Sydansk type test.
Initially, results can be divided between the first four tests and the last four tests. When increasing [Ca++] in 50 mg/L, results of final t of the reaction at 12 hours drop to values that make the gel an unviable option, therefore it could indicate that for these types of reactions this a high impact parameter.
Behavior of the reaction when varying [Fe++] is observed when comparing tests 1 - 2 (Figure 12), 3 - 4 (Figure 13), 5 - 6 (Figure 14) and 7 - 8 (Figure 15). In all four cases it is observed that final t of gel does not vary by modifying [Fe++], therefore, at least under parameters of study, [Fe++] does not directly influences final gel obtained.
Behavior of the reaction when varying [Mg++] is observed when comparing 1 - 3 (Figures 12 and 13), 2 - 4 (Figures 12 and 13), 5 - 7 (Figures 14 and 15) and 6 - 8 (Figures 14 and 15). In the first two cases, when [Ca++] is at its lowest point, final t of gel decreases when [Mg++] increases. In the following cases, when [Ca++] is at its highest point, final t of gel does not change when increasing [Mg++]. The above seems to indicate that increasing [Mg++] affects negatively gel obtained.
In the presence of divalent ions response of UV analysis is not as clear as without them, absorbance values show great variation, so its use in these types of waters may not be the best option, being the qualitative approach the best option for these case. To improve relevance of ultraviolet spectrum it will be necessary to build baselines for polymer solutions without the presence of x-linker and divalent ions because them may also cross-link the polymer.
Rheology of Polymer Gels
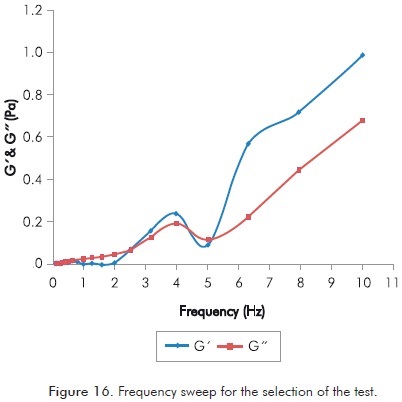
Rheological analysis of polymer gels begins by identifying the appropriate frequency at which selected rheometer must work. Annex Table A6 shows a frequency sweep using Rx02 mixture ([poly] 4000 / [x-linker] 20:1 / [NaCl] 4000), which is identified as the most appropriate to observe involved phenomena. Test temperature was maintained at all times at 67°C.
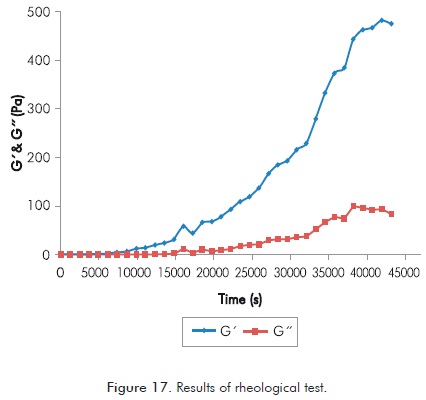
Figure 16 corresponds to values of G’ (storage modulus) and G’’ (loss modulus) showed in Annex Table A6.
In Figure 16 and annex Table A6, it is observed that trends of G’ and G’’ begin to separate over 0.6 Hz and their response does not clearly change. This justifies the selection of a frequency lower than 0.6 Hz (0.5 Hz in this case) for rheological studies in polymer solutions with x-linker.
Using the frequency selected in the previous step the rheological test is conducted during 12 hours to determine G’ and G’’. Duration of the test corresponds to the search of scientific consistency with short Sydansk type and UV tests performed with the same reaction.
Annex Table A7 shows part of results obtained in the rheological test, Figure 17 represents said information.
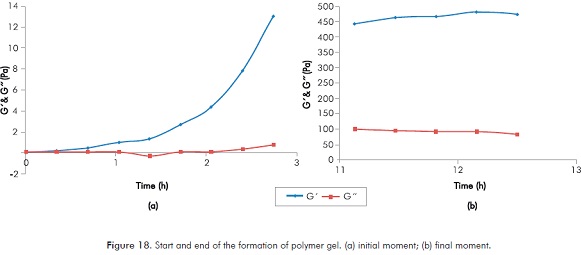
Figure 17 is a classic image of the expected response in measurements of G’ and G’’ in solutions that increases their viscosity with time. In this image can be observed for both parameters the start point of the formation of gel and a final stage in which the formation of gel has finished. Figure 18 shows the detail of both described moments, the figure shows a clear identification of a period of formation of gel (Figure 18a shows initial moment of the polymer gel formation and Figure 18b final moment of the gel formation).
For the conditions of the reaction under study the start point of the formation of gel is close to 0.55 hours, while the end of the formation of gel is closed to 11 hours. These values become important references when monitoring in the field injection processes of polymer gels.
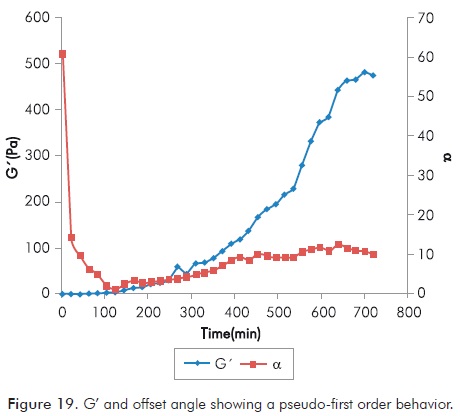
An important variable to be analyzed is the formation rate of gel. This rate is studied as a function of the storage modulus during the same. In this analysis the offset angle (α) between storage and loss modulus is also important. Annex Table A8 shows the information obtained during the rheological test and calculated to determine the reaction rate as a function of G’.
Figure 19 shows the values of G’ and offset angle (α) as a function of time. Behavior obtained allows to classify the formation of polymer gel as a pseudo-first order reaction, taking into account that polymer is in excess in the solution, therefore the stoichiometry is not considered in the same. In the same figure, behavior of offset angle is important. When it decreases and remains at low values shows the trend of gel to behave as a solid rather than as a fluid.
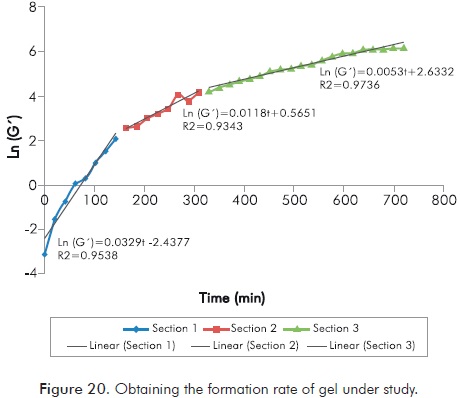
Figure 20 details the procedure for obtaining the reaction rate under study, from the natural logarithm of G’. As it shows different linear trends the approximation is divided into sections, as shown in the Figure 20.
From the Figure 20 can be determined that the reaction analyzed shows a rate that decreases until ending the same, rates obtained are 0.0329 min-1, 0.0118 min-1 and 0.0053 min-1.
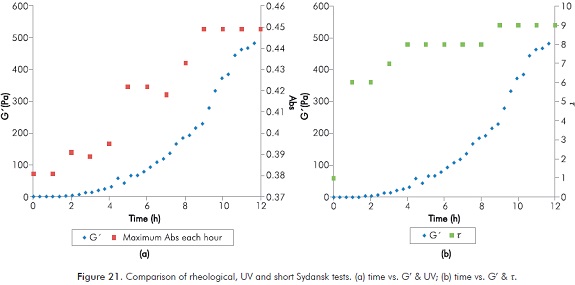
Analysis presented in this paper allow to conclude that the monitoring of polymer solutions can be performed more accurately by using rheological studies, however, the results of ultraviolet and Sydansk type tests are consistent with those obtained by rheology. Figure 21 compares results obtained with the different techniques for the same reaction. (Figure 21a time vs. G’ & UV and Figure 21b time vs. G’ & t).
It is clear that UV and Sydansk techniques consistently detect start and end of the formation of gel process, and its trend over time. As concluded above, for an appropriate design of polymer gel for field application, long-term tests (greater than one month) are needed; rheological tests can demand many resources. On the contrary, a Sydansk type test, which consist on a qualitative test that does not requires equipment, becomes a fundamental option. Use of ultraviolet spectrum can be considered as an intermediate option, however, the time needed for data analysis may be demanding.
5. CONCLUSIONS
- Sydansk type tests allow to determine the formation of polymer gel for a set of specific properties, however, do not provide a clear response on its behavior in the event of any variation of properties of the system.
- Polymer gel consistency (t) is not directly proportional to the concentration of polymer or x-linker. Formation of gel does not tend to stabilize its consistency; on the contrary, variations in the system outside an undetermined range always result in loss of consistency. The above indicates that once a gel is formed interactions that can destabilize it continue, making operation mechanisms unclear.
- There are clear operational risks in the technology studied; the most important are the possible no formation of gel at low temperatures (close to environment) and accelerated formation of the same when temperature increases.
- Equivalent salinity of water for preparation is not relevant for the formation of gels in short periods of time, it becomes significant for its stability and, therefore, for durability over time of an actual treatment. Polymer concentration responds to the same principle, high values contribute more to duration over time of gel than to its formation and t initially reached. Long-term tests, Sydansk type, are important for determining stability of gel over time; however, in the same should be evident that t is rapidly reached, otherwise soak time may be too long and treatment will be economically unviable.
- In general, in the presence of divalent ions qualitative approach of Sydansk type analysis is favored. Response of UV analysis is not clear in these cases, therefore its use in these types of waters may not be the best option.
- Reaction rate of a polymer gel specific formulation in this study was obtained using storage and loss modulus, identifying pseudo-first order behaviors. Given the excess of HPAM in solutions, stoichiometry of the same is not relevant, therefore kinetic concepts of reaction are inaccurate for modeling these phenomena.
- Experimental type errors, especially at high temperatures, can affect the results of this type of study, however, the above further justifies the need to develop quantitative procedures.
ACKNOWLEDGEMENTS
The authors express their gratitude to Ecopetrol S.A. and the Universidad Industrial de Santander for their consent to the publication of this paper. Additional acknowledgement to chemist Manuel Fernando Roa for his valuable contributions and discussions during the course of this research.
REFERENCES
Al-Muntasheri, G., Nasr-El-Din, H. & Zitha, P. (2008). Gelation kinetics and performance evaluation of an organically crosslinked gel at high temperature and pressure. SPE J., 13(03), 337-345. [ Links ]
American Petroleum Institute. (1990). Recommended practices for evaluation of polymers used in enhanced oil recovery operations. RP 63. 1 Ed. Washington, USA. [ Links ]
Gall, J. (1973). U.S. Patent No. 3.762.476. Subterranean formation permeability correction. Bartlesville. [ Links ]
Goetz, D. (1968). U.S. Patent No. 3.383.307. Geling agents, gels and methods for forming gels. Minneapolis. CL: 252-316. [ Links ]
Hiemenz, P. & Rajagopalan, R. (1997). Principles of colloid and surface chemistry. 3 ed. New York: Marcel Dekker. [ Links ]
Klaveness, T. & Ruoff, P. (1994). Kinetics of the cross-linking of polyacrylamide with Cr(III). Analysis of possible mechanisms. J. Phys. Chem., 98(40), 10119-10123. [ Links ]
Klaveness, T., Ruoff, P. & Kolnes, J. (1995). Kinetics of the cross-linking of polyacrylamide with Cr(III). Rheological measurements of the gelation. J. Phys. Chem., 99(20), 8255-8259. [ Links ]
Lockhart, T. (1994). Chemical properties of chromium/polyacrilamide gels. SPE Advanced Technology Series, 2(2), 199-205. [ Links ]
Norman, C., Turner, B., Romero, J., Centeno, G. & Muruaga, E. (2006). A review of over 100 polymer gel injection well conformance treatments in Argentina and Venezuela: Design, field implementation and evaluation. International Oil Conference and Exhibition in Mexico, Cancun, Mexico. SPE 101781. [ Links ]
Sheng, J. (2011). Modern chemical enhanced oil recovery: Theory and practice. 1 ed. Burlington: Elsevier. [ Links ]
Sydansk, R. & Argabright, P. (1987). U.S. Patent No. 4.683.949. Conformance improvement in a subterranean hydrocarbon-bearing formation using a polymer gel. Findlay. [ Links ]
Sydansk, R. & Moore, P. (1990). Production responses in Wyoming's big horn basin resulting from application of acrylamide-polymer/Cr(III)-Carboxilate gels. SPE-21894-MS. [ Links ]
Sydansk, R. & Romero-Zeron, L. (2011). Reservoir conformance improvement. 1 ed. Richardson: Society of Petroleum Engineers. [ Links ]
Terry, R. Huang, C. Green, D. Michnick, M. & Willhite G. (1981). Correlation of gelation times for polymer solutions used as sweep improvement agents. SPE J., 21(2), 229-235. [ Links ]
Wouterlood, C., Falcigno, E. & Norman, C. (2002). Metodología y resultados de proyectos de inyección de geles para incrementar la recuperación en un reservorio heterogéneo y multicapa de la Cuenca Neuquina de Argentina. INGEPET. [ Links ]
AUTHORS
Gustavo-Adolfo Maya-Toro
Affiliation: Ecopetrol S. A. - Instituto Colombiano del Petróleo (ICP)
Petroleum Engineer, Universidad Nacional de Colombia
M. Sc. in Petroleum Engineering, Universidad Industrial de Santander
e-mail: gustavo.maya@ecopetrol.com.co
Rubén-Hernán Castro-García
Affiliation: Ecopetrol S. A. - Instituto Colombiano del Petróleo (ICP)
Petroleum Engineer, Universidad de América
M. Sc. in Petroleum Engineering, Universidad Industrial de Santander
e-mail: rubenhe.castro@ecopetrol.com.co
Robinson Jiménez-Díaz
Affiliation: Ecopetrol S. A. - Instituto Colombiano del Petróleo (ICP)
Petroleum Engineer, Universidad Industrial de Santander
M. Sc. in Petroleum Engineering, Universidad Industrial de Santander
e-mail: robinson.jimenezDi@ecopetrol.com.co
Samuel-Fernando Muñoz-Navarro
Affiliation: Universidad Industrial de Santander
Petroleum Engineer, Universidad Industrial de Santander
M. Sc. in Hydrocarbons, Universidad Industrial de Santander
M. Sc. in Petroleum Engineering, Colorado School of Mines
e-mail: samuel@uis.edu.co