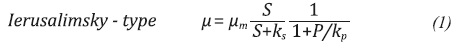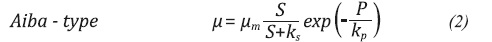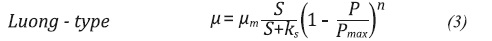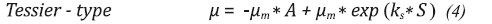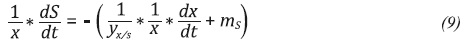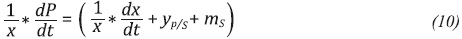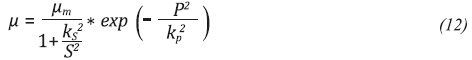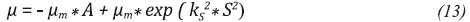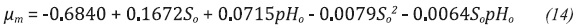Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383
C.T.F Cienc. Tecnol. Futuro vol.6 no.1 Bucaramanga Jan./June 2015
UNSTRUCTURED KINETIC MODEL FOR BATCH FERMENTATION OF USP GLYCEROL FOR LACTIC ACID PRODUCTION
MODELO CINÉTICO NO-ESTRUCTURADO DE FERMENTACIÓN DISCONTINUA DE GLICEROL USP PARA LA PRODUCCIÓN DE ÁCIDO LÁCTICO
MODELO CINÉTICO DE GLICEROL DESESTRUTURADO NONCONTINUOUS DE FERMENTAÇÃ0 USP PARA A PRODUÇÃO DE ÁCIDO LÁCTICO
Jimy-Alexander Gamboa-Rueda1, Víctor-Alexis Lizcano-González1, Mario-Andrés Ordoñez-Supelano2, José-Andrés Pérez-Mendoza3, Carolina Guzmán-Luna2 and Luis-Javier López-Giraldo4*
1Escuela de Ingeniería Química, Universidad Industrial de Santander, Bucaramanga, Santander, Colombia
2Escuela de Microbiología, Universidad Industrial de Santander, Bucaramanga, Santander, Colombia
3Programa de Ingeniería Química, Universidad del Atlántico, Barranquilla, Atlántico, Colombia
4Escuela de Ingeniería Química, Grupo de Investigación - CICTA, AgroBiotech Research Center-ABC, Universidad Industrial de Santander, Bucaramanga, Santander, Colombia, e-mail: ljlopez@uis.edu.co
How to cite: Gamboa-Rueda, J. A., Lizcano-González, V. A., Ordoñez-Supelano, M. A., Pérez-Mendoza, J. A., Guzmán-Luna, C. & López-Giraldo, L. J. (2015). Unstructured kinetic model for batch fermentation of USP glycerol for lactic acid production. CT&F - Ciencia, Tecnología y Futuro, 6(1), 81-94.
*To whom correspondence should be addressed
(Received: Feb. 06, 2015; Accepted: Jun. 02, 2015)
ABSTRACT
This study show the capability of Lactobacillus rhamnosus ATCC 7469 to produce lactic acid using crude glycerol from biodiesel production as carbon source; in addition, a kinetic model that describes the behaviour of the fermentation process using USP glycerol as substrate was proposed and developed. The strain was adapted to the new carbon source by doing successive cultures, the substrate conversion was up to 94.5% after 24 hours of fermentation using crude glycerol as under initial conditions of substrate concentration (crude glycerol) and pH of 8 g/L and 6.5, respectively. Then, the influence of initial pH (pHo) and concentration of substrate (So) were evaluated by fermentation tests of USP glycerol. The optimal value of volumetric lactic acid productivity (Qv) achieved from this study was 0.087 g.L-1.h-1 for initial pHo = 6.5 and initial substrate concentration So = 6 g/L. Lastly, the kinetics parameters of an unstructured and not segregated model (Aiba type equation for growth rate expression), were adjusted with an average fit degree of 88% for all the initial conditions, using USP glycerol. In addition, the kinetic parameters are laid out as function of initial pH and substrate concentration.
Keywords: Inhibition, Crude glycerol, Kinetics, Lactobacillus rhamnosus, Fermentation, Lactic acid.
RESUMEN
En este trabajo se demostró la capacidad de Lactobacillus rhamnosus ATCC 7469 para producir ácido láctico usando glicerol crudo proveniente de la industria del biodiesel como fuente de carbono, además se propuso y se desarrolló un modelo cinético que describe el proceso de fermentación usando glicerol USP como sustrato. La cepa fue adaptada a la nueva fuente de carbono utilizando cultivos sucesivos, la conversión de sustrato fue de hasta 94.5% después de 24 horas de fermentación en condiciones iniciales de sustrato de 8 g/L y pH inicial de 6.5 respectivamente. Posteriormente, la influencia del pH inicial (pHo) y la concentración de sustrato inicial (So) fueron evaluadas mediante ensayos fermentativos de glicerol USP. El valor óptimo de productividad volumétrica de ácido láctico (Qv) alcanzado en este trabajo fue de 0.087 g.L-1.h-1 para un pH inicial de pHo = 6.5 y una concentración inicial de sustrato So = 6 g/L. Finalmente los parámetros cinéticos de un modelo no estructurado y no segregado (ecuación tipo Aiba para la velocidad de crecimiento), fueron optimizados alcanzando un grado de ajuste de 88% para todas las condiciones iniciales, usando glicerol USP. Adicionalmente, los parámetros cinéticos son presentados como función del pH inicial y la concentración inicial de sustrato.
Palabras clave: Inhibición, Glicerol crudo, Cinética, Lactobacillus rhamnosus, Fermentación, Ácido láctico
RESUMO
Este trabalho demonstrou a capacidade do Lactobacillus rhamnosus ATCC 7469 para a produção de ácido láctico usando glicerol quando oriundo da indústria do biodiesel como fonte de carbono, adicionalmente, um modelo cinético foi proposto e desenvolvido com a finalidade de descrever o processo de fermentação usando glicerol USP como substrato. A cepa foi adaptada à nova fonte de carbono utilizando culturas sucessivas, a conversão do substrato foi de até 94.5% após 24 horas de fermentação em condições iniciais de substrato de 8 g/L e pH inicial de 6.5, respectivamente. Posteriormente, a influência do pH inicial (pHo) e a concentração de substrato inicial (So) foram avaliadas mediante ensaios fermentativos de glicerol USP. O valor ótimo de produtividade volumétrica de ácido láctico (Qv) conseguido neste trabalho foi de 0.087 g.L-1.h-1 para um pH inicial de pHo = 6.5 e uma concentração inicial de substrato So = 6 g/L. Finalmente, os parâmetros cinéticos de um modelo não estruturado e não segregado (equação tipo Aiba para a velocidade de crescimento), foram otimizados atingindo um grau de ajuste de 88% para todas as condições iniciais, usando glicerol USP. Adicionalmente, os parâmetros cinéticos são apresentados como função do pH inicial e a concentração inicial do substrato.
Palavras-chave: Inibição, Glicerol cru, Cinética, Lactobacillus rhamnosus, Fermentação, Ácido láctico.
1. INTRODUCTION
The global production of biofuels (bioethanol and biodiesel) grew 0.7% from 2010 to 2011, with a remarkable increase of 7.32% in biodiesel production. Hence, the biodiesel industry in 2011 reached a production of 1.5x107 tons of equivalent oil (British Petroleum, 2012). In Colombia the biodiesel production was 4.4x105 metric tons, which represents an increase of 31.19% compared to 2010 (Fedebiocombustibles, 2012). The biodiesel process produces in average 10% in weight of glycerol (Posada, Rincón & Cardona, 2012; Cerrate et al., 2006), which represented in 2011 an approximate production of crude glycerol of 4.9x104 tons in Colombia.
The overproduction of crude glycerol (industrial waste) and its low cost has prompted its use as raw material in other processes. Different biotechnological processes have been reported in which crude glycerol is converted into metabolites such as: 1,3-propanediol, citric acid, ethanol, buthanol (Dobson, Gray & Rumbold, 2012) and polyhidroxialcanoates (Yáñez, 2013). Nevertheless, there are few studies where lactic acid is the main metabolite.
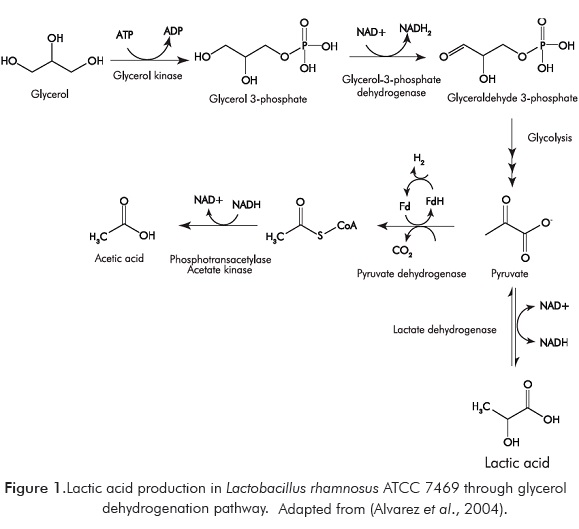
The biotechnological production of lactic acid from glycerol using Lactobacillus species is the result of several enzymatic steps. The first step is the formation of Glyceraldehide-3-Phospate (G3P) through glycerol dehydrogenation pathway and/or through glycerol dehydratase pathway. Lactobacillus rhamnosus ATCC 7469 only follows the dehydrogenation route, as it is shown in Figure 1 (Alvarez et al., 2004). Afterwards G3P is converted into pyruvate by glycolysis, finally pyruvate is dehydrogenated by the bacteria using the lactate dehydrogenase enzyme to produce lactic acid.
This pathway is an innovative and competitive option due to the vast amount of applications of this organic acid in the food, pharmaceutical, cosmetic and chemical industries (Wee et al., 2005; Hofvendahl & Hahn-Hägerdal, 2000). Furthermore, the low cost of glycerol compared with glucose (common substrate used in the majority of fermentation processes that produce lactic acid) suggest that biotechnological processes for this kind of substrate are of great economic interest.
In this topic, Hong et al. (2009), determined optimal fermentation conditions for Escherichia coli AC-521 reaching lactic acid concentration up to 85.8 g/L when fed-batch culture was applied with pure glycerol as carbon source. Choubisa et al. (2012), produced lactic acid from crude glycerol in batch cultures using strains of Lactobacillus delbrueckii reaching a concentration of 4.37 g/L and from pure glycerol using Lactococcus lactis, reaching concentrations of 2.26 g/L. Mazumdar et al. (2013), working with a genetically modified Escherichia coli in fed-batch culture were able to produce lactic acid from crude glycerol with concentrations up to 50 g/L. In spite of these promising results, none of the aforementioned articles includes the development of a kinetic model of the process.
In order to design the fermenters and analyze the dynamic behavior (Almquist et al., 2014) of lactic acid production, the next step is the kinetic modeling of the fermentation process. Therefore, the development of microbial kinetic models with the capacity of describing and predicting the concentration of products, biomass and substrate during the process is required (Doran, 1995). Kinetic models can also be applied in the stability analysis of fermentation (Castellanos, Matallana & López, 2014).
Although many different approaches could be used, in this study an unstructured and non-segregate model was selected in order to describe the fermentative system with a high degree of accuracy using the experimental information available (Banga, Versyck & Van Impe, 2000; Berry et al., 1999; Pinelli et al., 1997; Zhang et al., 1998).
Lactobacillus rhamnosus ATCC 7469 was selected on the basis of (Alvarez et al., 2004):
- It is possible to produce lactic acid from glycerol using this strain.
- The metabolic pathway of this fermentation was described.
At a first stage and with the purpose of taking into account its possible industrial applicability, the capability of the strain to metabolize crude glycerol obtained from the biodiesel process nationwide was evaluated, without any pretreatment or a prior step of refining. Subsequently, an unstructured and not segregated kinetic model that describes the behavior of the USP glycerol fermentation to produce lactic acid was proposed, considering the influence of the initial substrate concentration and pH. This proposed model would be a new approach because there are not available studies about the kinetic description for this specific type of fermentation.
2. KINETIC MODEL FRAMEWORK
Unstructured and not segregated models are defined as a differential equation system, which is based on the description of the specific growth rate (µ). This variable µ can be adjusted to different functions depending on different factors. In cases where inhibitor substrates and products are involved, the µ expression tends to be based on one of the following common models (Doran, 1995; Napoli et al., 2011; Shuler & Kargi, 2009):
The bacterial growth is usually described by: (Vlysidis et al., 2011).
The increasing product rate may be described by the Luedeking-Piret model (Vlysidis et al., 2011) considering growth and the non growth associated terms.
Lastly, the substrate variation is constructed as a mass balance considering consumption for cell maintenance, product and biomass formation (Vlysidis et al., 2011).
3. MATERIALS AND METHODS
Reagents
All the reagents used were of analytical grade, including liquid chromatography standards, salts for medium preparations, reagents for microbiological control etc. The crude glycerol was obtained from a Colombian biodiesel factory and its composition is detailed in Table 1 (Yáñez, 2013).
Fermentation Cultures
Man Rogosa Sharpe (MRS) media was used for growth and fermentation with the detailed formulation available in the microbiologic basic manual Cultimed (Panreac, 2012). In this research, glycerol (USP and crude) was used instead of glucose as carbon source. All cultures were carried out at 25 mL in 50 mL glass bottles, with 90% v/v of culture media and 10% v/v of inoculum with an Optical Density (OD) value of 0.2 units (the OD value is equivalent to an approximately start cell concentration of 107 CFU/mL in the broth). The optical density was measured at 540 nm in a spectrophotometer GENESYSTM 20 (ThermoSpectronic). Fermentation conditions were set to 37°C and 200 rpm in an orbital shaker Excella®E24 (New Brunswick Scientific).
Microorganism and Adaptation to the New Carbon Source
The strain Lactobacillus rhamnosus ATCC 7469 was purchased from the company Labcare de Colombia. The adaptation to the new carbon source followed a series of consecutive cultures with a gradual increase of substrate concentration from 4 g/L to 16 g/L. The bacterial cultures were intercalated between liquid and solid medium. Samples were taken after 24 hours in order to quantify the substrate conversion. Each test was done twice.
Effect of Initial pH and Substrate Concentration on the Volumetric Productivity
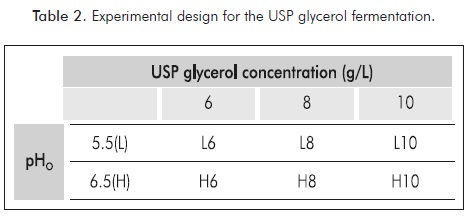
The effects of the initial conditions of pH (pHo) and substrate concentration (So) were evaluated following the experimental design shown in the Table 2.
For all conditions, destructive assays were employed by triplicate. The monitoring was performed at 0, 5, 9, 11, 13 and 15 hours of fermentation. For all tests biomass concentration, lactic acid, glycerol concentration and pH were measured. Finally, the influence of the pHo and So factors were studied through an ANOVA analysis having the volumetric productivity as the response variable; using software STATGRAPHICS®Centurion XV (trial version).
Analytical Techniques
Metabolites Monitoring
Glycerol and lactic acid concentration were quantified through High Performance Liquid Chromatography (HPLC) using a Coregel 107H column coupled to a UFLC LC 20AD (Shimadzu) equipment; the detector was a refraction index detector RID-10A. The chromatography equipment was operated in isocratic mode at 80°C, 0.6 mL/min flow. Sulfuric acid 8 mm was used as mobile phase. The analysis time was 25 minutes. Standards of the metabolites were used to calibrate the equipment. Prior to the analysis the samples were filtered using PVDF 0.45 µm membranes.
Biomass Monitoring
The biomass quantification was done using the lineal relation between the dry weight and the optical density. To determine the dry weight, samples were taken at different times and centrifuged at 6000 rpm during 15 minutes in a centrifuge MLW T23 (Janetski); afterwards the pellet was dried at 60°C until constant weight was reached.
Kinetic Parameter Estimation
First, for all conditions, the specific growth rate was calculated according to the Equation 8.
The function that describes the relation between the µ and the substrate concentration for all experiments was determined using the MATLAB’s fmincon function operating an interior point algorithm as an optimization problem. The maintenance constants (ms,mp) and the theoretical yields (yx/s,yp/x) were calculated using Equations 9 and 10.
Subsequently the adjusted model was integrated using the ODE45 tool of the software MATLAB (MathWorks®) 7.12.0 version. The parameters yx/s, ks and μm were optimized using the fmincon function operating an interior point algorithm coupled to the previously mentioned integrator. Finally, the accuracy and validity of each model was evaluated by means of the correlation coefficient (R2) and the average relative deviation, described in Equation 11.
4. RESULTS AND DISCUSSION
Strain Adaptation
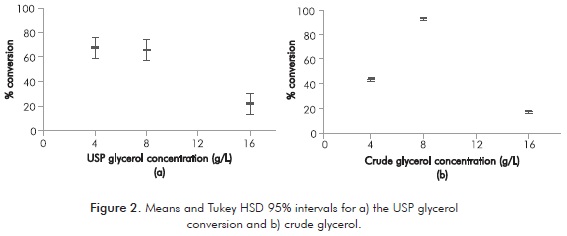
Adaptation process led to a better strain response to the new carbon source, which was represented in qualitative aspects as the number and size of the colonies in solid medium. It was also observed in quantitative aspects as the high grades of glycerol conversion reached (Figure 2).
Figure 2 shows that there is a significant conversion increase only when crude glycerol concentration was raised up from 4 g/L to 8 g/L. It is remarkable the high percentage of crude glycerol conversion (94.30 ± 0.15). For L rhamnosus ATCC 7469 both types of glycerol show substrate inhibition when concentration was raised up from 8 g/L to 16 g/L. From the data shown in the Figure 2 it may be inferred that L. rhamnosus has the potential to consume both USP glycerol and crude glycerol to produce lactic acid. Furthermore, crude glycerol has a great potential as substrate in lactic acid production.
The fundamental hypothesis behind why crude glycerol showed higher conversion rates is that impurities like diglycerides, monoglycerides and some free fatty acids may act as nutrients and enhancers for microbial growth (Choubisa et al., 2012).
Experimental Data for Kinetic Adjustment
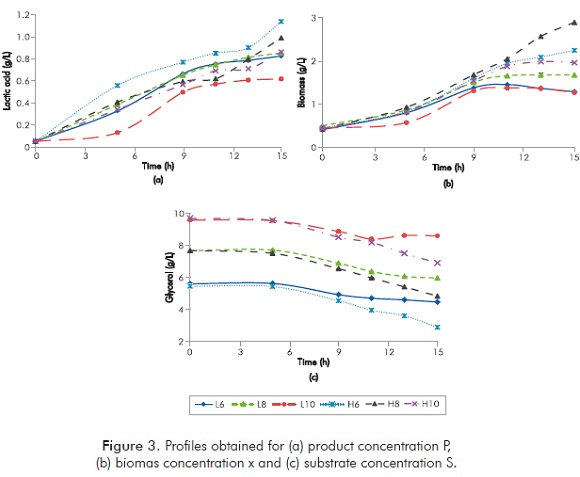
The experimental data was obtained following the experimental design described in the section 3. Profiles for substrate concentrations S (USP glycerol), biomass x (microorganisms) and product P (lactic acid) are shown in Figure 3.
Figure 3b shows a typical behavior of microorganisms, specifically there are two distinguishable patterns.
- Case 1: at low initial pH (pH0=5.5; represented by the letter L) and all the initial substrate concentrations evaluated, the lag phase took 5 h approximately, the exponential growth zone length was 4 h approximately and the stationary phase started at 9 h of fermentation.
- Case 2: at high initial pH (pH0=6.5; represented by the letter H) and all the initial substrate concentrations, the maximum exponential growth zone length was 10 h approximately and the stationary phase started after the 11th h of fermentation.
It is clear that the exponential growth phase was longer for case 2, which allowed higher productions of lactic acid (Figure 3a) and higher conversion of glycerol (Figure 3c). These observations are consistent with the kinetics or dynamics (Equation 7) for substrate consumption involved in the production of primary metabolites as lactic acid.
It is remarkable that even when not detecting glycerol consumption (first 5 hours of fermentation), lactic acid production was detected indeed, which indicates that the strain has the ability to synthetize this metabolite using only the culture media (Figure 3a and 3c). With regard to the above, it is known that some substances that are present in the MRS culture media such as peptone, yeast extract, meat extract and tween 80, can be considered carbon sources (Garland & Mills, 1991). A statistical data analysis was performed using the “Multiple response optimization” tool of the Statgraphics software (trial version). The maximum volumetric productivity calculated was 0.087 g.L-1.h-1 for the initial conditions pHo= 6.5 and So = 6 g/L and a fermentation time of 9.6 h. This volumetric productivity is similar to the one obtained for Choubisa et al. (2012) using Lactobacillus delbrueckii (0.091 g.L-1.h-1).
It should be noted that although the maximum glycerol conversion was around 50%, this research was aimed to obtain the kinetic parameters of the process, which constitutes valuable information for further studies. These ideas and results are consistent with the study by Rivaldi et al. (2013).
Estimation of Kinetic Parameters
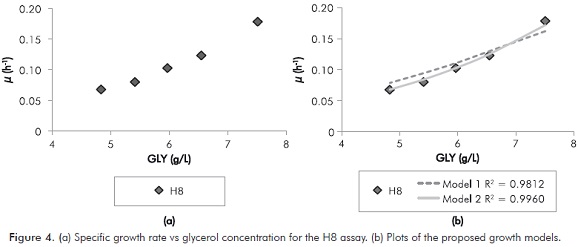
This procedure has taken into account the exponential growth phase and it was conducted for all the initial conditions. The Figure 4a shows the relation between µ and glycerol concentration for the exponential growth phase in the H8 assay. The increasing tendency of the specific growth rate in relation to the glycerol concentration was similar for all assays. Equations 12 and 13 show modifications of the growth models of the Aiba and Tessier type (Shuler & Kargi, 2009). These models were the ones that described the relation between µ and substrate concentration in the best way (see Figure 4b). Table 3 summarizes the values of the calculated parameters and the fit error for all the initial conditions.
From the results of adjustment, the following trends can be observed: For model 1 (Equation 12), in all cases studied, the maximum specific growth rate (μm) was greater for higher initial pH. This trend is consistent with the results reported in Figure 3. Using model 2 (Equation 13) as fit model it is discarded because it is not possible to make a physical analysis of the fermentation, in addition, the mean absolute error was higher than the one in model 1.
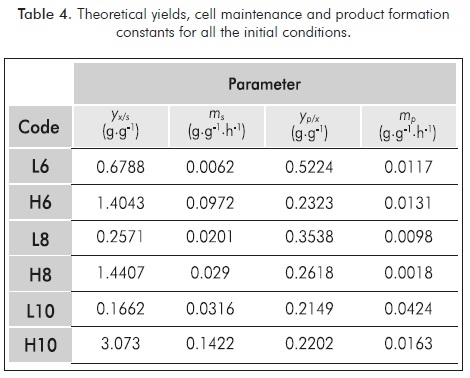
The kinetic-stoichiometric parameters yx/s, ms, yp/x and mp were calculated applying a linear regression to Equations 8 and 9, as it was explained in section 3. Values are presented in Table 4. The microorganism parameters were integrated to the differential Equations 5-7, employing the model 1 of microbial growth (Table 3). To reduce the error of the proposed model in relation to the experimental values, the optimization of the μm, ks and yx/s values was proposed, having as initial values the ones shown in Table 3 and Table 4.
Table 5 shows the post-optimization values for the kinetic parameters, the model error and the R2 coefficient value. As a result of parameter optimization it was possible to reduce the average error of the model from 31.53% to 12.24%, hence the R2 value increased 6.55% in average (Table 5).
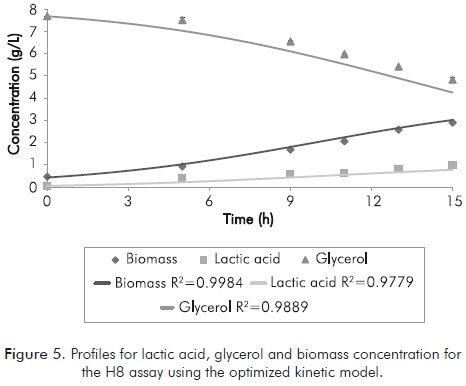
Figure 5 shows the integration of the not segregated and unstructured proposed model, using the optimized parameters for H8 assay. It is worth noting the grade of fit of the model for the H8 treatment, which represents an error of 9.3%.
According to the parameter obtained there are a directly proportional relation between the biomass - substrate yield and the initial pH of the medium. That relation is inversely proportional in most of cases for the product-biomass yield. Theoretical yields yx/s greater than one could be explained partially by the fact that this analysis does not separate the biomass formed from glycerol of the one formed just from the culture medium. The low values for the maintenance constants (m and mp) have their explanation in the fact that for facultative anaerobic strain as it is L. rhamnosus ATCC 7469 these values are typically low or even negligible (Vlysidis et al., 2011). In the case of the inhibition constants (ks and kp) it is not possible to make an extensive discussion since there is not available information of kinetic studies of the same strain with the same carbon source, because of the novelty character of this research. However, it is possible to state that the order of magnitude is comparable with similar kinetic studies that have been using glycerol as carbon source or lactic acid as the main product. In this regard, the fitted values of μm are in average 50% lower than the reported for glucose fermentation, which could be considered normal due to USP glycerol is a substrate more difficult to assimilate by microorganisms (Berry et al., 1999; Vlysidis et al., 2011).
To extend the applicability of the proposed model within the interval of established conditions, regression functions were developed in order to relate each parameter with the input variables pHo and So, obtaining the functions shown in Equations 14 to 20.
Equations 14-20 regression functions of the kinetic parameters in respect to pHo and So
The integrated profiles for all the initial conditions using the previously described regression functions are shown in Figure 6. It becomes evident that the profiles are consistent with the trend that describe the physical behavior of the bioprocess, hence, showing the good fit that represents the kinetic model proposed with mean values for error of 12.45% and 0.955 for the R2.
5. CONCLUSION
Lactobacillus rhamnosus ATCC 7469 was able to assimilate crude glycerol successfully with a substrate conversion close to 95% after 24 h of fermentation. Additionally the substrate conversion (USP glycerol) and the lactic acid production were favored by high initial pH (6.5) and low glycerol concentration (8 g/L). Hence, it was found evidence of a direct relation between the length of the exponential growth phase and the lactic acid concentration. For the initial conditions evaluated it was possible to identify that the optimum value for the volumetric productivity is around 9.6 h of fermentation with a value of Qv = 0.087 g.L-1.h-1. Finally, an unstructured and not segregated kinetics model that describes the fermentation process with a mean fit grade of 88% was proposed for the six initial conditions. It is noteworthy that the maximum glycerol conversion experimentally achieved was around fifty percent.
ACKNOWLEDGEMENTS
The authors wish to thank the Vicerrectoría de Investigación y Extensión of the Universidad Industrial de Santander for the financial support through the project entitled: Escalamiento de los procesos de producción de bio-alcoholes y procesamiento de subproductos provenientes de la producción de biocombustibles por vía fermentativa (Code 5452), the Bacteriology school of the Universidad Industrial de Santander for all the locations and equipment provided, and the Chemical Engineering school for allowing us to conduct all the HPLC analysis.
REFERENCES
Almquist, J., Cvijovic, M., Hatzimanikatis, V., Nielsen, J. & Jirstrand, M. (2014). Kinetic models in industrial biotechnology - Improving cell factory performance. Metab. Eng., 24: 38-60. [ Links ]
Alvarez, M., Medina, R., Pasteris, S., Strasser de Saad, A. & Sesma, F. (2004). Glycerol metabolism of Lactobacillus rhamnosus ATCC 7469: Cloning and expression of two glycerol kinase genes. J. Mol. Microbiol. Biotechnol., 7(4),170-181. [ Links ]
Banga, J. R., Versyck, K. J. & Van Impe, J. F. (2000). Numerical strategies for optimal experimental design for parameter identification of non-linear dynamic (Bio-)chemical processes. Comput. Aided Chem. Eng., 8: 37-42. [ Links ]
Berry, A., Franco, C., Zhang, W. & Middelberg, A. (1999). Growth and lactic acid production in batch culture of Lactobacillus rhamnosus in a defined medium. Biotechnol. Letters. 21(2), 163-167. [ Links ]
British Petroleum. (2012). BP Statistical Review of World Energy., London, England. 45pp. [ Links ]
Castellanos, L. J., Matallana, L. G. & López. L. J., (2014). Análisis de estabilidad de un sistema de fermentación acetona-butanol-etanol (ABE) a partir de glucosa empleando Clostridium acetobutylicum ATCC 824. Rev. Mutis, 4(1), 15-23. [ Links ]
Cerrate, S., Yan, F., Wang, Z., Coto, C., Sacakli, P. & Waldroup, P. W. (2006). Evaluation of glycerine from biodiesel production as a feed ingredient for broilers. Int. J. Poult. Sci., 5(11), 1001-1007. [ Links ]
Choubisa, B., Patel, H., Patel, M. & Dholakiya, B. (2012). Microbial production of lactic acid by using crude glycerol from biodiesel. J. Microbiol. Biotech. Res., 2(1), 90-93. [ Links ]
Dobson, R., Gray, V. & Rumbold, K. (2012). Microbial utilization of crude glycerol for the production of value-added products. J. Ind Microbiol. Biotechnol., 39(2), 217-226. [ Links ]
Doran, P. M. (1995). Bioprocess engineering principles. 1 ed. England: Elsevier Science and Technologic Books. [ Links ]
Federación Nacional de Biocombustibles de Colombia. (2012). Cifras informativas de sector biocombustibles, biodiésel de palma de aceite. Informe Fedebiocombustibles, Bogotá, Colombia. 8pp. [ Links ]
Garland, J. L. & Mills, A. L. (1991). Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol., 57(8), 2351-2359. [ Links ]
Hofvendahl, K. & Hahn-Hägerdal, B. (2000). Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microbial Technol., 26(2-4), 87-107. [ Links ]
Hong, A. A., Cheng, K. K., Peng, F., Zhou, S., Sun, Y., Liu, C. M. & Liu, D. H. (2009). Strain isolation and optimization of process parameters for bioconversion of glycerol to lactic acid. J. Chem. Technol. Biotechnol., 84(10), 1576-1581. [ Links ]
Mazumdar, S., Blankschien, M. D., Clomburg, J. M. & Gonzalez, R. (2013). Efficient synthesis of L-lactic acid from glycerol by metabolically engineered Escherichia coli. Microb. Cell Fact., 12: 1-7 [ Links ]
Napoli, F., Olivieri, G., Russo, M., Marzocchella, A. & Salatino, P. (2011). Continuous lactose fermentation by Clostridium acetobutylicum- Assessment of acidogenesis kinetics. Bioresour. Technol. 102(2), 1608-1614. [ Links ]
Panreac. Cultimed (2012). Manual básico de microbiología. PANREAC. [ Links ]
Pinelli, D., González-Vara, A., Matteuzzi, D. & Magelli, F. (1997). Assessment of kinetic models for production of L- and D- lactic acid isomers by Lactobacillus casei DMS 20011 and Lactobacillus coryniformis DMS 20004 in continuous fermentation. J. Ferment. Bioeng., 83(2), 209-212. [ Links ]
Posada, J. A., Rincón, L. E. & Cardona, C. A. (2012). Design and analysis of biorefineries based on raw glycerol: Addressing the glycerol problem. Bioresour. Technol., 111: 282-293. [ Links ]
Rivaldi, J., Sousa, M., Duarte, L., Ferreira, A., Cordeiro, C., de Almeida, M., de Ponces, A. & de Mancilha, I. (2013). Metabolism of biodiesel-derived glycerol in probiotic Lactobacillus strains. Appl. Microbiol. Biotechnol., 97(4), 1735-1743. [ Links ]
Shuler, M. L. & Kargi, F. (2009). Bioprocess engineering basic concepts. 2 ed. United States: Prentice Hall PTR. [ Links ]
Vlysidis, A., Binns, M., Webb, C. & Theodoropoulos, C. (2011). Glycerol utilization for the production of chemicals: Conversion to succinic acid, a combined experimental and computational study. Biochem. Eng. J. 58-59: 1-11. [ Links ]
Yáñez, C. (2013). Extracción y caracterización de polihidroxibutirato producido a partir de Bacillus megaterium ATCC 14581 utilizando glicerol residuo de la industria de biodiesel como fuente de carbono. Tesis de pregrado, Escuela de Química, Universidad Industrial de Santander, Bucaramanga, Colombia, 63pp. [ Links ]
Wee, Y. J., Kim, J. N., Yun, J. S. & Ryu, H. W. (2005). Optimum conditions for the biological production of lactic acid by a newly isolated lactic acid bacterium, Lactobacillus sp. RKY2. Biotechnol. Bioprocess Eng., 10(1), 23-28. [ Links ]
Zhang, X. W., Sun, T., Sun, Z. Y., Liu, X. & Gu, D. X. (1998). Time-dependent kinetic models for glutamic acid fermentation. Enzyme Microbiol. Technol., 22(3), 205-209. [ Links ]
AUTHORS
Jimy-Alexander Gamboa-Rueda
Affiliation: Universidad Industrial de Santander
Chemical Engineering, Universidad Industrial de Santander
e-mail: jimygamboa@gmail.com
Víctor-Alexis Lizcano-González
Affiliation: Universidad Industrial de Santander
e-mail: victorlizcano@outlook.com
Mario-Andrés Ordoñez-Supelano
Affiliation: Universidad Industrial de Santander
e-mail: mario.ordonhez@gmail.com
José-Andrés Pérez-Mendoza
Affiliation: Universidad del Atlántico
Chemical Engineering, Universidad Industrial de Santander
M. Sc. Chemistry, Universidad Industrial de Santander
e-mail: jandrespmen@gmail.com
Carolina Guzmán-Luna
Affiliation: Universidad Industrial de Santander
Bacteriologist, Universidad Industrial de Santander
e-mail: cgluna74@gmail.com
Luis-Javier López-Giraldo
Affiliation: Universidad Industrial de Santander
Chemical Engineer, Universidad Nacional de Colombia
Ph. D. in Chemistry, Biochemistry and Food Science, École Superieur Agronomique Montpellier
e-mail: ljlopez@uis.edu.co