Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383
C.T.F Cienc. Tecnol. Futuro vol.6 no.1 Bucaramanga Jan./June 2015
THERMOPHYSICAL PROPERTIES OF CASTOR OIL (RICINUS COMMUNIS L.) BIODIESEL AND ITS BLENDS
PROPIEDADES TERMOFÍSICAS DE BIODIESEL DE ACEITE DE RICINO (RICINUS COMMUNIS L.) Y SUS MEZCLAS
PROPIEDADES TERMOFÍSICAS DE BIODIESEL A PARTIR DE ÓLEO DE RICINO (RICINUS COMMUNIS L.) E SUAS MISTURAS
Osman Gokdogan1*, Tanzer Eryilmaz2 and Murat Kadir Yesilyurt2
1Department of Biosystems Engineering, Nevsehir Haci Bektas University, Nevsehir, Turkey, e-mail: osmangokdogan@gmail.com
2Department of Biosystems Engineering, Bozok University, Yozgat, Turkey
How to cite: Gokdogan, O., Eryilmaz, T. & Yesilyurt, M. K. (2015). Thermophysical properties of castor oil (Ricinus communis L.) biodıesel and its blends. CT&F - Ciencia, Tecnología y Futuro, 6(1), 95-128.
*To whom correspondence should be addressed
(Received: Mar. 02, 2015; Accepted: Jun. 03, 2015)
ABSTRACT
In this study, biodiesel (methyl ester) was produced from Castor Oil (Ricinus communis L.) (CO) using sodium hydroxide (NaOH) and methanol (CH3OH) by the two-step transesterification method. Nine different fuel blends (2, 5, 10, 20, 30, 40, 50, 60 and 75% by volume blending with diesel) were prepared. The density values of Castor Oil Biodiesel (COB) and its blends were measured at the temperature range from 0 to 93°C in steps of 5°C and the kinematic viscosity values of COB and its blends were measured at the temperature range from 30 to 100°C in the steps of 5°C. The results showed that the density, kinematic viscosity, calorific value, flash point, pH, copper strip corrosion and water content of COB are 932.40 kg.m-3, 15.069 mm2.s-1, 38.600 MJ.kg-1, 182°C, 7, 1a and 1067.7 mg.kg-1, respectively. The density and kinematic viscosity of fuel samples decrease as temperature increases; and also these properties decrease as a result of the increase in the amount of diesel in the blends.
Keywords: Castor oil, Transesterification, Fuel property, Biodiesel, Density, Kinematic viscosity.
RESUMEN
En este estudio, se produjo biodiesel (metil éter) a partir de aceite de ricino (Ricinus communis L.) (CO, de sus siglas en ingles) utilizando hidróxido de sodio (NaOH) y metanol (CH3OH) a través del método de transesterificación en dos pasos. Se prepararon nueve mezclas diferentes (2, 5, 10, 20, 30, 40, 50, 60 and 75% dependiendo del volumen de la mezcla con biodiesel. Se estimaron los valores de densidad del Biodiesel de Aceite de Ricino (COB, de sus siglas en ingles) y sus mezclas en un rango de temperatura de 0 a 93°C en intervalos de 5°C y también se estimaron los valores de viscosidad cinemática de COB y sus mezclas dentro del rango temperatura comprendido entre 30 y 100°C en intervalos de 5°C. Los resultados mostraron que la densidad, la viscosidad cinemática, el valor calorífico, el punto de ignición, pH, la corrosión de la franja de cobre y el contenido de agua del COB son 932.40 kg.m-3, 15.069 mm2.s-1, 38.600 MJ.kg-1, 182°C, 7, 1a y 1067.7 mg.kg-1, respectivamente. La densidad y la viscosidad cinemática de las muestras de combustible disminuyen a medida que aumenta la temperatura; y también estas propiedades disminuyen como resultado del aumento en la cantidad de biodiesel en las mezclas.
Palabras clave: Aceite de Ricino, Transesterificación, Propiedades del Combustible, Biodiesel, Densidad, Viscosidad Cinemática.
RESUMO
Neste estudo, o biodiesel (metil éter) foi produzido a partir do óleo de rícino (Ricinus communis L.) (CO, por suas siglas em inglês) usando hidróxido de sódio (NaOH) e metanol (CH3OH) através de um método de transesterificação de dois passos. Foram preparadas até nove misturas de combustível diferentes (2, 5, 10, 20, 30, 40, 50, 60 e 75% por volume de mistura com o diesel). Os valores de densidade do biodiesel de óleo de rícino (COB, por suas siglas em inglês) e as suas misturas foram calculados dentro do rango de temperatura de 0 a 93°C no passo de 5°C e os valores de viscosidade cinemática do COB e das misturas foram calculadas no rango de temperatura de 30 a 100°C no passo de 5°C. Os resultados demonstraron que a densidade, viscosidade cinemática, valor calorífico, ponto de fusão, PH, corrosão da faixa de cobre e conteúdo de água do COB eram de 932.40 kg.m-3, 15.069 mm2.s-1, 38.600 MJ.kg-1, 182°C, 7, 1a e 1067.7 mg.kg-1, respectivamente. Os valores de densidade e viscosidade cinemática das amostras de combustível diminuem na medida em que aumenta a temperatura; e também essas propriedades diminuem em decorrência do aumento na quantidade de diesel nas misturas.
Palavras-chave: Óleo de rícino, Transesterificação, Propriedades do Combustível, Biodiesel, Densidade, Viscosidade cinemática.
1. INTRODUCTION
Since the beginning of civilization, human beings are struggling for making progress in almost all domains of our life as a means to fulfill bare necessities like shelter, food, clothing and energy, etc. In addition to environmental and socio-economic concerns, the increasing gap between energy demand and supply coupled with the focus of limited fossil fuel resources and price inflation, have led researchers to develop biodiesel as an eco-friendly alternative to diesel fuel (Mumtaz et al., 2012). The requirements for diesel fuels include the ability to flow in the fuel feed system and to lubricate fuel injection pumps and fuel injectors. The viability to use vegetable oil as a diesel fuel was first demonstrated in 1895 by Rudolph Diesel, who operated a diesel engine with peanut oil. It was later verified that, in order to improve vegetable oils capacity to be used as a diesel fuel, it was necessary to remove glycerol from their molecules. Finally, biodiesel is obtained after the glycerol removal (Valente et al., 2010).
Biodiesel is defined as the mono-alkyl esters of vegetable oils or animal fats. Vegetable oils and fats as alternative engine fuels are all extremely viscous with viscosities ranging from 10 to 17 times greater than petroleum diesel fuel. Biodiesel is produced by transesterifying the parent oil or fat to achieve a viscosity close to that of diesel. The chemical conversion of the oil to its corresponding fatty ester (biodiesel) is called transesterification. The purpose of the transesterification process is to lower the viscosity of the oil (Demirbas, 2007).
There are more than 350 oil-bearing plants identified (with thousands of sub-species) that could be used to grow a new crop of fuel every year. The productivity of perennials is higher; they avoid erosion and can also be cultivated in mountain areas. Some species can be harvested more than once a year. The fuel potentialities of many vegetable oils were considered as early as 1939 (Salvi & Panwar, 2012; Jahirul et al., 2013). Table 1 shows main feedstocks of biodiesel production.
The production of biodiesel using non-edible crops, inappropriate for human consumption due to the presence of toxic compounds, has been researched over the last few years. Among the most studied crops are: jatropha (Jatropha curcas), karanja (Pongamia pinnata), castor (Ricinus communis), coriander (Coriandrum sativum), tobacco seed (Nicotiana tabacum), rubber (Hevea brasiliensis) and wild mustard (Brassica juncea) (Dias et al., 2013).
CO (castor bean, castor, castor oil plant, ricin, higuerilla, mamona, mamoeira, palma christi) is a member of the tropical spurge family (Euphorbiaceae) and can nowadays be found naturalized and cultivated in all temperate countries of the world. Castor is originally a tree or shrub that can grow above 10 m high, reaching an age of about 4 years. At present, the cultivated varieties grow to a height of 60-120 cm in 1 year, and several meters in perennial cultivation. Castor grows in the humid tropics to the subtropical dry zones (optimal precipitation 750-1000 mm, temperature 15-38°C) and can be also cultivated in southern Europe (Scholz & Nogueira, 2008).
CO, extracted from the seeds of Ricinus communis L., is viscous, pale yellow, non-volatile and non-drying oil (Hincapié, Mondragón & López, 2011). CO presents between 80-90% of ricinoleic acid (12-hydroxy-9-cis-octadecenoic acid). Such unique composition brings a disadvantage for its use for biodiesel production, since its viscosity is about 7 times higher than the one of other vegetable oils. Because CO is highly hygroscopic, water content might also be higher than desirable. To overcome such issues, its use in mixture with diesel has proven effective, namely in a 10% blend, to comply with specifications in standards (Dias et al., 2013).
Conceição et al. (2007) have stated that the oil ratio of CO plant changes at 47-49% range and its biodiesel cost is lower, in comparison with other plants. They found COB’s viscosity value as 13.75 mm2.s-1 (at 40°C), sulphur value as 0.0001%, density as 0.9279 g.cm-3 (at 15°C), ASTM colour as yellow, flash point as 120°C and copper strip corrosion value as 1.
In their study compiling the characteristics of CO, Scholz and Nogueira (2008) determined density as 950-974 kg.m-3 (at 15°C), flash point as 229-260°C, kinematic viscosity as 240-300 mm2.s-1 (at 40°C), net calorific value as 37.2-39.5 MJ.kg-1, water content as 0.15-0.30% and iodine number as 82-90 g.Iodine 100.g-1.
Albuquerque et al. (2009) have produced biodiesel from castor, soybean, canola and cotton oil, and studied the fuel characteristics. They reported COB’s specific weight as 920, kinematic viscosity as 13.5 cSt (at 40°C), iodine index as 85.2, acid value as 0.42 mg KOH.g-1, free glycerol as 0.015 %m.m-1 and combined glycerol as 0.018 %m.m-1.
Berman, Nizri and Wiesman (2011) produced biodiesel from CO, by using methanol and potassium hydroxide (KOH) through a single-stage transesterification process. They concluded COB’s cetane number (48.9), cloud point (-14°C), oxidation stability (44 h at 110°C) values met ASTM D6751 standards, while kinematic viscosity (at 40°C) and distillation temperature (15.17 mm2.s-1 and 398.7°C respectively) values did not meet the standards.
Valente et al., (2011) have produced biodiesels from frying oil and CO at 6:1 methanol to oil molar ratio, 0.5% of NaOH and 60°C reaction temperature, and they mixed these biodiesels with diesel fuel, at a ratio of 25, 50 and 75% to analyse density, kinematic viscosity, distillation temperature and sulphur contents. As a result, 35% COB concentration in diesel fuel met EN 14214 for biodiesel density, kinematic viscosity and distillation temperature at 90°C.
Knothe, Cermak and Evangelista (2012) showed that refined CO’s kinematic viscosity (at 40°C), oxidative stability (at 110°C) and acid values were 256.69 mm2.s-1, 74.80 h and 0.323 mg KOH.g-1 respectively. Analysing the fuel characteristics of COB, cetane number as 37.55, kinematic viscosity as 14.82 mm2.s-1 (at 40°C), oxidative stability as 5.87 h (at 110°C), yield point as -20°C, acid number as 0.148 mg KOH.g-1, lubrication as 186 μm, density as 927.7 kg.m-3 (at 15°C), water content as 640 mg.kg-1, phosphorous value as 0 mg.kg-1 and sulphur value as 0.2 mg.kg-1.
In their studies analysing the physico-chemical characteristics of biodiesels produced from jatropha and CO, Okullo et al., (2012) indicated COB’s kinematic viscosity as 17.10 mm2.s-1 (at 40°C), flash point as 178°C, acid value as 2.11 mg KOH.g-1, cloud point as -13°C, density as 910 kg.m-3 (at 15°C) and calorific value as 29.60 MJ.kg-1. In their studies optimizing biodiesel production from CO, Dias et al. (2013) reached maximum productivity at 73.62% for 6:1 methanol to oil molar ratio, 1% of KOH, 65°C and 8 h reaction conditions. Following the optimization studies, they found the acid value was between 0.92-1.87 mg KOH.g-1, kinematic viscosity value was between 18.3-60.9 mm2.s-1 (at 40°C), flash point was between 165-186.5°C and copper strip corrosion value was class 1.
In this present work, biodiesel from CO was produced via the two-step transesterification method. Methanol was used as an alcohol and NaOH was used as a catalyst. Different kind of blends, such as B2, B5, B10, B20, B30, B40, B50, B60, B75 and pure COB, were prepared. Then, fuel properties of COB and its blends with diesel fuel were determined. Additionally, the density values of COB and its blends were measured at the temperature range from 0 to 93°C in the steps of 5°C and the kinematic viscosity values of COB and its blends were measured at the temperature range from 30 to 100°C in the steps of 5°C. This study is aimed to identify the effect of temperature and biodiesel concentration on the density and kinematic viscosity of biodiesel blends as well as to develop a correlation for biodiesel concentration, temperature, density and kinematic viscosity.
2. MATERIALS AND METHODS
Biodiesel Production
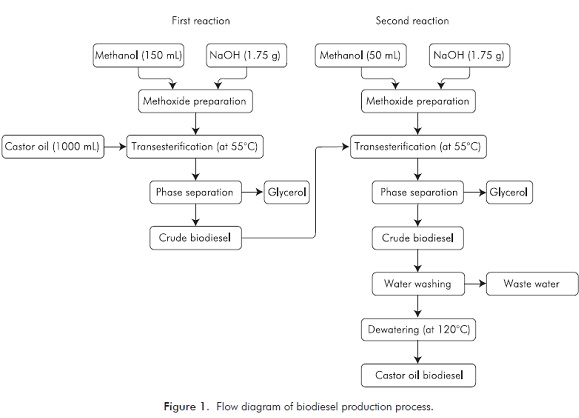
In this study, CO was purchased from a local oil plant. Ultraforce Euro Diesel was supplied from a petrol station for experiments. The experiment was performed in a laboratory scale apparatus. Biodiesel was produced from this vegetable oil by means of the two-step transesterification process. In this reaction, methanol and NaOH were used as an alcohol and a catalyst. The chemicals (methanol and NaOH), which were used during the experiments, were taken from Merck. The details of the transesterification process used in this experiment are shown in Figure 1.
For the first reaction, methanol (75% of oil) and NaOH (50% of oil), which is 150 mL of methanol and 1.75 g NaOH, were resolved in a magnetic mixer, obtaining methoxide. This mixture of methoxide was added to the 1000 mL CO mixed at 55°C, which is the best reaction temperature (the best esters yield). For the mixing, the circulation rate was set to 1000 min-1 and the mixture was mixed for 90 min. Later on, the mixer and heater were switched off. After waiting for 2 h for glycerol to subside, glycerol was removed (Figure 2). Later on, we proceeded to the second stage.
For the second reaction, methanol (25% of oil) and NaOH (50% of oil), which is 50 mL of methanol and 1.75 g NaOH, were resolved in a magnetic mixer, obtaining methoxide. The crude biodiesel, whose first reaction was attained, was heated up to 55ºC again by starting mixing and was submitted to reaction for 60 min. Then, the mixer and the heater were turned off. The resulting mixture was left to rest and glycerol was removed. The temperature of the crude biodiesel was increased up to 75ºC and methanol was removed. After waiting for 15 hours for glycerol to subside, glycerol was taken. Meanwhile, the pH value of the biodiesel was measured and repeatedly washed with distilled water until neutralization. It was submitted to a wash-off using the douching method (Figure 3).
The aim of the wash-off is to remove alcohol which does not get involved in reaction, remaining fatty acids, Na+, K+ ions, catalyst substance and glycerol which could have remained during separation. While washing, the temperature of biodiesel and distilled water was 55ºC and a total of 200 mL of distilled water was used in the process. After the washing process, it was rested for 12 h for water to subside. The subsided water was taken using a separating funnel. The washed biodiesel was taken to a magnetic mixer with heater again and was heated up to 120ºC which is where water goes beyond biodiesel. For biodiesel, drying was made at 120ºC for 2 h. Thus, biodiesel was produced from CO.
Preparing the Fuel Blends
Biodiesel can be used on its own, or by mixing it with diesel at any rate. Much of the world uses a system known as the “B” factor to state the amount of biodiesel in any fuel mix (Aksoy, Baydir & Bayrakçeken, 2010a; Aksoy, Baydir & Bayrakçeken, 2010b). For example, fuel containing 20% biodiesel is labeled B20. Pure biodiesel is referred to as B100. However, two special blends are important. These are B2 and B20 fuels. B2 is preferred for improving lubrication, while B20 mixtures are preferred for better lubrication as well as decreased exhaust emissions. On the other hand, B100 provides both benefits, and can replace diesel, as long as it can be found cheap and at sufficient quantities (Sekmen, 2007; Eryilmaz, 2009). Blends of 20% biodiesel with 80% diesel can generally be used in diesel engines without modification. Also pure biodiesel (B100) can be used in diesel engines but may require certain modifications to avoid maintenance and performance problems. The common international standards for biodiesel are EN 14214 and ASTM D 6751 (Demirbas & Demirbas, 2010).
When blending diesel and COB, first 98, 95, 90, 80, 70, 60, 50, 40 and 25% diesel was put in a glass beaker, then 2, 5, 10, 20, 30, 40, 50, 60 and 75% biodiesel were added, respectively. The blend was tested to become homogenous first with laboratory type IKA brand Yellow line OST basic model mixer at 1500 min-1, then with Yellow line brand DI 18 basic model homogenizer at 24000 min-1, for 7.5 min each, for a total of 15 min. Following this, B2, B5, B10, B20, B30, B40, B50 and B75 mixtures were acquired.
Measurement Procedure of Fuel Properties
Important physical and chemical properties such as density, kinematic viscosity, flash point, water content, calorific value, pH and copper strip corrosion of diesel, CO, COB and COB-diesel blends were determined and presented in this study. Table 2 shows the apparatus used in this study to measure and to perform this analysis.
Fatty acid composition of CO and COB were analyzed by gas chromatography-mass spectrometry (GC-MS). GC-MS analysis was performed on an Agilent 6890N Network GC system combined with Agilent 5975C VL MSD Network Mass Selective Detector. The GC-MS conditions were; column, DB Waxetr, is 60.0 m x 0.25 mm x 0.25 µm; oven temperature programed as the column held initially at 60°C for 1 min after injection, then increased to 185°C with 1°C.min-1 heating ramp for 10 min and increased to 200°C with 5°C.min-1 heating ramp without hold; injector temperature is 250°C; carrier gas is He; inlet pressure is 25.36 psi; linear gas velocity is 7 cm.sec-1; initial flow is 0.3 mL.min-1; split ratio is 30.0:1 and injected volume is 1.0 µL.
3. RESULTS AND DISCUSSIONS
The fuel properties of diesel, CO, COB and its blends with diesel fuel are given in Table 3. When the fuel properties of alternative fuel -biodiesel of CO- obtained as a result of transesterification were analyzed according to EN 14214 standard, and B2, B5, B10, B20, B30, B40, B50, B60, B75 fuel blends were analyzed according to EN 590, density, kinematic viscosity, calorific value, flash point, water content, copper strip corrosion analyses revealed values within limits up to B20 fuel blends. A cetane number is a characteristic of fuel that shows the ability of self-ignition in the cylinder of a diesel engine. The cetane number depends on fuel composition and influences the start of a diesel, the beginning of the combustion process, equal operation of a diesel, and the emission of exhaust gases. Cetane number (ignitability in diesel engines) of COB (80.2) is higher than standard diesel fuel (54) within in the standard values (Ozcanli et al., 2012). As well as COB-diesel fuel blends such as B2, B5, B10 and B20 can be used in diesel engine without any modification.
The composition and physico-chemical properties of oils have been found to vary depending on the plant location and the agricultural practices applied on the raw materials (Okullo et al., 2012). The composition of the raw material and biodiesel can be inferred by its fatty acid composition, analyzed by gas chromatography, as presented in Table 4. The obtained results match the composition expected according to the literature.
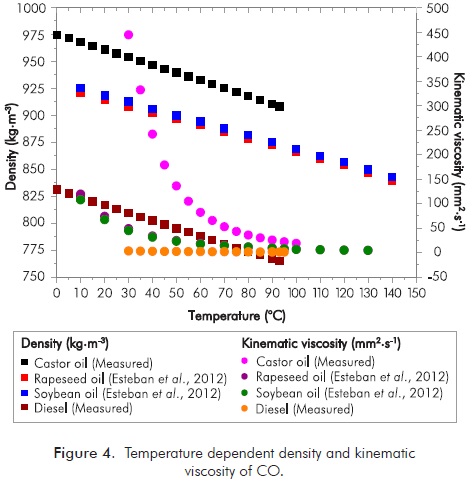
Temperature dependent density and kinematic viscosity of CO, rapeseed oil, soybean oil (Esteban et al., 2012) and diesel were shown in Figure 4. When compared, the density and kinematic viscosity of CO were higher than for the other vegetable oils. Densities of fatty acid methyl ester (FAME) with similar number of carbon atoms increase with an increased number of double bonds (for instance, ρC18:3 >ρC18:2 >ρC18:1 >ρC18:0) (Ramírez-Verduzco, 2013). Knothe (2005) showed that the kinematic viscosity of biodiesel is influenced by structural arrangement of organic compounds present in the raw material. As can be seen in Table 4, CO and COB have approximately 90% ricinoleic acid, so the oil and biodiesel show more viscosity as compared to other oils and biodiesels.
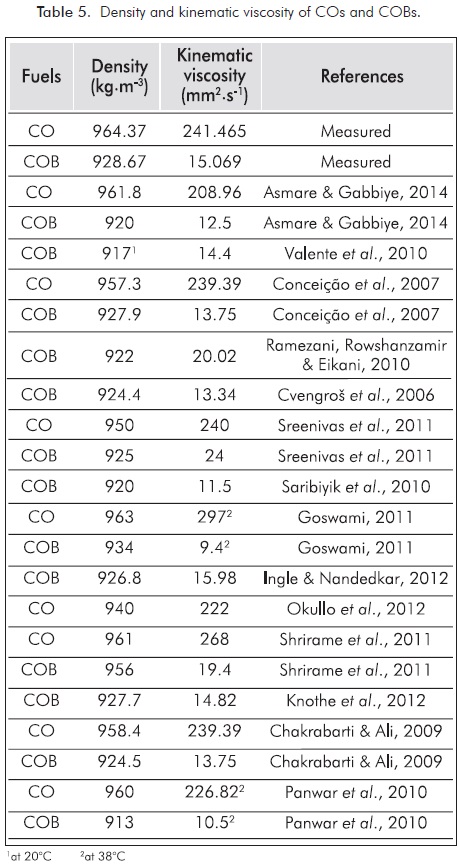
With two step transesterification method, CO was transformed to biodiesel. Therefore, the density and kinematic viscosity of CO was decreased from 964.37 kg.m-3 to 928.67 kg.m-3 at 15°C and from 241.465 mm2.s-1 to 15.069 mm2.s-1 at 40°C, respectively. Prior literature reported the density and the kinematic viscosity of CO and COB as shown in Table 5. Empirical and literature results indicate that, COB does not meet the density and kinematic viscosity standards. Seed and oil productivity, oil acid composition and oil-fuel relations are characteristics affected by type and ambient conditions (Eryilmaz et al., 2014a). With transesterification method, the density and kinematic viscosity values decreased about 4.35% and 94.02% (Asmare & Gabbiye, 2014), 3.07% and 94.26% (Conceição et al., 2007), 2.63% and 90.00% (Sreenivas, Mamilla & Sekhar, 2011), 3.01% and 96.65% (Goswami, 2011), 0.52% and 92.76% (Shrirame, Panwar & Bamniya, 2011), 3.54% and 94.26% (Chakrabarti & Ali, 2009), 4.90% and 95.37% (Panwar et al., 2010), respectively. We found out these ratios to be about 3.70% and 93.76%, respectively. The density and kinematic viscosity of COB exceeds the maximum specifications in standards, so that would only be possible to blend these esters with diesel fuel.
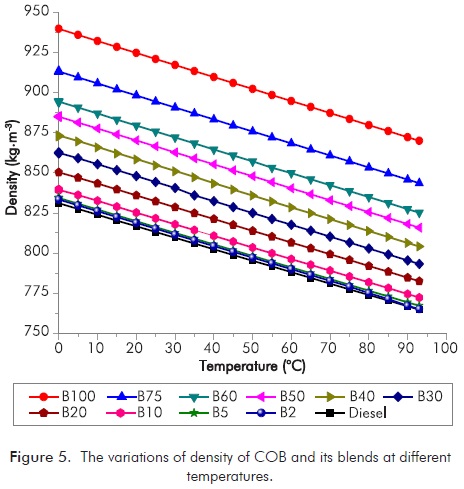
The density is specified in EN 14214 with a range of 860-900 kg.m-3 at 15°C and in EN 590 with a range of 820-845 kg.m-3 at 15°C. Figure 5 shows the density variations of diesel, COB, B2, B5, B10, B20, B30, B40, B50, B60, and B75 at a temperature range of 0 to 93°C. The densities of samples vary in the range of 822.43-902.11 kg.m-3 and higher than those of diesel fuel. As the density of COB is approximately 1.13 times higher than density of diesel fuel at 15°C, for the densities of B2, B5, B10, B20, B30, B40, B50, B60, and B75 are approximately 1.00, 1.00, 1.01, 1.02, 1.04, 1.05, 1.07, 1.08 and 1.10 times higher than density of diesel fuel in the same conditions, respectively. The maximum density values of each sample were measured at 0°C. In all cases, the density of biodiesels and its blends decreases as temperature increases; and also density decreases because of the increase in the amount of diesel in the blends. The blends with COB concentration up to 20% (for EN 590) could be acceptable.
Prediction of density and kinematic viscosity of biodiesel and the mixture of diesel fuel, which reduces time and workload at the same time, will allow preparation of the correct blending ratio for the diesel engines. A possible method for predicting the density of the biodiesel blends should be given by Ramírez-Verduzco et al., (2011), Rodenbush, Hsieh and Viswanath (1999), Esteban et al. (2012) at fixed concentration.
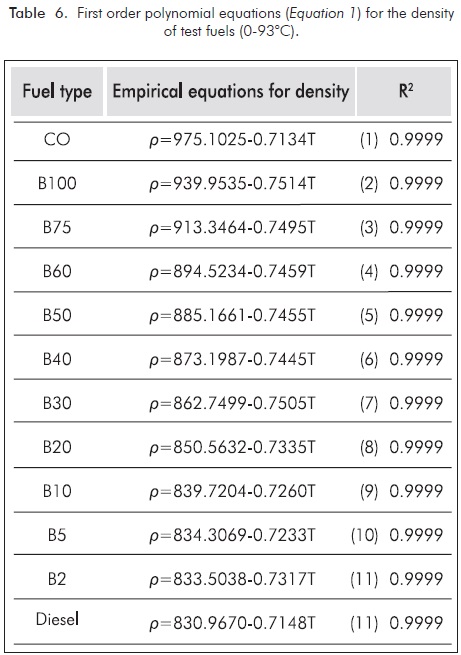
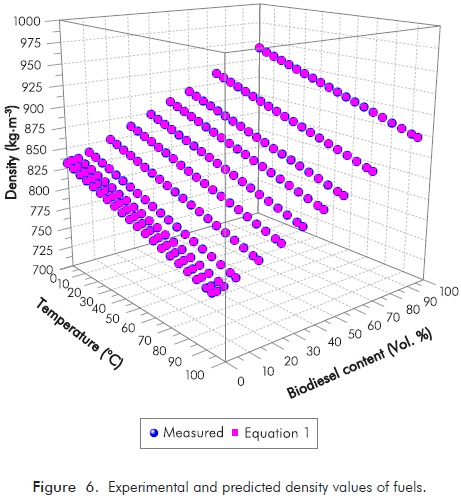
Where T is the temperature in °C, A and B are the adjustable parameters. Empirical equations for density prediction (Equation 1) of diesel, biodiesel and its blends related to blending ratio at different temperatures are given in Table 6. In Equation 1, the coefficient of determination (R2) is 0.9999 which indicates a very close match as compared to the measurements. Figure 6 shows those experimental and predicted density values of fuels. Error and percent relative error for density of biodiesel blends with these equations were shown in Figure 7.
For the remaining properties Kay’s mixing rule is used:
Where φB is the property of the blend and φi is the respective property of the ith component. Using volume fraction instead of molar fraction, Equation 2 for a binary mixture takes the form of an arithmetic volume average:
Where ρ is the density of the biodiesel blend, ρ1 and ρ2 are the density of the pure biodiesel and diesel in kg.m-3, respectively. V1 and V2 are the volume percentage of the pure biodiesel and diesel, respectively (Benjumea, Agudelo & Agudelo, 2008; Al-Hamamre & Al-Salaymeh, 2014; Eryilmaz, 2012; Eryilmaz et al., 2014b). Error and percent relative error of the fuel blends calculated from Kay’s mixing rule is given in Figure 8. It is interesting to note that the lower blend ratio fuel exhibited greater average error of the experimental values. Therefore, with the increase in temperature, linearly decreasing characteristics of density became more dramatic for lower biodiesel blends and thus the model predictions has an slightly higher average error.
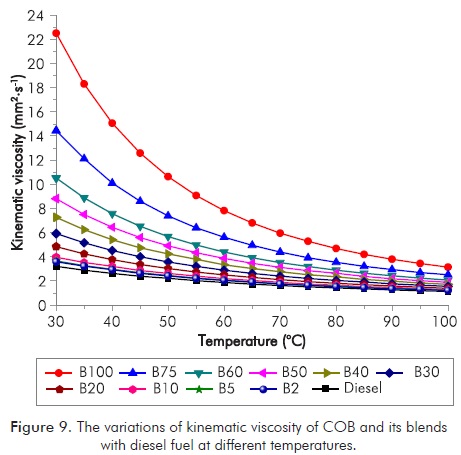
Figure 9 shows the kinematic viscosity variations of diesel, COB, B2, B5, B10, B20, B30, B40, B50, B60, and B75 at a temperature range of 30 to 100°C. The kinematic viscosities (at 40°C) of COB are 5.74 times that of diesel fuel, whereas the respective kinematic viscosity of COB was obtained to be 2.75 times that of diesel fuel by increasing temperature to 100°C.
To estimate the viscosity values of the mixtures, the equation suggested by Arrhenius and determined by Grunberg and Nissan has been used (Eryilmaz, 2012).
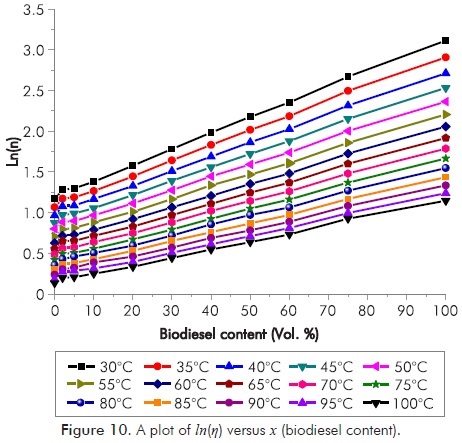
Where ηb represents the kinematic viscosity of blends at certain temperatures, η1 and V1 represents the kinematic viscosity of the first fuel used in the blend and the volumetric mixture rate, η2 and V2 the kinematic viscosity of the second fuel used in the blend and the volumetric mixture rate. A plot of ln(ηb) versus x (blend ratio) (Figure 10) should give a straight line that can be analysed using linear regression to confirm the correlation from calculation of the slope is equal to ln(ηbiodiesel/ηdiesel) and intercept is equal to ln(ηdiesel).
Table 7 shows the linear fitting of biodiesel kinematic viscosity values versus blend ratio. R2 values ranges from 0.9957 to 0.9989 and these formulas can be used to predict kinematic viscosities of COB and its blends with diesel fuel. Besides, for confirmation of this correlation, we calculated ln(ηbiodiesel/ηdiesel) and ln(ηdiesel). After comparing the intercepts and slopes of those linear regression and ln(ηbiodiesel/ηdiesel) and ln(ηdiesel), both of them proved to match each other.
Error and percent relative error of the fuel blends calculated from Equation 4 is shown in Figure 11. The errors are ranged between -0.01-0.08 and the percent relative errors are ranged between 0-20.
Kinematic viscosity depending on the temperature change can be found using Andrade equation (Esteban et al., 2012; Ramírez-Verduzco et al., 2011; Rodenbush et al., 1999; Eryilmaz, 2012; Kimilu, Nyang’aya & Onyari, 2011; Tat & Van Gerpen, 1999; Yuan et al., 2005; Yuan, Hansen & Zhang, 2009; Kerschbaum & Rinke, 2004; Aksoy et al., 2014). 2 and 3 constant Andrade equations are;
where A, B and C are constant coefficients for the fluid, η is kinematic viscosity and T refers to the temperature.
Aksoy, Yabanova & Bayrakçeken (2011) and Aksoy et al., (2014) suggested equations for estimating the viscosity for biodiesels. These equations are:
In these equations T is temperature and A, B and C are constants.
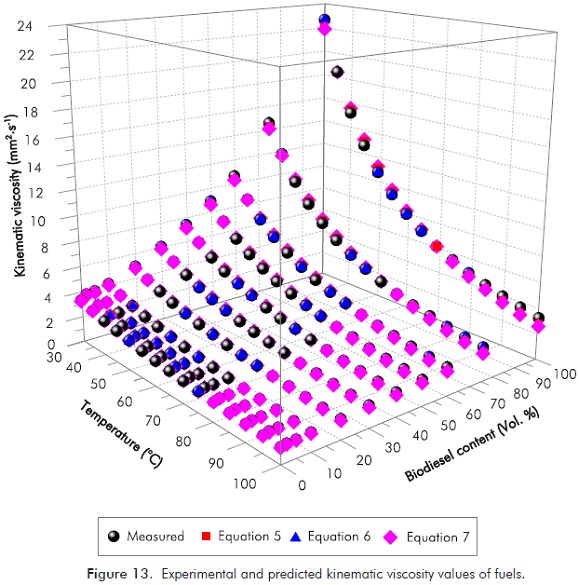
The correlation coefficients of the Equations 5, 6 and 7 for each fuel sample were given in Table 8, and error and percent relative error were shown in Figure 12. As can be seen in Figure 12, Equation 6 yields excellent results and minimum errors; however Equation 7 shows the lowest agreement with experimental values. Figure 13 shows the experimental and predicted kinematic viscosity values of fuel. The whole set of experimental results, including those for density and kinematic viscosity are shown in Annex A and Annex B.
4. CONCLUSION
CO can be used as a biodiesel feedstock with its high oil content (40-55%) and its non-edible characteristics. In the present work, methyl ester was derived from CO and density, kinematic viscosity, water content, flash point, pH and copper strip corrosion properties of CO, COB and its blends with diesel fuel have been outlined. Fuel blends with up to 20% COB concentration in diesel fuel will meet present specifications for biodiesel density, kinematic viscosity, water content, flash point and copper strip corrosion. It was not possible to obtain a product conforming EN 14214 and EN 590, as expected, mainly due to the high density and kinematic viscosity. The density and kinematic viscosity of COB and its blends with diesel fuel decrease as temperature increases; and also density and kinematic viscosity decrease because of the increase in the amount of diesel in the blends. The results obtained are valuable to continue studies regarding both the use CO in Turkey and the application of CO for biodiesel production.
ACKNOWLEDGMENTS
The authors would like to thank the Bozok University (Yozgat, Turkey) for analyzing the fuels.
REFERENCES
Aksoy, F., Baydir, S. A. & Bayrakçeken, H. (2010a). An investigation on the effect in the viscosity of canola and corn oil biodiesels at a temperature range of 0 to 100°C. Energ. Source. Part A, 32(2), 157-164. [ Links ]
Aksoy, F., Baydir, S. A. & Bayrakçeken, H. (2010b). The viscosity at different temperatures of soybean and sunflower biodiesels and diesel fuel blends. Energ. Source. Part A, 32(2), 148-156. [ Links ]
Aksoy, F., Yabanova, I. & Bayrakçeken, H. (2011). Estimation of dynamic viscosities of vegetable oils using artificial neural networks. IJCT, 18(3), 227-233. [ Links ]
Aksoy, F., Yabanova, I., Bayrakçeken, H. & Aksoy, L. (2014). Estimating the dynamic viscosity of vegetable oils using artificial neural networks. Energ. Source. Part A, 36(8), 858-865. [ Links ]
Albuquerque, M. C. G., Machado, Y. L., Torres, A. E. B., Azevedo, D. C. S., Cavalcante, Jr., C. L., Firmiano, L. R. & Parente, Jr., E. J. S. (2009). Properties of biodiesel oils formulated using different biomass sources and their blends. Renew. Energ., 34(3), 857-859. [ Links ]
Al-Hamamre, Z. & Al-Salaymeh, A. (2014). Physical properties of (jojoba oil + biodiesel), (jojoba oil + diesel) and (biodiesel + diesel) blends. Fuel, 123: 175-188. [ Links ]
Asmare, M. & Gabbiye, N. (2014). Synthesis and characterization of biodiesel from castor bean as alternative fuel for diesel engine. Amer. J. Energ. Eng., 2(1), 1-15. [ Links ]
ASTM D6751-15a. Standard specification for biodiesel fuel blend stock (B100) for middle distillate fuels. ASTM International, West Conshohocken, PA, 2015. [ Links ]
Atabani, A. E., Silitonga, A. S., Badruddin, I. A., Masjuki, H. H. & Mekhilef, S. (2012). A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sust. Energ. Rev., 16(4), 2070-2093. [ Links ]
Balaji, G. & Cheralathan, M. (2013). Potential of various sources for biodiesel production. Energ. Source. Part A, 35(9), 831-839. [ Links ]
Benjumea, P., Agudelo, J. & Agudelo, A. (2008). Basic properties of palm oil biodiesel-diesel blends. Fuel, 87(10-11), 2069-2075. [ Links ]
Berman, P., Nizri, S. & Wiesman, Z. (2011). Castor oil biodiesel and its blends as alternative fuel. Biomass Bioenergy, 35(7), 2861-2866. [ Links ]
Borugadda, V. B. & Goud, V. V. (2012). Biodiesel production from renewable feedstocks: Status and opportunities. Renew. Sust. Energ. Rev., 16(7), 4763-4784. [ Links ]
Chakrabarti, M. H. & Ali, M. (2009). Performance of compression ignition engine with indigenous castor oil biodiesel in Pakistan. Ned. Univ. J. Res., 6(1), 10-19. [ Links ]
Conceição, M. M., Candeia, R. A., Silva, F. C., Bezerra, A. F., Fernandes, Jr, V. J. & Souza, A. G. (2007). Thermoanalytical characterization of castor oil biodiesel. Renew. Sust. Energ. Rev., 11(5), 964-975. [ Links ]
Cvengroš, J., Paligová, J. & Cvengrošová, Z. (2006). Properties of alkyl esters base on castor oil. Eur. J. Lipid Sci. Technol., 108(8), 629-635. [ Links ]
Demirbas, A. (2007). Importance of biodiesel as transportation fuel. Energ. Policy, 35(9), 4661-4670. [ Links ]
Demirbas, A. (2008a). Biodiesel: A realistic fuel alternative for diesel engines. London: Springer-Verlag London. [ Links ]
Demirbas, A. (2008b). Comparison of transesterification methods for production of biodiesel from vegetable oils and fats. Energ. Convers. Manage., 49(1), 125-130. [ Links ]
Demirbas, T. & Demirbas, A. H. (2010). Bioenergy, green energy. Biomass and biofuels. Energ. Source. Part A, 32(12), 1067-1075. [ Links ]
Dias, J. M., Araújo, J. M., Costa, J. F., Alvim-Ferraz, M. C. M. & Almeida, M. F. (2013). Biodiesel production from raw castor oil. Energy, 53: 58-66. [ Links ]
DIN 51900-1. Testing of solid and liquid fuels - Determination of gross calorific value by the bomb calorimeter and calculation of net calorific value - Part 1: Principles, apparatus, methods. 2000-04. [ Links ]
DIN 51900-2. Testing of solid and liquid fuels - Determination of the gross calorific value by the bomb calorimeter and calculation of the net calorific value - Part 2: Method using isoperibol ot static, jacket calorimeter. 2003-05. [ Links ]
DIN 51900-3. Testing of solid and liquid fuels - Determination of gross calorific value by the bomb calorimeter and calculation of net calorific value - Part 3: Method using adiabatic jacket. 2005-01. [ Links ]
EN 590. Automotive fuels-Diesel-Requirements and test methods. 2004. [ Links ]
EN 14214. Automotive fuels. Fatty Acid Methyl Esters (FAME) for diesel engines. Requirements and test methods. 2008. [ Links ]
EN ISO 2160. Petroleum products-Corrosiveness to copper-Copper strip test. 1998. [ Links ]
EN ISO 2719. Determination of flash point-Pensky-Martens closed cup method. 2002 [ Links ]
EN ISO 3104. Petroleum products-Transparent and opaque liquids-Determination of kinematic viscosity and calculation of dynamic viscosity. 1994 [ Links ]
EN ISO 3675. Crude petroleum and liquid petroleum products-Laboratory determination of density-Hydrometer method. 1998. [ Links ]
EN ISO 3679. Determination of flash point-Rapid equilibrium closed cup method. 2004. [ Links ]
EN ISO 12185. Crude petroleum and petroleum products-Determination of Density- Oscillating U-tube method, 1996/Cor.1:2001. [ Links ]
EN ISO 12937. Petroleum products-Determination of water-Coulometric Karl Fischer titration method. 2000. [ Links ]
Eryilmaz, T. (2009). The effect of the different mustard oil biodiesel blending ratios on diesel engines performance. PhD Thesis, Natural and Applied Science, Selcuk University, Konya, Turkey, 130pp. [ Links ]
Eryilmaz, T. (2012). Investigation of temperature dependent kinematic viscosity variations of animal fat methyl ester and its blends. EEST Part A, 28(2), 1191-1198. [ Links ]
Eryilmaz, T., Yeşilyurt, M. K., Cesur, C., Yumak, H., Aydin, E., Çelik, S. A. & Yildiz, A. K. (2014a). Determination of fuel properties of biodiesel produced from safflower (Carthamus tinctorius L.) Dincer species grown in Yozgat province conditions. JAFAG, 31(1), 63-72. [ Links ]
Eryilmaz, T., Yeşilyurt, M. K., Yumak, H., Arslan, M. & Şahin, S. (2014b). Determination of the fuel properties of cottonseed oil methyl ester and its blends with diesel fuel. IJAET, 3(2), 79-90. [ Links ]
Esteban, B., Riba, J. R., Baquero, G., Rius, A. & Puig, R. (2012). Temperature dependence of density and viscosity of vegetable oils. Biomass Bioenergy, 42: 164-171. [ Links ]
Goswami, A. (2011). An alternative eco-friendly avenue for castor oil biodiesel: Use of solid supported acidic salt catalyst. In: Stoytcheva, M. (Ed.). Biodiesel - Feedstocks and processing technologies. Croatia: InTech, (18), 379-396 [ Links ]
Hincapié, G., Mondragón, F. & López, D. (2011). Conventional and in situ transesterification of castor seed oil for biodiesel production. Fuel, 90(4), 1618-1623. [ Links ]
Ingle, S.S. & Nandedkar, V. M. (2012). Indigenous castor oil biodiesel an alternative fuel for diesel engine. IJMIE, 2(2), 62-64. [ Links ]
Issariyakul, T. & Dalai, A. K. (2014). Biodiesel from vegetable oils. Renew. Sust. Energ. Rev., 31: 446-471. [ Links ]
Jahirul, M. I., Brown, R. J., Senadeera, W., O'Hara, I. M. & Ristovski, Z. D. (2013). The use of artificial neural networks for identifying sustainable biodiesel feedstocks. Energies, 6(8), 3764-3806. [ Links ]
Kafuku, G. & Mbarawa, M. (2010). Biodiesel production from Croton megalocarpus oil and its process optimization. Fuel, 89(9), 2556-2560. [ Links ]
Karmakar, A., Karmakar, S. & Mukherjee, S. (2010). Properties of various plants and animals feedstocks for biodiesel production. Bioresour. Technol., 101(19), 7201-7210. [ Links ]
Kerschbaum, S. & Rinke, G. (2004). Measurement of the temperature dependent viscosity of biodiesel fuels. Fuel, 83(3), 287-291. [ Links ]
Kibazohi, O. & Sangwan, R. S. (2011). Vegetable oil production potential from Jatropha curcas, Croton megalocarpus, Aleurites moluccana, Moringa oleifera and Pachira glabra: Assessment of renewable energy resources for bio-energy production in Africa. Biomass Bioenergy, 35(3), 1352-1356. [ Links ]
Kilic, M., Uzun, B. B., Putun, E. & Putun, A. E. (2013). Optimization of biodiesel production from castor oil using factorial design. Fuel Process. Technol., 111, 105-110. [ Links ]
Kimilu, R. K., Nyang'aya, J. A. & Onyari, J. M. (2011). The effects of temperature and blending on the specific gravity and viscosity of jatropha methyl ester. ARPN J. Eng. Appl. Sci., 6(12), 97-105. [ Links ]
Knothe, G. (2005). Dependence of biodiesel fuel properties on the structure of fatty acid alkyl ester. Fuel Process. Technol., 86(10), 1059-1070. [ Links ]
Knothe, G., Cermak, S. C. & Evangelista, R. L. (2012). Methyl esters from vegetable oils with hydroxyl fatty acids: Comparison of lesquerella and castor methyl esters. Fuel, 96: 535-540. [ Links ]
Lin, L., Cunshan, Z., Vittayapadung, S., Xiangqian, S. & Mingdong, D. (2011). Opportunities and challenges for biodiesel fuel. Appl. Energy, 88(4), 1020-1031. [ Links ]
Mumtaz, M. W., Adnan, A., Anwar, F., Mukhtar, H., Raza, M. A., Ahmad, F. & Rashid, U. (2012). Response surface methodology: An emphatic tool for optimized biodiesel production using rice bran and sunflower oils. Energies, 5: 3307-3328. [ Links ]
Okullo, A., Temu, A. K., Ogwok, P. & Ntalikwa, J. W. (2012). Physico-chemical properties of biodiesel from jatropha and castor oils. IJRER, 2(1), 47-52. [ Links ]
Ozcanli, M., Serin, H., Saribiyik, O.Y., Aydin, K. & Serin, S. (2012). Performance and emission studies of castor bean (Ricinus Communis) oil biodiesel and its blends with diesel fuel. Energ. Source. Part A, 34(19), 1808-1814. [ Links ]
Panwar, N. L., Shrirame, H. Y., Rathore, N. S., Jindal, S. & Kurchania, A. K. (2010). Performance evaluation of a diesel engine fueled with methyl ester of castor seed oil. Appl. Therm. Eng., 30(2-3), 245-249. [ Links ]
Ramezani, K., Rowshanzamir, S. & Eikani, M. H. (2010). Castor oil transesterification reaction: A kinetic study and optimization of parameters. Energy, 35(10), 4142-4148. [ Links ]
Ramírez-Verduzco, L. F. (2013). Density and viscosity of biodiesel as a function of temperature: Empirical models. Renew. Sust. Energ. Rev., 19: 652-665. [ Links ]
Ramírez-Verduzco, L. F., García-Flores, B. E., Rodríguez-Rodríguez, J. E. & Jaramillo-Jacob, A. (2011). Prediction of the density and viscosity in biodiesel blends at various temperatures. Fuel, 90(5), 1751-1761. [ Links ]
Rodenbush, C. M., Hsieh, F. H. & Viswanath, D. S. (1999). Density and viscosity of vegetable oils. JAOCS, 76(12), 1415-1419. [ Links ]
Salvi, B. L. & Panwar, N. L. (2012). Biodiesel resources and production technologies - A review. Renew. Sust. Energ. Rev., 16(6), 3680-3689. [ Links ]
Saribiyik, O. Y., Özcanli, M., Serin, H., Serin, S. & Aydin, K. (2010). Biodiesel production from Ricinus Communis oil and its blends with soybean biodiesel. Stroj. Vestn. J. Mech. Eng., 56(12), 811-816. [ Links ]
Saxena, P., Jawale, S. & Joshipura, M. H. (2013). A review on prediction of properties of biodiesel and blends of biodiesel. Procedia Engineering, 51, 395-402. [ Links ]
Scholz, V. & Nogueira da Silva, J. (2008). Prospects and risks of the use of castor oil as a fuel. Biomass Bioenergy, 32(2), 95-100. [ Links ]
Sekmen, Y. (2007). Use of watermelon and flax seed oil methyl esters as a fuel in a diesel engine. Technology, 10(4), 295-302. [ Links ]
Shahid, E. M. & Jamal, Y. (2011). Production of biodiesel: A technical review. Renew. Sust. Energ. Rev., 15(9), 4732-4745. [ Links ]
Shrirame, H. Y., Panwar, N. L. & Bamniya, B. R. (2011). Biodiesel from castor oil - a green energy option. Low Carbon Economy, 2(1), 1-6. [ Links ]
Singh, S. P. & Singh, D. (2010). Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sust. Energ. Rev., 14(1), 200-216. [ Links ]
Sreenivas, P., Mamilla, V.R. & Sekhar, K.C. (2011). Development of biodiesel from castor oil. Int. J. Energ. Sci., 1(3), 192-197. [ Links ]
Tat, M. E. & Van Gerpen, J. (1999). The kinematic viscosity of biodiesel and its blends with diesel fuel. JAOCS, 76(12), 1511-1513. [ Links ]
Valente, O. S., José da Silva, M., Pasa, V. M. D., Belchior, C. R. P. & Sodré, J. R. (2010). Fuel consumption and emissions from a diesel power generator fuelled with castor oil and soybean biodiesel. Fuel, 89(12), 3637-3642. [ Links ]
Valente, O. S., Pasa, V. M. D., Belchior, C.R.P. & Sodré, J. R. (2011). Physical-chemical properties of waste cooking oil biodiesel and castor oil biodiesel blends. Fuel, 90(4), 1700-1702. [ Links ]
Yuan, W., Hansen, A. C. & Zhang, Q. (2009). Predicting the temperature dependent viscosity of biodiesel fuels. Fuel, 88(6), 1120-1126. [ Links ]
Yuan, W., Hansen, A. C., Zhang, Q. & Tan, Z. (2005). Temperature-dependent kinematic viscosity of selected biodiesel fuels and blends with diesel fuel. JAOCS, 82(3), 195-199. [ Links ]
Zuleta, E. C., Rios, L. A. & Benjumea, P. N. (2012). Oxidative stability and cold flow behavior of palm, sacha-inchi, jatropha and castor oil biodiesel blends. Fuel Process. Technol., 102: 96-101. [ Links ]
AUTHORS
Osman Gokdogan
Affiliation: Nevsehir Haci Bektas University
Biosystems Engineering, Nevsehir Haci Bektas University
M. Sc. in Agricultural Machinery, Suleyman Demirel University
Ph. D. in Agricultural Machinery, Selcuk University
e-mail: osmangokdogan@gmail.com
Tanzer Eryilmaz
Affiliation: Bozok University
Biosystems Engineering, Bozok University
M. Sc. in Agricultural Machinery, Selcuk University
Ph. D. in Agricultural Machinery, Selcuk University
e-mail: tanzer.eryilmaz@bozok.edu.tr
Murat Kadir Yesilyurt
Affiliation: Bozok University
Biosystems Engineering, Bozok University
M. Sc. in Mechanical Engineering, Kirikkale University
Ph. D. Student in Mechanical Engineering, Bozok University
e-mail: kadir.yesilyurt@bozok.edu.tr