Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
CT&F - Ciencia, Tecnología y Futuro
versión impresa ISSN 0122-5383
C.T.F Cienc. Tecnol. Futuro vol.6 no.2 Bucaramanga jul./dic. 2015
SUPERCRITICAL TRANSESTERIFICATION OF BEEF TALLOW FOR BIODIESEL PRODUCTION IN A BATCH REACTOR
PRODUCCIÓN DE BIODIESEL A PARTIR DE SEBO VACUNO POR MEDIO DE TRANSESTERIFICACIÓN SUPERCRÍTICA EN UN REACTOR BATCH
PRODUÇÃO DE BIODIESEL A PARTIR DE SEBO DE BOVINO ATRAVÉS DA TRANSESTERIFICAÇÃO SUPERCRÍTICA EM UM REATOR BATCH
Paola-Andrea Marulanda-Buitrago1 and Víctor-Fernando Marulanda-Cardona1*
1Environmental and Sanitary Engineering Program, Universidad de La Salle, Bogotá, Cundinamarca, Colombia
e-mail: vfmarulanda@unisalle.edu.co
*To whom correspondence should be addressed
How to cite: Marulanda-Buitrago, P. A. & Marulanda-Cardona, V. F. (2015). Supercritical transesterification of beef tallow for biodiesel production in a batch reactor. CT&F - Ciencia, Tecnología y Futuro, 6(2), 57-68.
(Received: Jul. 21, 2015; Accepted: Oct. 28, 2015)
ABSTRACT
Supercritical transesterification experiments of beef tallow with ethanol were carried out in a batch reactor in order to assess the effect of temperature (350 - 400°C), reactant molar ratio (9:1 - 15:1) and reaction time (8 - 40 min). Composition of the produced biofuel was assessed in terms of the percentage of peak area of the long chain Fatty Acid Ethyl Esters (FAEEs) identified, including also the decomposition products generated as a result of thermal cracking or cis-trans isomerization reactions. Thermal decomposition of FAEEs produced from beef tallow included short chain ethyl esters (C8 - C13), ethyl pentadecanoate (C15:0), heptadecanoate (C17:0), not initially present or prevalent in triglycerides sources, as well as ethyl stearate (C18:0) and n-alkanes. Glycerol decomposition reactions were also verified by the presence of glycerol ethers and water content in the produced biodiesel. The highest conversion of triglycerides was verified at 400°C, 15:1 triglycerides to ethanol molar ratio and 40-minute reaction time. At these conditions, ethyl oleate (C18:1) decomposed completely to form cis-trans isomers as well as ethyl octadecanoate (C18:0), which was attributed to double bond hydrogenation reactions. Short chain ethyl esters and glycerol decomposition products have the potential to benefit certain properties of the fuel such as viscosity and cold flow.
Keywords: Biodiesel, Transesterification, Vegetable oils, Fatty acids, Hydrotreating, Glycerol.
RESUMEN
Los experimentos de transesterificación supercrítica de sebo vacuno con etanol se llevaron a cabo en un reactor batch con el fin de evaluar el efecto de la temperatura (350 - 400°C), relación molar de reactantes (9:1-15:1) y tiempo de reacción (8 - 40 min). La composición del biocombustible producido se evaluó en términos del porcentaje de área de picos de los esteres etílicos de ácidos grasos o FAEEs (Fatty Acid Ethyl Esters) así como los productos de descomposición generados de reacciones de cracking o isomerización. La descomposición térmica de los FAEEs producidos incluyó esteres etílicos de cadena corta (C8 - C13), etil pentadecanoato (C15:0), heptadecanoato (C17:0), los cuales no proceden de ácidos grasos presentes en el sebo, así como etil estearato (C18:0) y n-alcanos. De la misma forma, se verificó la ocurrencia de reacciones de descomposición de la glicerina por medio de la identificación de éteres y del contenido de agua en el biodiesel. La máxima conversión de triglicéridos se verificó a 400°C, con una relación molar de 15:1 y 40 minutos de tiempo de reacción. A estas condiciones, el etil oleato (C18:1) se descompuso completamente para formar isómeros cis-trans así como etil octadecanoato (C18:0). Los esteres etílicos de cadena corta y los productos de descomposición del glicerol tienen el potencial de beneficiar ciertas propiedades del biocombustible.
Palabras clave: Biodiesel, Transesterificación, Aceites vegetales, Ácidos grasos, Hidrotratamiento, Glicerina.
RESUMO
Os experimentos de transesterificação supercrítica de sebo de bovino com etanol foram realizados em um reator batch no intuito de avaliar o efeito da temperatura (350 – 400°C), relação molar de reagentes (9:1-15:1) e tempo de reação (8 - 40 min). A composição do biocombustível produzido foi avaliada em termos de porcentagem de área de picos dos ésteres etílicos de ácidos graxos ou FAEEs (Fatty Acid Ethyl Esters), bem como os produtos de decomposição produzidos pelas reações de cracking ou isomerização. A decomposição térmica dos FAEEs produzidos incluiu ésteres etílicos de caída curta (C8 – C13) etil pentadecanoato (C15:0), heptadecanoato (C17:0), não procedentes de ácidos graxos presentes no sebo, bem como etil estearato (C18:0) e n-alcanos. De igual modo, verificou-se a ocorrência de reações de decomposição da glicerina através da identificação de éteres e do conteúdo de água no biodiesel. A máxima conversão de triglicérides foi verificada a 400°C, com uma relação molar de 15:1 e 40 minutos de tempo de reação. Sob essas condições, o etil oleato (C18:1) decompôs-se completamente para formar isómeros cis-trans, bem como etil octadecanoato (C18:0). Os ésteres etílicos de cadeia curta e os produtos da decomposição do glicerol têm o potencial de beneficiar certas propriedades do biocombustível.
Palavras-chave: Biodiesel, Transesterificação, Aceites vegetales, Ácidos grasos, Hidrotratamiento, Glicerina.
1. INTRODUCTION
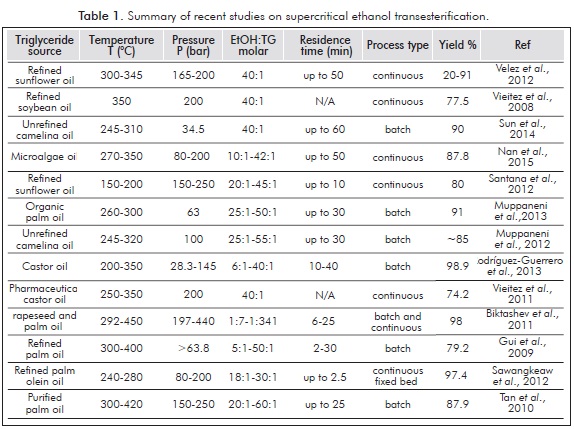
Supercritical transesterification, also known as supercritical alcoholysis, is a process usually carried out in the temperature range from 300 to 400°C, with pressures generally higher than 100 bars and high alcohol to oil molar ratios. The supercritical processing of fats and oils for biodiesel production takes advantage of the enhanced miscibility of alcohol and triglycerides at these conditions to conduct the transesterification reaction in a homogeneous phase without using a catalyst (Sawangkeaw, Bunyakiat & Ngamprasertsith, 2010). Therefore, and in contrast to the limitations evidenced by the conventional base catalyzed process, the supercritical transesterification process can make an efficient use of cheaper low quality triglyceride sources such as waste oils and animal fats (Pinnarat & Savage, 2008). In addition, downstream processing of biodiesel produced is simpler because there is no need to neutralize and remove the catalyst or other unwanted saponification products that can turn the separation of the byproduct glycerol into a very difficult task. In recent years, intense research efforts have been conducted towards establishing the optimal set of temperature and pressure conditions for the supercritical processing of different vegetable oils in batch and continuous reactors (Sawangkeaw et al., 2010), as well as the effects of the type of alcohol used (Sawangkeaw et al., 2011; Sun et al., 2014), the presence of water (Kusdiana & Saka, 2004; Vieitez et al., 2008) and free fatty acids (Tan, Lee & Mohamed, 2010). More recently, in-situ supercritical processing of algal biomass for biodiesel production has also been reported with very promising results (Nan et al., 2015).
It is well known that biodiesel production costs could be significantly reduced if waste oils and animal fats were used, since raw materials account for more than 70% of total production costs (Zhang et al., 2003). Yet, reports on the use of low quality feedstock are scarce in comparison to the reported research using refined vegetable oils. Besides the inherent implications of the required equipment for working at high temperature and pressure conditions, the supercritical transesterification process still struggles with the high alcohol to oil molar ratios employed, usually 42:1, which translates into high pumping, preheating, and recycling costs (Marulanda et al., 2010a). From a renewability standpoint, biodiesel currently produced by means of the conventional method cannot be considered 100% renewable or a second generation biofuel due to the use of methanol in the transesterification reaction.
Although ethanol, currently produced from fermentation of sugar biomass, could be used to replace methanol and make it completely renewable, its use has been somewhat hindered by the difficult processing that arises from the mutual solubility of glycerol and ethyl esters in the presence of ethanol, which complicates the spontaneous phase separation (Mendow et al., 2011). In addition, rectified or absolute ethanol, currently used as a gasoline additive up to 10 vol%, is also costlier than methanol (Vieitez et al., 2011). Yet, use of ethanol instead of methanol in the transesterification reaction offers several advantages such as a higher oxidative stability, better lubricity, lower cloud and pour points (Stamenković, Veličković & Veljković, 2011), as well as an increased yield due to the extra carbon atom (Mendow et al., 2011) and improved emission profile and engine performance (Yilmaz & Sanchez, 2012).
Table 1 summarizes some of the most recent studies on the supercritical transesterification process from different raw materials and ethanol. Only a few works have reported the use of triglyceride sources different from refined vegetable oils. Several authors have reported the decomposition of unsaturated esters at temperatures around 350°C (Bunyakiat et al., 2006; Saka & Kusdiana, 2001), and therefore, reported yields are lower than those obtained at lower temperatures (Gui, Lee & Bhatia, 2009; Vieitez et al., 2011). Other authors have reported the biodiesel production by supercritical transesterification at temperatures near 400°C and alcohol to oil molar ratios from near stoichiometric (6:1) to a moderate excess (9:1 - 20:1). These works reported the occurrence of decomposition reactions of the main C16-C18 esters to shorter chain esters and linear alkanes and alkenes (Marulanda et al., 2010a; Sawangkeaw et al., 2011), which does not necessarily mean a loss in productivity. In fact, an additional yield between 5% to 10% depending on the type of alcohol used was reported (Sawangkeaw et al., 2011), as well as the potential to produce biofuels with improved properties (Lin, Zhu & Tavlarides, 2014; Marulanda et al., 2010a). Consequently, this work reports on the batch supercritical transesterification of bovine fat, also known as beef tallow, at temperatures 350 - 400°C and molar ratios 6:1 - 15:1, in order to assess the effect of process variables such as temperature, reaction time and molar ratio of the reactants; highlight the characteristics of the produced biofuel in terms of long chain FAEEs and the products that arise from the esters decomposition, either shorter esters or hydrocarbons as well as the best set of experimental conditions to attain the highest amount of FAEEs and decomposition products in terms of sample percentage composition.
2. MATERIALS AND METHODS
Beef tallow for the study was acquired in a local slaughterhouse in the city of Bogotá, Colombia, without any treatment or purification and was placed in a cooler to avoid degradation. The grease, yellowish in color, is solid at room temperature due to the high content of Saturated Fatty Acids (SFA), 45.6%, in comparison to chicken fat, which is partially liquid at room temperature and has a SFA content of 32% and liquid soybean oil with a SFA content of 15% (Banković-Ilić et al., 2014). Tallow is mainly composed of myristic (3 - 6 wt%), palmitic (24 - 32 wt%), stearic (20 - 25 wt%) and oleic acids (37 - 43 wt%) and a ratio of saturated to unsaturated fatty acids between 1.2 to 1.4 (Karmakar, Karmakar & Mukherjee, 2010). The grease was melted at 65°C and filtrated in order to remove unwanted impurities such as meat and skin residues and the exact amount of grease needed to load in the reactor was weighted. Ethanol absolute (99.5% v/v, 0.5% water) for the transesterification reaction was purchased from Panreac. Alcohol and beef tallow humidity, as well as water produced in the reaction, were measured by a Mettler Toledo Karl Fisher titrant model K20.
Experimental Setup
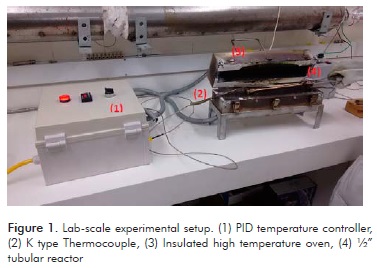
Experimental runs were carried out in a batch reactor made of 316SS Swagelok high pressure tubing and fittings, 1.27 cm OD, 0.1651 cm wall thickness and 15 cm long, for a volume of 10.4 cm3. In a typical run, 6.2 g of beef tallow were loaded in the reactor. The required amount of ethanol was determined on the basis of the loaded moles of beef tallow and the triglycerides to ethanol molar ratio for that run in the experimental design, assuming the molecular weight of triolein, 885.4 g/mol for beef tallow. This approach is based on the triolein intermediate properties between saturated and unsaturated fatty acids. Accordingly, 0.007 moles of beef tallow and 0.084 moles of ethanol, for a 12:1 molar ratio, were loaded in the reactor. With the total moles loaded and the reactor volume, the molar volume was calculated. Pressure at reaction conditions of the ethanol-beef tallow mixture was calculated with the Peng-Robinson equation of state and Chueh and Prausnitz mixing rules. Triolein was used as a model compound for beef tallow triglycerides, due to its intermediate properties between unsaturated and saturated triglycerides, a common approach for simulation purposes of supercritical transesterification processes (Marulanda, 2012). In all runs, it was verified that the pressure ranged from 23 to 27 MPa due to the difference in molar ratios and temperatures, well below the safety working pressure and above the supercritical conditions of ethanol. Before the run, the reactor was purged with nitrogen to remove air, closed and immersed in an insulated high temperature oven, which permits temperatures of 350-400 ± 2°C, previously preheated at the desired reaction temperature. The reactor was left in the oven for a total time corresponding to that in the experimental design and upon completion extracted and quickly quenched in a water bath to stop the reaction. Figure 1 shows the used lab-scale experimental setup. Reactor contents were emptied in glass tubes for further analysis.
Preliminary Supercritical Transesterification Runs
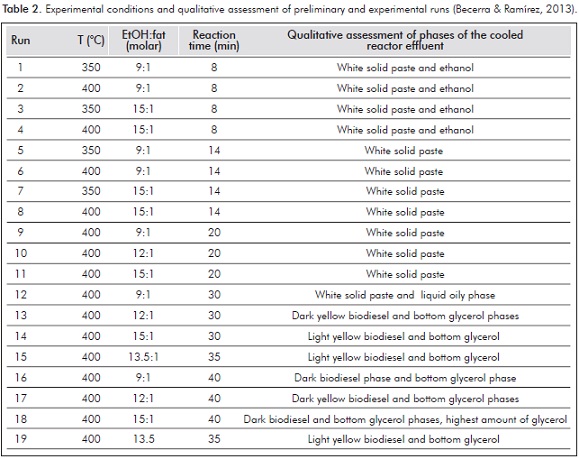
Preliminary runs were carried out in the temperature range from 350 - 400°C, reaction times of 8 and 12 minutes and alcohol to triglyceride molar ratios of 9:1 and 15:1 (Becerra & Ramírez, 2013). At similar experimental conditions, previous batch supercritical transesterification studies have reported high biodiesel yields from chicken fat (Marulanda et al., 2010b) and palm oil (Sawangkeaw et al., 2011). However, the reaction did not proceed as expected and instead of a liquid phase indicating the production of biodiesel, a solid paste difficult to extract from the reactor was produced, indicating a poor triglyceride conversion, which could be attributed to the lower reactivity of SFA in comparison to unsaturated fatty acids (Warabi, Kusdiana & Saka, 2004). Only those runs carried out at 400°C produced a mixture of a solid and liquid phase. Therefore, additional preliminary runs were carried out at 400°C and longer reaction times while assessing the disappearance of the solid phase (Becerra & Ramírez, 2013).
Experimental Design
Based on the qualitative assessment of the preliminary runs, the experimental methodology assessed the effect of molar ratios 12:1 and 15:1 and reaction times of 30 and 40 minutes, while keeping temperature at 400°C, organized according to a 22 factorial design. As previously calculated in the preliminary experiments, pressure was kept in the range 24 - 27 MPa, with variations attributed to the difference in molar ratios and temperatures. Each run was replicated twice and two central points were also run, for a total of 10 experimental runs.
Sample Preparation and Analytical Methods
After each experimental run a biodiesel sample for chromatography analysis was prepared by bubbling nitrogen during 1 hour in order to remove the excess alcohol as well as other volatile products that might have formed during the reaction. However, no water wash was performed to avoid loss of other compounds of interest that might have been formed due to decomposition reactions. Qualitative assessment of the reaction products was performed by HP 5971 Mass-Selective Detector (MSD) in a HP-1 MS cross linked methyl siloxane column with dimensions of 30 m × 0.32 mm I.D. × 0.25 µm film thickness, with helium as carrier gas. The temperature program started at 100°C, held for 1 min and a subsequent ramp of 5°C/min to 200°C, held for 3 min, 3°C/min to 250°C, held for 4 min and 5°C/min to 300°C, held for 5 min. MSD transfer line was kept at 300°C.
3. RESULTS AND DISCUSSION
The initial qualitative assessment of the reactor effluent consisted on the identification of the formed product phases. Since beef tallow is solid at room temperature due to the high concentration of tristearin and tripalmitin SFA (Banković-Ilić et al., 2014; Chakraborty, Gupta & Chowdhury 2014; Karmakar et al., 2010), a solid phase in the product can be related to a very low raw material conversion. Similarly, the absence of a solid phase and the formation of a glycerol bottom liquid phase account for a high raw material conversion. In Table 2, runs 1-11, preliminary runs, produced a solid paste indicating a low triglyceride conversion, whereas runs carried out at 400°C and reaction times longer than 30 minutes, runs 12-17, produced a liquid effluent that formed two liquid phases, an upper biodiesel phase and a lower glycerol phase, indicating glycerol separates spontaneously and its separation through decantation is simple.
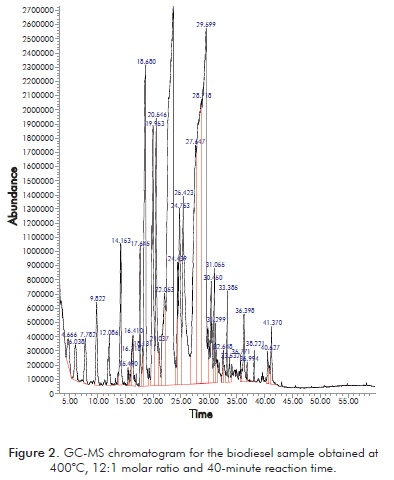
Figure 2 shows the GC-MS chromatogram for the biodiesel sample obtained at 400°C, 12:1 molar ratio and 40-minute reaction time. Most abundant peaks, those with retention times between 18 and 30 minutes, correspond to C14-C18 FAEEs present in the original raw material, whereas peaks with retention times shorter than 18 minutes or longer than 30 minutes correspond to shorter (C8-C13) FAEEs or glycerol decomposition products.
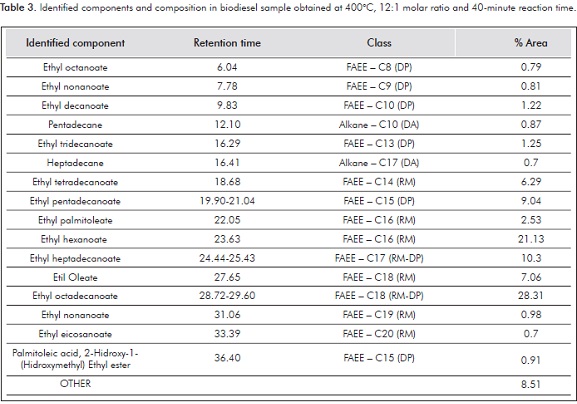
Table 3 shows the most relevant components in the biodiesel sample chromatogram classified as decomposition FAEE-DP if those components were not produced from triglycerides present in the raw material, Decomposition Alkanes (DA) and FAEE produced from triglycerides present in the Raw Material (RM). Identified peaks are those with a minimum identification quality of 90 and a percentage area higher than 0.5%. All the other peaks with a lower identification quality are lumped in other compounds. In some cases several peaks are identified as the same component, which can be attributed to isomeric or structurally similar overlapping compounds which might not be present in the equipment databases and therefore identified as the most similar available. In such cases the range of retention times in which those components elude and the name of the most probable compound is given. These results agree with those reported by Quesada-Medina and Olivares-Carrillo (2011), who reported thermal decomposition as a broad single peak proportional to the disappearance of the polyunsaturated fatty acids which contain 2 or more double bonds.
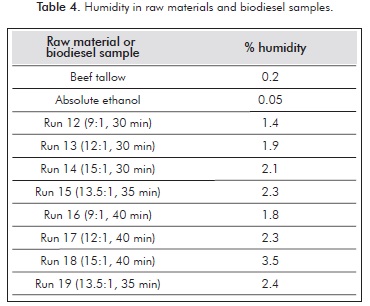
Glycerol decomposition products, glycerol ethers mainly, were also identified at retention times between four and five minutes, but could not be properly quantified due to its proximity to the solvent delay time in the GC-MS method. Nevertheless, glycerol decomposition reactions were verified by means of biodiesel humidity after the reaction, before bubbling nitrogen to remove alcohol in excess. Humidity results are shown in Table 4, which shows that the measured humidity was produced during the transesterification reaction.
As shown in the literature (Marulanda et al., 2010a; Sawangkeaw et al., 2011), at supercritical processing conditions, glycerol suffers etherification reactions with methanol to produce water. In fact, some authors have referred to this process as glycerol free, eliminating practically the necessity of washing the produced biodiesel (Aimaretti el al., 2009). Therefore, the water content in biodiesel samples can be associated to etherification reactions of glycerol. As shown in Table 4, humidity increases with an increase in molar ratio and reaction time, which can be associated to a higher availability of ethanol for glycerol etherification reactions in the first case or a higher conversion of triglycerides to produce glycerol which further reacts to produce glycerol ethers and water. Water has been proven to have a positive effect in the transesterification reactions at supercritical conditions (Kusdiana & Saka, 2004; Vieitez et al., 2008) whereas glycerol ethers are well known fuel additives, acting as octane boosters and reducing fuel viscosity (Rahmat, Abdullah & Mohamed, 2010), which suggests these decomposition products can be part of the biodiesel and enhance some of its properties. However, glycerol ethers are easily stripped together with excess alcohol from biodiesel. Some authors have reported an improved performance regarding combustion characteristics and emission profiles of diesel-biodiesel-ethanol-water blends over diesel-biodiesel blends (Lee et al., 2011; Qi et al., 2010). In any case, the feasibility of using the produced biodiesel without any additional treatment or purification in biodiesel-diesel blends still needs to be further addressed by means of engine tests.
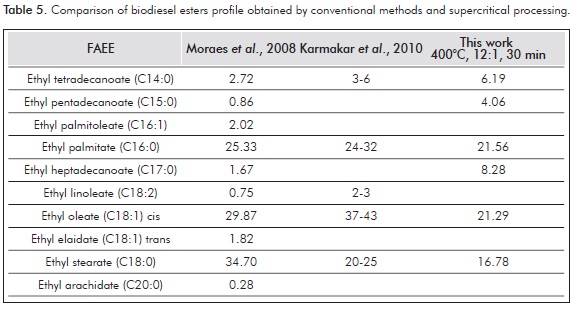
Table 5 shows a comparison of the tallow biodiesel FAEE profile obtained by supercritical processing and conventional catalyzed methods. Several papers have reported the occurrence of decomposition reactions at supercritical conditions. Whereas some authors refer to a decreased yield due to the decomposition of esters at certain temperature and residence time conditions (Bertoldi et al., 2009; Quesada-Medina & Olivares-Carrillo, 2011), some others have reported increased yields due to the production of several decomposition products from esters and glycerol (Marulanda et al., 2010a; Sawangkeaw et al., 2011). The difference can be mainly attributed to the yield calculation based on the FAEE quantification with respect to hexadecanoic acid ester, which is assumed not to be prone to decomposition. The identified components in Table 3 agree with the decomposition results reported by Lin, Zhu and Tavlarides, (2013), who carried out batch thermal stressing experiments of a commercial biodiesel sample in the temperature range 250 - 425°C and found that pyrolysis, also known as thermal cracking or thermal decomposition reactions, and cis-trans isomerization occur at temperatures >350°C and the main decomposition products are C5:0 - C15:0 and C17:0 methyl esters and C8 - C17 n-alkanes. Table 5 shows the biodiesel obtained by means of supercritical transesterification has a high percentage composition of pentadecanoic and heptadecanoic FAEE generally not prevalent in natural sources. In fact, methyl heptadecanoate (C17:0) is the internal standard used to determine the total FAME according to ASTM standards. The high content of C15:0 and C17:0 in contrast to biodiesel obtained by conventional methods can be attributed mostly to the decomposition of oleic acid ethyl ester, as it is prone to suffer reactions that involve the double bond in comparison to saturated FAEE, which is deemed more stable.
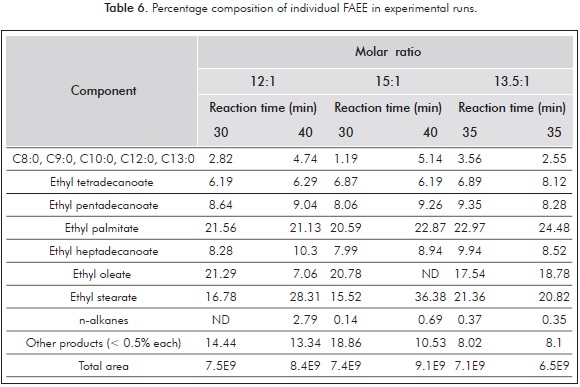
Table 6 presents the percentage composition of individual esters in each of the experimental runs as well as that for decomposition products represented in other compounds not identified or present in low concentrations.
As shown in Table 6, percentage composition of pentadecanoic (C15:0) and heptadecanoic (C17:0) FAEEs increases with reaction time at 12:1 and 15.1 molar ratios. It can also be observed that saturated FAEEs such as ethyl tetradecanoate (C14:0) and ethyl hexadecanoate (C16:0) composition percentages are similar regardless of the reaction time and molar ratio, which indicates these FAEE are generally stable at experimental conditions and match the results previously published (Quesada-Medina & Olivares-Carrillo, 2011; Shin et al., 2011). However, ethyl oleate (C18:1), the only prevalent unsaturated FAEE in the raw material, decreases its composition sharply with the reaction time, going from 21.29 to 7.06% at 12:1 molar ratio and from 20.78 to non-detected at 15.1 molar ratio. The disappearance of C18:1 can be directly associated to the appearance of the short chain, C15:0 and C17:0 FAEE as well as the increase of ethyl octadecanoate (C18:0), which should not suffer decomposition in a similar manner to ethyl tetradecanoate and hexadecanoate, but almost double its composition with an increase in the residence time in both molar ratios. These results agree with those reported by Shin et al. (2011) who studied the thermal decomposition and stability of FAME in supercritical methanol and found that the main degradation products of methyl stearate (C18:0) and methyl oleate (C18:1) were low molecular FAMEs, 1-alkenes and n-alkanes for the first one, an methyl stearate for the second one, produced from the C-C double bond hydrogenation. In all runs, decomposition products with reduced detection quality or accounting less than 0.5% individually account for up 10% total peak area. The highest yield in terms of the accounting of percentage areas of FAEE and decomposition products was obtained with a 15:1 molar ratio and 40-minute reaction time. Repeatability of the experiments was generally good, as evidenced by the similar percentage area composition in both runs carried out at 13.5:1 molar ratio and 35-minute reaction time.
Thermal cracking reactions have the potential to improve cold flow and stability of biodiesel as reported by Seames et al. (2010), who conducted thermal cracking experiments of canola methyl esters in the temperature range 350 - 440°C and found a 20°C and 15°C reduction in the cloud and pour points and Lin et al. (2014), who suggested pyrolysis reactions benefit biodiesel properties and may be a potential method to produce diesel alternatives that have improved properties. Due to the resulting high percentage composition of saturated FAEE in biodiesel produced from animal fats as compared to vegetable oils through conventional methods, viscosity and cold filter plugging point are higher, which is a major concern for its utilization as fuel (Teixeira et al., 2010). Therefore, mixtures of biodiesel with high proportions of diesel are used to keep the fuel within the limits of viscosity and cold filter plugging point (da Cunha et al., 2009). The results indicate decomposition is a potential method to improve such properties for biodiesel produced from beef tallow, due to the formation of smaller chain FAEEs which have lower viscosity and melting point, thus making it possible to increase the biodiesel to diesel ratio in commercial mixtures. Nevertheless, such claims must be thoroughly assessed through appropriate tests, not performed at this stage of the research.
4. CONCLUSION
Batch supercritical transesterification experiments of beef tallow as triglyceride source and ethanol were carried out to assess the composition of the produced biofuel in terms of produced FAEEs and decomposition products formed through pyrolysis and cis-trans isomerization reactions, which are known to occur at supercritical processing conditions. Experimental results showed beef tallow requires more severe reaction conditions, mainly a longer reaction time and higher molar ratios than biodiesel produced from vegetable oils by means of supercritical transesterification, which can be attributed to the high content of saturated fatty acids of beef tallow. GC-MS analysis of biodiesel samples showed the presence of esters derived from triglycerides in the raw material, palmitic, oleic and stearic, as well as shorter chain ethyl esters and linear alkanes. The disappearance of ethyl oleate, the only prevalent unsaturated FAEE in the raw material, could be directly associated to the appearance of pentadecanoic and heptadecanoic FAEE, as well as the increase of ethyl octadecanoate (C18:0). Glycerol ethers detected in biodiesel samples could be produced from glycerol etherification reactions with ethanol, as was evidenced by means of Karl Fisher humidity measurements. The highest yield of esters, whether initially present as triglycerides or formed through decomposition reactions, was measured at 400°C, 15:1 molar ratio and a 40-minute reaction time. Under these conditions, ethyl oleate practically disappeared whereas other ethyl esters such as pentadecanoate and heptadecanoate increased considerably. Glycerol phase was recovered by decantation, whereas glycerol ethers were detected in the biodiesel phase.
ACKNOWLEDGEMENTS
The authors wish to thank the Colombian Institute of Science and Technology (COLCIENCIAS) for a young researcher grand awarded to engineer Paola Marulanda to carry out research at Universidad de La Salle in Bogotá for one year.
REFERENCES
Aimaretti, N., Manuale, D. L., Mazzieri, V. M., Vera, C. R. & Yori, J. C. (2009). Batch study of glycerol decomposition in one-stage supercritical production of biodiesel. Energy Fuels, 23(2), 1076-1080. [ Links ]
Banković-Ilić, I. B., Stojković, I. J., Stamenković, O. S., Veljkovic, V. B. & Hung, Y. T. (2014). Waste animal fats as feedstocks for biodiesel production. Renew. Sust. Energ. Rev., 32: 238-254. [ Links ]
Becerra, Y. V. & Ramírez, N. E. (2013). Estudio experimental de la utilización de sebo vacuno para la producción de biodiesel por medio del proceso de transesterificación supercrítica. Tesis de Grado, Ingenieria Ambiental y Sanitaria. Universidad de La Salle. Bogotá. 79pp. [ Links ]
Bertoldi, C., Da Silva, C., Bernardon, J. P., Corazza, M. L., Filho, L. C., Oliveira, J. V., & Corazza, F. C. (2009). Continuous production of biodiesel from soybean oil in supercritical ethanol and carbon dioxide as cosolvent. Energy Fuels, 23(10), 5165-5172. [ Links ]
Biktashev, S. A., Usmanov, R. A., Gabitov, R. R., Gazizov, R. A., Gumerov, F. M., Gabitov, F. R., Abdulagatov, I. M., Yarullin, R. S. & Yakushev, I. A. (2011). Transesterification of rapeseed and palm oils in supercritical methanol and ethanol. Biomass Bioenerg., 35(7), 2999-3011. [ Links ]
Bunyakiat, K., Makmee, S., Sawangkeaw, R. & Ngamprasertsith, S. (2006). Continuous production of biodiesel via transesterification from vegetable oils in supercritical methanol. Energy Fuels, 20(2), 812-817. [ Links ]
Chakraborty, R., Gupta, A. K. & Chowdhury, R. (2014). Conversion of slaughterhouse and poultry farm animal fats and wastes to biodiesel: Parametric sensitivity and fuel quality assessment. Renew. Sust. Energ. Rev., 29: 120-134. [ Links ]
Da Cunha, M. E., Krause, L. C., Moraes, M. S. A., Faccini, C. S., Jacques, R. A., Almeida, S. R., Rodrigues, M. R. A & Caramão, E. B. (2009). Beef tallow biodiesel produced in a pilot scale. Fuel Process. Technol., 90(4), 570-575. [ Links ]
Gui, M. M., Lee, K. T. & Bhatia, S. (2009). Supercritical ethanol technology for the production of biodiesel: Process optimization studies. J. Supercrit. Fluids, 49(2), 286-292. [ Links ]
Karmakar, A., Karmakar, S. & Mukherjee, S. (2010). Properties of various plants and animals feedstocks for biodiesel production. Bioresour. Technol., 101(19), 7201-7210. [ Links ]
Kusdiana, D. & Saka, S. (2004). Effects of water on biodiesel fuel production by supercritical methanol treatment. Bioresour. Technol., 91(3), 289-295. [ Links ]
Lee, W. J., Liu, Y. C., Mwangi, F. K., Chen, W. H., Lin, S. L., Fukushima, Y., Liao, C. N. & Wang, L. C. (2011). Assessment of energy performance and air pollutant emissions in a diesel engine generator fueled with water-containing ethanol-biodiesel-diesel blend of fuels. Energy, 36(9), 5591-5599. [ Links ]
Lin, R., Zhu, Y. & Tavlarides, L. L. (2013). Mechanism and kinetics of thermal decomposition of biodiesel fuel. Fuel, 106: 593-604. [ Links ]
Lin, R., Zhu, Y. & Tavlarides, L. L. (2014). Effect of thermal decomposition on biodiesel viscosity and cold flow property. Fuel, 117: 981-988. [ Links ]
Marulanda, V. F. (2012). Biodiesel production by supercritical methanol transesterification: Process simulation and potential environmental impact assessment. J. Cleaner Production, 33: 109-116. [ Links ]
Marulanda, V. F., Anitescu, G. & Tavlarides, L. L. (2010a). Biodiesel fuels through a continuous flow process of chicken fat supercritical transesterification. Energy Fuels, 24(1), 253-260. [ Links ]
Marulanda, V. F., Anitescu, G. & Tavlarides, L. L. (2010b). Investigations on supercritical transesterification of chicken fat for biodiesel production from low-cost lipid feedstocks. J. Supercrit. Fluids, 54(1), 53-60. [ Links ]
Mendow, G., Veizaga, N. S., Sánchez, B. S. & Querini, C. A. (2011). Biodiesel production by two-stage transesterification with ethanol. Bioresour. Technol., 102(22), 10407-10413. [ Links ]
Moraes, M. S. A., Krause, L. C., Da Cunha, M. E., Faccini, C. S., de Menezes, E. W., Veses, R. C., Rodrigues, M. R. A. & Caramão, E. B. (2008). Tallow biodiesel: Properties evaluation and consumption tests in a diesel engine. Energy Fuels, 22(3), 1949-1954. [ Links ]
Muppaneni, T., Reddy, H. K., Patil, P. D., Dailey, P., Aday, C. & Deng, S. (2012). Ethanolysis of camelina oil under supercritical condition with hexane as a co-solvent. Appl. Energ., 94: 84-88. [ Links ]
Muppaneni, T., Reddy, H. K., Ponnusamy, S., Patil, P. D., Sun, Y., Dailey, P. & Deng, S. (2013). Optimization of biodiesel production from palm oil under supercritical ethanol conditions using hexane as co-solvent: A response surface methodology approach. Fuel, 107: 633-640. [ Links ]
Nan, Y., Liu, J., Lin, R. & Tavlarides, L. L. (2015). Production of biodiesel from microalgae oil (Chlorella protothecoides) by non-catalytic transesterification in supercritical methanol and ethanol: Process optimization. J. Supercrit. Fluids, 97: 174-182. [ Links ]
Pinnarat, T. & Savage, P. E. (2008). Assessment of noncatalytic biodiesel synthesis using supercritical reaction conditions. Ind. Eng. Chem. Res., 47(18), 6801-6808. [ Links ]
Qi, D. H., Chen, H., Matthews, R. D. & Bian, Y. Z. (2010). Combustion and emission characteristics of ethanol-biodiesel-water micro-emulsions used in a direct injection compression ignition engine. Fuel, 89(5), 958-964. [ Links ]
Quesada-Medina, J. & Olivares-Carrillo, P. (2011). Evidence of thermal decomposition of fatty acid methyl esters during the synthesis of biodiesel with supercritical methanol. J. Supercrit. Fluids, 56(1), 56-63. [ Links ]
Rahmat, N., Abdullah, A. Z. & Mohamed, A. R. (2010). Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sust. Energ. Rev., 14(3), 987-1000. [ Links ]
Rodríguez-Guerrero, J. K., Rubens, M. F. & Rosa, P. T. V. (2013). Production of biodiesel from castor oil using sub and supercritical ethanol: Effect of sodium hydroxide on the ethyl ester production. J. Supercrit. Fluids, 83: 124-132. [ Links ]
Saka, S. & Kusdiana, D. (2001). Biodiesel fuel from rapeseed oil as prepared in supercritical methanol. Fuel, 80(2), 225-231. [ Links ]
Santana, A., Maçaira, J. & Larrayoz, M. A. (2012). Continuous production of biodiesel from vegetable oil using supercritical ethanol/carbon dioxide mixtures. Fuel Process. Technol., 96: 214-219. [ Links ]
Sawangkeaw, R., Bunyakiat, K. & Ngamprasertsith, S. (2010). A review of laboratory-scale research on lipid conversion to biodiesel with supercritical methanol (2001-2009). J. Supercrit. Fluids, 55(1), 1-13. [ Links ]
Sawangkeaw, R., Teeravitud, S., Bunyakiat, K. & Ngamprasertsith, S. (2011). Biofuel production from palm oil with supercritical alcohols: Effects of the alcohol to oil molar ratios on the biofuel chemical composition and properties. Bioresour. Technol., 102(22), 10704-10710. [ Links ]
Sawangkeaw, R., Tejvirat, P., Ngamcharassrivichai, C. & Ngamprasertsith, S. (2012). Supercritical transesterification of palm oil and hydrated ethanol in a fixed bed reactor with a CaO/Al2O3 catalyst. Energies, 5(4), 1062-1080. [ Links ]
Seames, W., Luo, Y., Ahmed, I., Aulich, T., Kubátová, A., Št'ávová, J. & Kozliak, E. (2010). The thermal cracking of canola and soybean methyl esters: Improvement of cold flow properties. Biomass Bioenerg., 34(7), 939-946. [ Links ]
Shin, H. Y., Lim, S. M., Bae, S. Y. & Oh, S. C. (2011). Thermal decomposition and stability of fatty acid methyl esters in supercritical methanol. J. Anal. Appl. Pyrol., 92(2), 332-338. [ Links ]
Stamenković, O. S., Veličković, A. V. & Veljković, V. B. (2011). The production of biodiesel from vegetable oils by ethanolysis: Current state and perspectives. Fuel, 90(11), 3141-3155. [ Links ]
Sun, Y., Reddy, H. K., Muppaneni, T., Ponnusamy, S., Patil, P. D., Li, C., Jiang, L & Deng, S. (2014). A comparative study of direct transesterification of camelina oil under supercritical methanol, ethanol and 1-butanol conditions. Fuel, 135: 530-536. [ Links ]
Tan, K. T., Lee, K. T. & Mohamed, A. R. (2010). Effects of free fatty acids, water content and co-solvent on biodiesel production by supercritical methanol reaction. J. Supercrit. Fluids, 53(1-3), 88-91. [ Links ]
Teixeira, L. S. G., Couto, M. B., Souza, G. S., Filho, M. A., Assis, J. C. R., Guimarães, P. R. B., Pontes, L. A. M, Almeida, S. Q. & Teixeira, J. S. R. (2010). Characterization of beef tallow biodiesel and their mixtures with soybean biodiesel and mineral diesel fuel. Biomass Bioenerg., 34(4), 438-441. [ Links ]
Velez, A., Soto, G., Hegel, P., Mabe, G. & Pereda, S. (2012). Continuous production of fatty acid ethyl esters from sunflower oil using supercritical ethanol. Fuel, 97: 703-709. [ Links ]
Vieitez, I., da Silva, C., Borges, G. R., Corazza, F. C., Oliveira, J. V., Grompone, M. A. & Jachmanián, I. (2008). Continuous production of soybean biodiesel in supercritical ethanol-water mixtures. Energy Fuels, 22(4), 2805-2809. [ Links ]
Vieitez, I., Pardo, M. J., Da Silva, C., Bertoldi, C., De Castilhos, F., Oliveira, J. V., Grompone, M. A. & Jachmanián, I. (2011). Continuous synthesis of castor oil ethyl esters under supercritical ethanol. J. Supercrit. Fluids, 56(3), 271-276. [ Links ]
Warabi, Y., Kusdiana, D. & Saka, S. (2004). Reactivity of triglycerides and fatty acids of rapeseed oil in supercritical alcohols. Bioresour. Technol., 91(3), 283-287. [ Links ]
Yilmaz, N. & Sanchez, T. M. (2012). Analysis of operating a diesel engine on biodiesel-ethanol and biodiesel-methanol blends. Energy, 46(1), 126-129. [ Links ]
Zhang, Y., Dubé, M. A., McLean, D. D. & Kates, M. (2003). Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresour. Technol., 90(3), 229-240. [ Links ]
AUTHORS
Paola-Andrea Marulanda-Buitrago
Affiliation: Universidad de La Salle
Environmental and Sanitary Engineer, Universidad de La Salle
e-mail: pmarulandab@lasalle.edu.co
Victor-Fernando Marulanda-Cardona
Affiliation: Universidad de La Salle
Chemical Engineer, Universidad Nacional de Colombia
Doctor in Engineering, Universidad del Valle
e-mail: vfmarulanda@unisalle.edu.co