Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
CT&F - Ciencia, Tecnología y Futuro
versión impresa ISSN 0122-5383
C.T.F Cienc. Tecnol. Futuro vol.6 no.2 Bucaramanga jul./dic. 2015
OPERATING CONDITIONS INFLUENCE ON VMD AND SGMD FOR ETHANOL RECOVERY FROM AQUEOUS SOLUTIONS
INFLUENCIA DE LAS CONDICIONES DE OPERACIÓN EN VMD Y SGMD PARA LA RECUPERACIÓN DE ETANOL DE SOLUCIONES ACUOSAS
INFLUÊNCIA DAS CONDIÇÕES DE OPERAÇÃO EM VMD E SGMD PARA A RECUPERAÇÃO DE ETANOL DE SOLUÇÕES AQUOSAS
Ricardo-Javier Cotamo-De la Espriella1, Fredy-Wsbaldo Barón-Núñez1 and Carlos-Jesús Muvdi-Nova1*
1Chemical Engineering School, Universidad Industrial de Santander (UIS), Bucaramanga, Santander, Colombia.
e-mail: cjmuvdi@uis.edu.co
How to cite: Cotamo-De la Espriella, R. J., Barón-Núñez, F. W. & Muvdi-Nova, C. J. (2015). Operating conditions influence on VMD and SGMD for ethanol recovery from aqueous solutions. CT&F - Ciencia, Tecnología y Futuro, 6(2), 69-80.
*To whom correspondence should be addressed
(Received: Nov. 17, 2014; Accepted: Sep. 30, 2015)
ABSTRACT
This work focuses on Vacuum Membrane Distillation (VMD) and Sweeping Gas Membrane Distillation (SGMD) as a separating technique of ethanol from aqueous solutions. VMD was studied at moderate temperature (30, 40 and 50°C) and pressure (0.11, 0.20 and 0.30 atm) conditions, whereas SGMD was studied at different temperatures (50 and 70°C) and air-flow rates (10x10-6 and 20x10-6 m3·min-1). These techniques were experimentally studied using prepared ethanol-water solutions and fermented broths, with ethanol at 10% w/w. Under these operating conditions and using prepared ethanol-water solutions, an average total flux of 22.61 and 1.6 kg·m-2·h-1, and concentration factors of 2.3 and 1.7 were obtained for VMD and SGMD, respectively. For fermented broths, total flux of 17.66 and 0.9 kg·m-2·h-1, and concentration factors of 1.8 and 1.9 were obtained for VMD and SGMD, respectively. The fouling impact was also studied, finding a significant effect of pressure (vacuum) for VMD technique; mainly due to the biomass presence in the solution. Experimental results show that applying pressurization/depressurization cycles decreases membrane fouling, stabilizing flux and concentration in the permeate. While for SGMD configuration, the incidence of fouling was significantly lower.
Keywords: Membrane distillation, Separation process, Ethanol, VMD, SGMD.
RESUMEN
Este trabajo se enfoca en la Destilación con Membranas con Vacío (VMD, por sus siglas en inglés) y con Gas de Arrastre (SGMD, por sus siglas en inglés) como técnica para separar el etanol de soluciones acuosas. La VMD fue estudiada utilizando condiciones moderadas de temperatura (30, 40 y 50°C) y presión (0.11, 0.20 y 0.30 atm), mientras que la SGMD fue estudiada a temperaturas de 50 y 70°C y flujos de aire de 10x10-6 y 20x10-6 m3·min-1. Las técnicas fueron estudiadas experimentalmente usando soluciones etanol:agua preparadas y caldos de fermentación, ajustando la concentración de etanol al 10% p/p. Bajo estas condiciones de operación y utilizando soluciones etanol:agua preparadas, se obtuvieron flux totales promedio de 22.61 y 1.6 kg·m-2·h-1, y factores de concentración de 2.3 y 1.7 para VMD y SGMD, respectivamente. Para el caso de los caldos de fermentación, se obtuvieron flux totales promedio de 17.66 y 0.9 kg·m-2·h-1, y factores de concentración de 1.8 y 1.9 para VMD y SGMD, respectivamente. El impacto del ensuciamiento también fue estudiado, encontrando un efecto significativo de la presión (de vacío) para la técnica VMD; principalmente debida a la presencia de biomasa en la solución. Pruebas experimentales muestran que aplicando ciclos de presurización/despresurización se disminuye el ensuciamiento de la membrana, estabilizando el flujo y la concentración en el permeado. Mientras para la configuración SGMD, el impacto del ensuciamiento fue considerablemente menor.
Palabras clave: Destilación con membranas, Procesos de separación, Etanol, VMD, SGMD.
RESUMO
Este trabalho está focado na Destilação com Membranas em Vácuo (VMD em inglês) e com Gás de Arrastre (SGMD, em inglês) como técnica para a separação do etanol de soluções aquosas. A VMD foi estudada utilizando condições moderadas de temperatura (30, 40 e 50°C) e pressão (0.11, 0.20 e 0.30 atm), enquanto a SGMD foi estudada em temperaturas de 50 e 70°C e fluxos de ar de 10x10-6 e 20x10-6 m3·min-1. As técnicas foram estudadas de forma experimental utilizando soluções etanol:água preparadas e caldos de fermentação, ajustando a concentração de etanol a 10% w/w. Sob estas condições de operação e utilizando soluções etanol:água preparadas, conseguimos obter flux totais médios de 22.61 e 1.6 kg·m-2·h-1, e fatores de concentração de 2.3 e 1.7 para VMD e SGMD, respectivamente. No caso dos caldos de fermentação, obtivemos flux totais médios de 17.66 e 0.9 kg·m-2·h-1, e fatores de concentração de 1.8 e 1.9 para VMD e SGMD, respectivamente. O impacto da incrustação também foi estudado, encontrando um efeito significativo da pressão (de vácuo) para a técnica VMD; principalmente, causada pela presença de biomassa na solução. Provas experimentais mostram que aplicando ciclos de pressurização/despressurização diminuímos a incrustação da membrana, estabilizando o fluxo e a concentração no permeado. Enquanto que para a configuração SGMD, o impacto da incrustação foi consideravelmente menor.
Palavras-chave: Destilação com membranas, Processos de separação, Etanol, VMD, SGMD.
1. INTRODUCTION
One of the problems that arises in batch fermentation processes for bioethanol production is the inhibition of microorganisms due to the bioethanol concentration. This inhibition stops fermentation, reducing the process efficiency and increasing production costs (Jaramillo, Gómez & Fontalvo, 2012; Stanley et al., 2010).
Membrane Pervaporation (PV) is one of the most studied unconventional separation techniques. This technique uses dense membranes and presents low permeability flux. It has been reported that PV is more suitable for concentration-dehydration of solutions than for separation (Chapman et al., 2008). A comparison between reported research works about ethanol separation from aqueous solutions shows Membrane Distillation (MD) permeate flux values are 100 times higher than those obtained by PV (Cerneaux et al., 2009; Qiu et al., 2009). This is due to the porous nature of membranes used in MD, which allow higher permeability under same operating conditions.
MD is a technique that could be used in situ for bioethanol separation. This separation technique is advantageous as it uses low temperature operation and compact design as compared to "traditional” separation systems as distillation or vacuum evaporation (Lee & Hong, 2001).
MD is based on separation of volatile compounds from a solution (an aqueous one) by using a hydrophobic porous membrane (Baker, 2004). The hydrophobic nature of membrane prevents the liquid leakage and at the same time allows the passage of volatile compounds as vapor. The driven force in this technique is the partial vapor pressure difference on both sides of the membrane, directly linked to the process temperature (Rivier et al., 2002). The vapor passing through the membrane (permeate) can be assured in several ways: Direct Contact Membrane Distillation (DCMD) (Cerneaux et al., 2009; Hwang et al., 2011; Khayet, Mengual & Matsuura, 2005), Air Gap Membrane Distillation (AGMD) (Alkhudhiri, Darwish & Hilal, 2012), Vacuum Membrane Distillation (VMD) (Cerneaux et al., 2009) or using Sweeping Gas Membrane Distillation (SGMD) (Cojocaru & Khayet, 2011). From these configurations, VMD and SGMD are the ones having the largest industrial potential application, due to an easier recovery implementation principle (vacuum and sweep gas). Most studies have focused on VMD, using mostly prepared solutions (in most of cases using only water) and working under high vacuum conditions (3x10-3 atm). These two aspects show a lack of knowledge about fouling effect and moderate conditions influence on VMD process application performance. In contrast, there are few studies concerning SGMD configuration, therefore, it is not easy to explain this tendency. Additionally, there are no studies comparing these two techniques under similar conditions (membrane used and operating conditions), which would facilitate the analysis and discussion concerning the advantages of each of these techniques in a more accurate and rigorous way.
Therefore, this work analyzed and compared these two configurations, VMD and SGMD, using prepared water-ethanol solutions and water-ethanol mixtures from fermented broths. At first, we considered working under moderate vacuum conditions for VMD, and using membranes with pore diameter up to 5 µm for SGMD. Also, this is the first study reporting the comparison of the two techniques under similar operating conditions.
2. MATERIALS AND METHODS
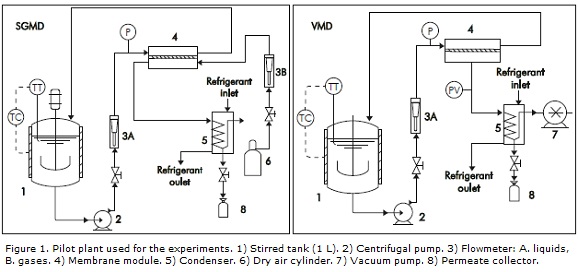
Module and Membranes
For this work, a membrane module was designed. This module works with 47 mm diameter flat membranes. Commercial polytetrafluoroethylene (PTFE) membranes were supplied by Whatman®, with a 34 mm diameter (filtration area = 9.08x10-4 m2). Pore diameters and other characteristics of the membranes used in this work are shown in Table 1. For pressure measurement bourdon manometers were used.
Experimental set-up
Figure 1 shows the experimental arrangement used and its components.
Fluids used
Feed solutions were prepared-ethanol water solutions and S. cerevisiae fermented broths (0.5% w/w biomass and 7.5 °Brix). For all tests, ethanol concentration was adjusted at 10% w/w (inhibitory concentration reported for microorganisms used for ethanol production) (Lin & Tanaka, 2006). Solutions were prepared using Ethanol A.R Merck and distilled water. Ethanol concentration in the broths (8% w/w) was adjusted up to 10% w/w.
Operating Conditions Influence on Process using Prepared Ethanol-water Solutions as Feed
SGMD
Experiments were conducted in order to determine the influence of feed temperature (50 and 70°C) and air-flow rate (10x10-6 and 20x10-6 m3·min-1 at 25°C and 1 atm) on flux and ethanol concentration in the permeate side. Temperatures were chosen based on preliminary tests to ensure permeate flux through the membrane and to avoid membrane deterioration. For this technique, the results obtained with PTFE3 and PTFE2 membranes are presented. For PTFE1 membrane, it was not possible to obtain permeate in any tested conditions. Experiments were realized twice and lasted 3 h.
VMD
Influence of temperature feed (30, 40, 50°C) and pressure (0.11, 0.2 and 0.3 atm) on flux and ethanol concentration in the permeate side was analyzed. These levels were established to evaluate mild vacuum operating conditions. For this technique, PTFE2 and PTFE1 membranes were used. PTFE3 membrane (for all evaluated pressures) and PTFE2 (at 0.11 atm) were discarded due to leakage of liquid feed in the permeate (transmembrane pressures higher to the intrusion pressures). Experiments were realized twice and lasted 3 h.
SGMD and VMD Performance in Time
The effect of non-soluble compounds in feed solution on MD performance was analyzed using fermented broths. For this, permeate flux for both configurations (SGMD and VMD) were monitored. Ethanol concentration in fermented broths was adjusted to 10% w/w. Feed flow rate and temperature were 2.64x10-6 m3·s-1 and 40°C (S. cervisiae approximate fermenting temperature). VMD worked under 0.11 atm pressure and for SGMD a sweeping gas flow rate of 20x10-6 m3·min-1 was used. These were the best study conditions found with prepared ethanol water solutions. All tests lasted 6 h. Dry air was used (with 25 ppm of water) as sweeping gas, which was supplied by Praxair Inc.
Characterization Parameters
Total and Ethanol Flux
Total flux was determined by measuring permeate mass per unit time and membrane surface. Ethanol flux was calculated using total flux and ethanol concentration in permeate.
Ethanol Concentration
Ethanol concentration was analyzed by HPLC, UFLC method with a SHIMADZU LC-20AD equipment. Parameters were as follows: oven temperature 80°C, isocratic flow rate of H2SO4, 8 mM from 0.6 mL·min-1; analysis time 45 min.
3. ANALYSIS AND DISCUSSION
Process Performance of VMD with Prepared Solutions
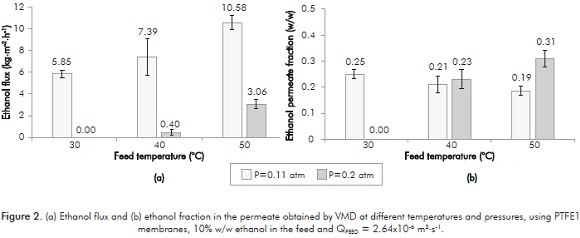
Figure 2 shows the results obtained by using the PTFE1 membrane. This figure shows that ethanol flux increases with temperature and decreases with pressure in the permeate compartment. This behavior is characteristic of this configuration, since the two variables directly affect the ethanol partial pressure and water difference on both membrane sides. A temperature increment generates increments between 25 and 670% on ethanol flux. Similarly, ethanol flux increases from 245 to 1748% when pressure in the permeate side decreases. The increase in both operating variables (temperature and transmembrane pressure) generates an increase on the vapor pressure of the components, facilitating compounds volatilization and passage through the membrane (ethanol in particular). This was also observed in different VMD studies such as the one performed by Izquierdo-Gil and Jonsson (2003), obtaining a flux ethanol increment of 51% increasing the feed temperature (from 35 to 45°C) (Izquierdo-Gil & Jonsson, 2003). On the other hand, Bowen, Noble and Falconer (2004) reported ethanol permeate flux increments up to 22% and a concentration factor of 5%, increasing feed temperature (from 40 to 50°C) with ethanol flux of 0.16 kg·m-2·h-1.
On the other hand, as to ethanol molar fraction in the permeate side, a significant influence occurred only for the pressure in the permeate compartment of 0.2 atm and temperature of 50°C, where the ethanol fraction increased up to 63%. It is important to note that tests with PTFE1 membrane at 0.3 atm in the permeate compartment did not generate any permeate.
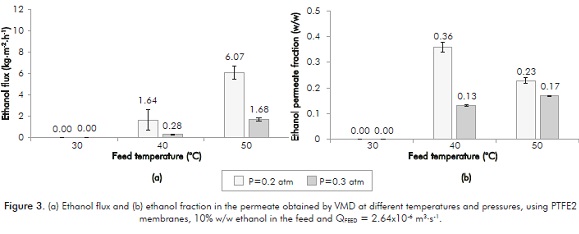
Similar results were observed in the PTFE2 membrane for permeate flux (see Figure 3). Increasing the transmembrane pressure caused ethanol flux increments from 260% (at 50°C) and 485% (at 40°C); increasing temperature induced increments between 270% (at 0.2 atm) and 500% (at 0.3 atm).
PTFE2 membrane tests at 0.11 atm were not possible due to the feed solution filtration (at these conditions transmembrane pressure is close to the membrane intrusion pressure, ΔPintrusion = 0.9 atm, Whatman®).
With respect to the ethanol fraction in the permeate, contrary trends have been observed with the PTFE1 membrane when varying feeding temperature and pressure in the permeate compartment. This behavior may be due to the different characteristics of the membranes used (PTFE2 membrane has a higher diameter and a lower thickness) and dependent on the different pressure levels used for the two membranes; on one hand absence of permeate (at 0.3 atm with PTFE1 membranes) and on the other hand aqueous filtered solution (at 0.11 atm with PTFE2 membranes). Results obtained do not allow us to conclude on the variable transmembrane pressure influence or on temperature influence on the ethanol fraction in the permeate.
In all cases, ethanol concentration in permeates were at least 2.5 times more concentrated than feed concentration (10% w/w ethanol). It is worth mentioning that this study reported the use of moderate vacuum pressures in the permeate compartment for VMD for the first time.
Process Performance of VMD with Real Solutions
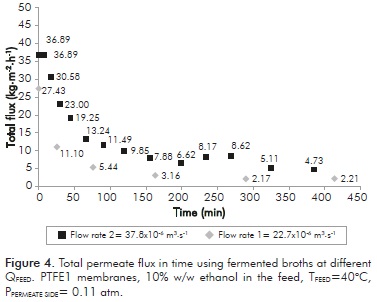
Based on the results obtained with prepared solutions, experiments were carried out with fermented broths using PTFE1 membranes and varying the feed rate. Total permeate flux variation in time can be observed in Figure 4. This figure shows that permeate flux decreases significantly (over 82% for both feed flow rates); being faster for the lower feed rate evaluated (22.7x10-6 m3·s-1). It is important to mention that formation of an organic layer on the membrane surface could be observed at the end of the tests.
This behavior is due to the pores fouling with insoluble material (biomass) that was deposited on membrane surface, forming a layer that prevents the normal flow through it.
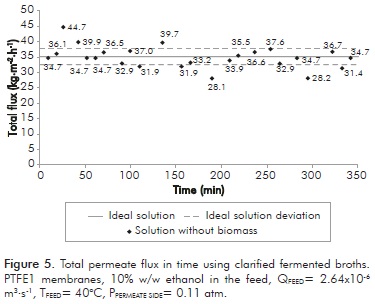
Besides the flux loss, permeate ethanol concentration was also affected. For flow rate of 22.7x10-6 m3·s-1, a permeate with 1.67% w/w of ethanol was obtained, whereas for flow rate of 37.8x10-6 m3·s-1 concentration was 6.79% w/w ethanol. Both values are below the ethanol feed concentration (10% w/w ethanol). This can also be explained by considering the adjacent layer formed by the biomass. This layer deposited on the membrane loses water and the remaining ethanol and prevents the passage of any other compound through it. This result is reinforced by subsequent tests made using clarified real solutions (no biomass presence). Figure 5 shows the results obtained using clarified fermented broths (without biomass).
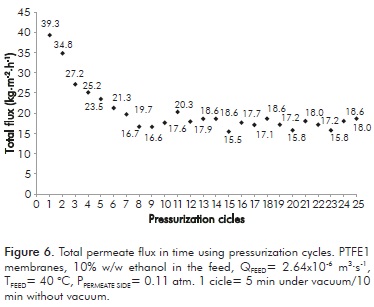
This figure shows that there is no decrease in permeability. Moreover, no deposition on the membrane was observed, confirming that this single layer formed by the biomass presence in the solution is the cause of membrane fouling. Based on the results reported by Lewandowicz et al. (2011), it could be possible that vacuum conditions are the reason of membrane fouling. These authors did not use vacuum as driving force for the separation; by using the DCMD method with hollow fiber polypropylene membranes, no fouling was observed during experiments (over 50 h). To corroborate this, experiments were conducted by turning off vacuum during 10 min each 5 min of distillation process. Results are presented in Figure 6. This figure shows that flux stabilized itself at a higher level value than shown in Figure 4, although a loss of approximately 50% occurred from the initial permeate flux. This indicates that (vacuum) pressure significantly promotes membrane fouling.
Regarding ethanol concentration in this test, the system was stabilized after cycle10 on a mean value of 17.65% w/w ethanol. This value is 15.9% lower with respect to prepared solutions (21.0% w/w ethanol) but 2.6 times higher than the value obtained when vacuum is not removed from the system periodically (6.78% w/w ethanol).
These results show the fouling layer (biomass) deposited on the membrane is partially swept by the feed stream during periods with no vacuum, like it happens with the back-flushing strategy implemented for membrane systems with high fouling problems (Zsirai et al., 2012). Further studies concerning this aspect should be carried out in order to improve VMD as an ethanol recovery technique from fermented broths. Specially, analyzing the effect of cycle frequency and application time on both fouling and process productivity.
Process Performance of SGMD with Prepared Solutions
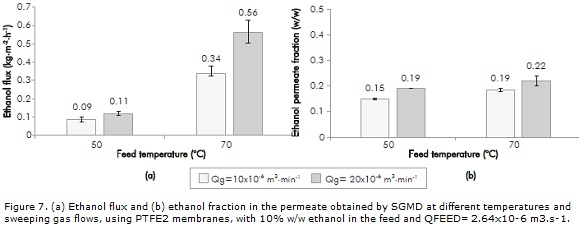
The PTFE1 membrane results are not presented due to the absence of permeate with all the operating conditions studied. That could be explained by the characteristics of the membrane (pore diameter, thickness and porosity) that generate a mass transfer resistance that could not be overcome by the vapor pressure gradient, generating a minimal membrane flux, that was not perceived during the three hours of the experiment (in SGMD, system is at atmospheric pressure). The results obtained with PTFE2 are shown in Figure 7.
Based on this figure, it can be noticed that flux values and ethanol fractions of the permeate increase with higher temperature, as well as with higher sweeping gas flow rate. Increments in temperature from 50 to 70°C produce ethanol flux increases between 270 and 410 %. As to the sweeping gas flow rate, ethanol flux increased from 22 to 65%. On the other hand, ethanol fraction in the permeate improved its value between 22 and 27 % with the temperature, and between 16 to 20% with sweeping gas flow rate. This behavior is explained by considering that as feed temperature increases, the vapor pressure of both compounds makes them volatilize and cross through the membrane (particularly for ethanol). This behavior is reported in other studies dealing with alcohol separation such as isopropanol, butanol, and volatile compounds such as ammonia (Khayet, Godino & Mengual, 2000; Xie et al., 2009). Lee and Hong (2001), who worked in the isopropanol separation by SGMD using 10% w/w solutions, reported increments of isopropanol flux in 64% approx. by changing temperature from 40 to 45°C, and an isopropanol concentration factor of 6.3.
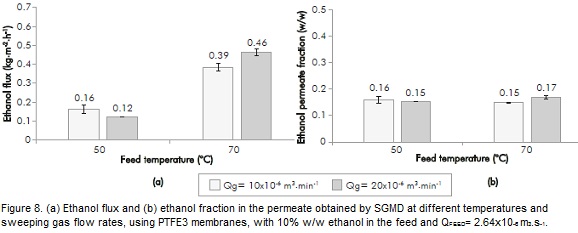
Figure 8 presents the results obtained for PTFE3 membrane under the same operating conditions. This figure shows that a temperature increment produces, as in the PTFE2 membrane, an increment in the ethanol permeate flux (144 - 283%). Likewise, the influence of sweeping gas at 70°C is observed, although in both cases results are significantly lower than for PTFE2. Neither the sweeping gas, nor the temperature showed a significant influence on the ethanol concentration of the permeate (unlike those obtained with the PTFE2 membrane). These differences can be attributed to both the pore diameter and porosity being greater for the PTFE3 membrane, which facilitates the passage of water through the membrane. As found with the VMD technique, it is possible to observe how the membrane characteristics determine the membrane separation process, inducing different behaviors for all tested membranes.
Performance of Membrane Distillation process with SGMD using Fermented Broths
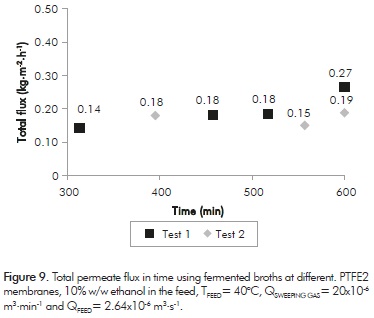
Figure 9 shows the results obtained for the total permeate flux versus time. All tests lasted 10 h. This figure shows that the total flux remains constant in time. This indicates no fouling phenomenon occurs during the experimentation time. Concerning ethanol concentration in the permeate, it was 22% w/w, reaching a concentration factor of 2.2; which represents a 22.7% increment compared to those obtained with prepared solutions.
These results suggest that working under the SGMD configuration would significantly decrease membrane fouling. It is important to remember that this aspect was the main VMD limitation process.
Comparison between the studied Membrane Distillation Techniques (VMD and SGMD)
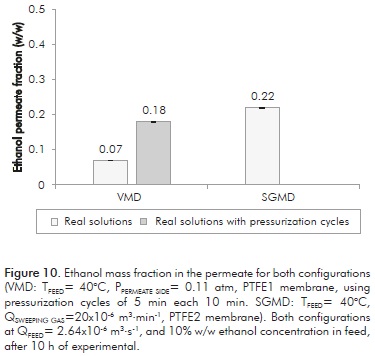
Figure 10 shows ethanol fractions of the permeate obtained by both techniques using fermented broths with an ethanol concentration of 10% w/w at a feed temperature of 40°C. It must be remembered that with VMD technique, permeate ethanol fraction is influenced by fouling phenomena generated by the biomass in the solution and vacuum pressure, which causes a drop from 18 to 7% w/w. For SGMD configuration, ethanol fraction does not significantly change when using fermented broths (maintained at 22% w/w). The system under SGMD configuration does not evidence biomass significant effect on the process, and therefore, on the ethanol fraction in the permeate.
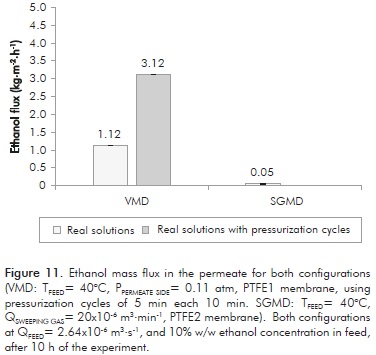
Figure 11 shows ethanol flux values in the permeate obtained through both techniques using fermented broths with ethanol concentration of 10% w/w and a feed temperature of 40°C. As seen on the figure, the VMD technique (with pressurization cycles) provides permeate flux higher than those obtained with SGMD (with similar ethanol permeate concentrations between the two techniques). But on a practical point of view, the possible membrane weakening when working with pressurization-depressurization cycles must be considered, as it reduces its useful life, leading to increased maintenance costs.
In the other hand, no fouling was observed with the SGMD configuration, at a flow rate of 2.64x10-6 m3·s-1. Although permeate flux obtained with this technique is inferior to VMD, productivity can be improved by increasing membrane surface. It is worth noting that regarding to the ethanol rate separation from the fermented broth, it is important to remove this compound at the same rate it was produced (preventing its accumulation into the system).
4. CONCLUSION
This work allowed us to compare for the first time two techniques of great potential for the ethanol continuous separation produced by fermentation. It was established that the VMD technique can be performed under moderate vacuum conditions and temperatures (0.11 to 0.3 atm and 30-50°C). Average ethanol flux values of 10.58 kg·m-2·h-1 were obtained on the permeate, with concentration factors higher than 2.5 with respect to feed concentration. Moreover, a significant influence of biomass on membrane fouling was observed by using fermented broths. This impact decreased with the implementation of pressurization cycles during VMD. With regards to SGMD, this technique shows the capacity to separate ethanol solutions (10% w/w) with a permeate ethanol flux up to 0.56 kg·m-2·h-1, with concentration factors higher than 1.5 (for feeding temperatures between 50 and 70°C and sweeping gas flow rate between 10 and 20x10-6 m3·min-1). Finally, during separation of ethanol from fermented broths by SGMD, it was found that membrane fouling can be significantly diminished when feed rate is adjusted at appropriate levels; aspect that would give a technical advantage to it regarding the VMD configuration.
ACKNOWLEDGEMENTS
The authors thank the Vicerrectoría de Investigación y Extensión of Universidad Industrial de Santander for supporting this study (VIE5444 codes).
REFERENCES
Alkhudhiri, A., Darwish, N. & Hilal, N. (2012). Membrane distillation: A comprehensive review. Desalination, 287: 2-18. [ Links ]
Baker, R. W. (2004). Membrane technology and applications. Menlo Park: WILEY, 2nd edition. [ Links ]
Bowen, T. C., Noble, R. D. & Falconer, J. L. (2004). Fundamentals and applications of pervaporation through zeolite membranes. J. Membr. Sci., 245(1-2), 1-33. [ Links ]
Cerneaux, S., Strużyńska, I., Kujawski, W. M., Persin, M. & Larbot, A. (2009). Comparison of various membrane distillation methods for desalination using hydrophobic ceramic membranes. J. Membr. Sci., 337(1-2), 55-60. [ Links ]
Chapman, P., Oliveira, T., Livingston, A. G. & Li, K. (2008). Review: Membranes for the dehydration of solvents by pervaporation. J. Membr. Sci., 318(1-2), 5-37. [ Links ]
Cojocaru, C. & Khayet, M. (2011). Sweeping gas membrane distillation of sucrose aqueous solutions: Response surface modeling and optimization. Sep. Purif. Technol., 81(1), 12-24. [ Links ]
Hwang, H. J., He, K., Gray, S., Zhang, J. & Moon, I. S. (2011). Direct Contact Membrane Distillation (DCMD): Experiment study on the commercial PTFE membrane and modeling. J. Membr. Sci., 371(1-2), 90-98. [ Links ]
Izquierdo-Gil, M. A. & Jonsson, G. (2003). Factors affecting flux and ethanol separation performance in Vacuum Membrane Distillation (VMD). J. Membr. Sci., 214(1), 113-130. [ Links ]
Jaramillo, O., Gómez, M. & Fontalvo, J. (2012). Remoción de los inhibidores de la fermentación etanólica usando membranas de polidimetilsiloxano (PDMS) por pervaporación. Rev. Ion, 25(1), 51-59. [ Links ]
Khayet, M., Godino, P. & Mengual, J. I. (2000). Theory and experiments on sweeping gas membrane distillation. J. Membr. Sci., 165(2), 261-272. [ Links ]
Khayet, M., Mengual, J. I. & Matsuura, T. (2005). Porous hydrophobic/hydrophilic composite membranes: Application in desalination using direct contact membrane distillation. J. Membr. Sci., 252(1-2), 101-113. [ Links ]
Lee, C. H. & Hong, W. H. (2001). Effect of operating variables on the flux and selectivity in sweep gas membrane distillation for dilute aqueous isopropanol. J. Membr. Sci., 188(1), 79-86. [ Links ]
Lewandowicz, G., Bialas, W., Marczewski, B. & Szymanowska, D. (2011). Application of membrane distillation for ethanol recovery during fuel ethanol production. J. Membr. Sci., 375(1-2), 212-219. [ Links ]
Lin, Y. & Tanaka, S. (2006). Ethanol fermentation from biomass resources: Current state and prospects. Appl. Microbiol. Biotechnol., 69(6), 627-642. [ Links ]
Qiu, W., Kosuri, M., Zhou, F. & Koros, W. J. (2009). Dehydration of ethanol- water mixtures using asymmetric hollow fiber membranes from commercial polyimides. J. Membr. Sci., 327(1-2), 96-103. [ Links ]
Rivier, C. A., García-Payo, M. C., Marison, I. W. & Von Stockar, U. (2002). Separation of binary mixtures by thermostatic sweeping gas membrane distillation: I. Theory and simulations. J. Membr. Sci., 201(1-2), 1-16. [ Links ]
Stanley, D., Bandara, A., Fraser, S., Chambers, P. J. & Stanley, G. A. (2010). The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae. J. Appl. Microbiol., 109(1), 13-24. [ Links ]
Xie, Z., Duong, T., Hoang, M., Nguyen, C. & Bolto, B. (2009). Ammonia removal by sweep gas membrane distillation. Water Research, 43(6), 1693-1699. [ Links ]
Zsirai, T., Buzatu, P., Aerts, P. & Judd, S. (2012). Efficacy of relaxation, backflushing, chemical cleaning and clogging removal for an immersed hollow fiber membrane bioreactor. Water Research, 46(14), 4499-4507. [ Links ]
AUTHORS
Ricardo-Javier Cotamo-De la Espriella
Affiliation: Universidad Industrial de Santander
Chemical Engineer, Universidad Industrial de Santander
e-mail: uis.rijacodle@gmail.com
Fredy-Wsbaldo Barón-Núñez
Affiliation: Universidad Industrial de Santander
Chemical Engineer, Universidad Industrial de Santander
e-mail: fredy.wsbaldo.b@gmail.com
Carlos-Jesús Muvdi-Nova
Affiliation: Universidad Industrial de Santander
Chemical Engineer, Universidad Industrial de Santander
M. Sc. in Process Engineering, Université de Montpellier II
Ph. D. in Process Engineering, Université de Montpellier II
e-mail: cjmuvdi@uis.edu.co