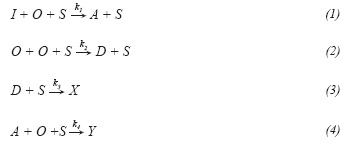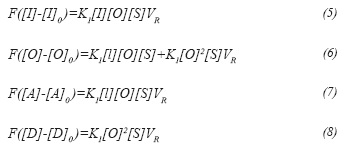Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383
C.T.F Cienc. Tecnol. Futuro vol.6 no.3 Bucaramanga Jan./June 2016
PRELIMINAR CONCEPT OF A REACTION SYSTEM FOR ALKYLATION OF ISOBUTANE ON A SOLID CATALYST
CONCEPTUALIZACIÓN PRELIMINAR DE UN SISTEMA DE REACCIÓN PARA ALQUILACIÓN DE ISOBUTANO USANDO UN CATALIZADOR SÓLIDO
CONCEPTUALIZAÇÃO PRELIMINAR DE UM SISTEMA DE REAÇÃO PARA ALQUILACÃO DE ISOBUTANO USANDO UM CATALIZADOR SÓLIDO
Hernando Salgado1*
1 Ecopetrol S.A. - Department of Process Engineering, Cartagena Refinery. Vía Mamonal KmlO, Cartagena, Colombia
e-mail: Hemando.Salgado@ecopetrol.com.co
How to cite: Salgado, H.,(2016). Preliminar Concept of a Reaction System for Alkylation of Isobutane on a Solid Catalyst. CT&F - Ciencia, Tecnología y Futuro, 6(3), 91 -104
* To whom correspondence should be addressed
(Received: Mar. 10, 2015; Accepted: May 31, 2016)
ABSTRACT
In oil refineries alkylation of isobutane with olefins, especially buthylenes, is carried out to produce alkylate, a high-octane component used in the preparation of premium gasolines. Alkylate production is mainly based on processes where the catalyst is a strong liquid acid, such as hydrofluoric or sulfuric acid, which might have potential impacts on process safety and environment. Therefore, a solid catalyst would be ideal to avoid the use of highly toxic and corrosive liquid acids, as well as to facilitate the separation steps, since the formation of strong hydrocarbon-acid emulsions is avoided.
Based on the state-of-the-art, simulations, and the application of a structured methodology for selecting reaction systems, in this study, a reaction system concept for alkylation of isobutane using a solid catalyst has been designed. The proposed reaction set up considers a combination of a structured catalyst in a staged CSTR-like configuration which simplifies the process, while maintaining selectivity to alkylate and product octane when compared to conventional alkylation processes. According to the literature consulted, zeolite β was found as the best alternative for an active phase catalyst that can replace liquid acids.
Keywords: Process Design, Solid Acid, Structured Catalyst, Refining Processes.
RESUMEN
En las refinerías de petróleo, la alquilación de isobutano con olefinas ligeras, principalmente butilenos, es usada para la producción de alquilato, un componente de alto octanaje usado en la preparación de gasolinas extras. La producción de alquilato se basa principalmente en procesos donde el catalizador es un ácido líquido fuerte, como el ácido fluorhídrico o el ácido sulfúrico, los cuales pueden tener impactos potenciales en la seguridad del proceso y el medio ambiente. Por lo tanto, un catalizador sólido sería ideal para evitarei uso de ácidos líquidos altamente tóxicos y corrosivos, y para facilitar adicionalmente las etapas de separación, dado que se evita la formación de emulsiones fuertes hidrocarburo-ácido.
Con base en una revisión del estado del arte, simulaciones y la aplicación de una metodología estructurada de selección de sistemas de reacción, en este trabajo se diseñó un concepto de un sistema de reacción para la alquilación de isobutano usando un catalizador sólido. El sistema de reacción propuesto considera la combinación de un catalizador estructurado en una configuración pseudo-CSTR en etapas, simplificando el proceso al tiempo que se mantiene la selectividad a alquilato y el octanaje del producto en comparación con los procesos de alquilación convencionales. De acuerdo con la literatura consultada se encontró que la zeolita β es la mejor alternativa como fase activa para un catalizador sólido que pueda remplazar a los ácidos líquidos.
Palabras clave: Diseño de Procesos, Ácido Sólido, Selección de Reactores, Catalizador Estructurado, Procesos de Refinación.
RESUMO
Nas refinarias de petróleo, a alquilação de isobutano com oleofinas leves, principalmente butilenos, é usada para a produção de alquilato, um componente de alta octanagem utilizado no preparo de gasolinas premium. A produção de alquilato está baseada principalmente em processos onde o catalizador é um ácido líquido forte, como o ácido fluorídrico ou o ácido sulfúrico, os quais podem causar impactos potenciais na segurança do processo e do meio ambiente. Portanto, um catalizador sólido seria ideal para evitar o uso de ácidos líquidos altamente tóxicos e corrosivos, além de facilitar as etapas de separação, evitando a formação de emulsões fortes hidrocarboneto-ácido.
Com base na revisão do estado da arte e simulações e através da aplicação de uma metodologia estruturada de seleção de sistemas de reação, neste trabalho foi desenhado um conceito de um sistema de reação para a alquilação de isobutano usando um catalizador sólido. O sistema de reação proposto considera a combinação de um catalizador estruturado em uma configuração pseudo-CSTR por etapas, simplificando o processo enquanto se mantém a seletividade ao alquilato e a octanagem do produto quando comparado com os processos de alquilação convencionais. Segundo a literatura consultada, verificou-se que a zeolita β é a melhor alternativa como fase ativa para um catalizador sólido que possa substituir os ácidos líquidos.
Palavras-chave: Design de Processos, Acido Sólido, Seleção de Reatores, Catalizador Estruturado, Processos de Refino.
1. INTRODUCTION
Alkylation is a conversion process based on the reaction of olefins (propylene, butylenes and amylenes) with isobutane in presence of an acid catalyst to produce high octane isoparaffins in the range of C7-C9, such as isooctane (2,2,4-trimethylpentane, TMP, with a Research Octane Number, RON, of 100); the end product of the process is a mixture of this isoparaffins called alkylate, which is used in oil refineries as a blending component in the gasoline pool.
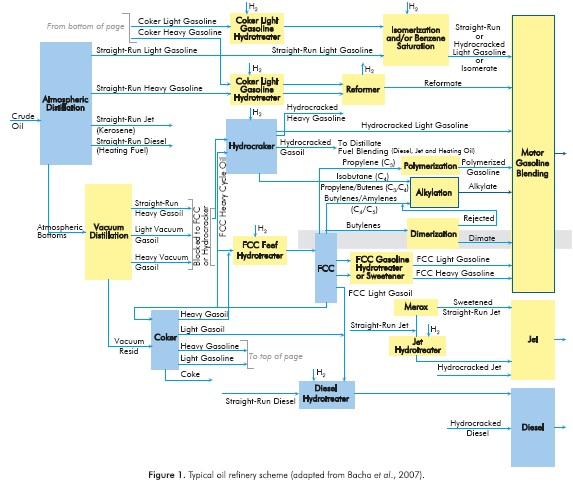
Figure 1 shows the location of an isobutane alkylation unit in a typical oil refinery. Typically, alkylation units use olefins, especially butylenes, coming from the FCC process as a feedstock:
As the reduction in the content of olefins, aromatics, and sulfur and nitrogen compounds is the current trend in gasoline formulation, and considering that alkylate is one of the few high-octane blending components that meets all these requirements, the alkylation process will continue playing an important role in the gasoline production within an oil refinery (Hommeltoft, 2001).
In the refining industry the alkylate production is based mainly on liquid acid processes, among which two acids have been used successfully: hydrofluoric (HF) and sulfuric acid (H2S04). On the other hand, several processes based on solid acid catalysts have been developed, but with no commercial applications so far (Albright, 2009; Akpabio & Neeka, 2013).
Since one of the main concerns are the safety and environmental problems related to liquid acid processes, the main aim of this study is to design a process concept, where the use of a solid acid is evaluated at conceptual level to produce alkylate. Besides safety and environmental concerns, other disadvantages linked to the conventional processes have been identified (Meyers, 2004):
- Equipment corrosion problems and high demand for sophisticated materials of construction.
- Acid separation and regeneration in different pieces of equipment required.
- Problems related with emulsion stability.
In the case of a solid acid process, rapid passivation of the catalyst can occur, which would require continuous regeneration of the catalyst (Hommeltoft, Ekelund & Zavilla, 1997).
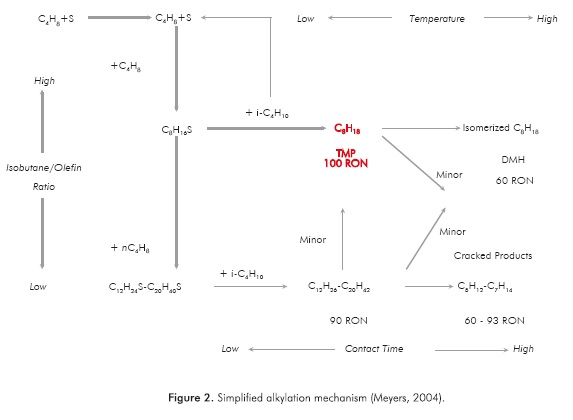
Regardless of the kind of acid catalyst used, the alkylation reactions mechanism follows the same pattern. Figure 2 shows a simplified mechanism, as well as the influence of the main variables. In this figure, S stands for a Brensted acid site.
As it can be observed in Figure 2, the formation of alkylate is favored at a high isobutane/olefin ratio, low temperature and short contact time. Moreover, since deactivation of the catalysis is one of the main concerns with solid acid catalyst, it would be desirable to operate under conditions that allow to reduce this problem. According to Hommeltoft et al. (1997), the optimal conditions for minimizing the deactivation would be achieved by using a catalyst with high acid site density, and at the same time by performing the alkylation reactions under low olefin concentration everywhere around the catalyst, in order to avoid olefin oligomerization.
Based on the weaknesses of liquid catalyst alkylation, and the considerations described above, a list of desired improvements and process needs was formulated, as follows:
- Avoid the use of a liquid acid as a catalyst.
- Minimize oligomerization reactions.
- Assure a high acid site density.
- Assure a short contact time of the olefins with the catalyst (operation under kinetic control is preferred).
To select the most appropriate reactor configuration and operation, the approach developed by Krishna and Sie (1994) was used. This approach consists in selecting independently the best configuration at three different levels: catalyst design, injection/dispersion strategy, and hydrodynamic operation. On the other hand, reaction kinetics and mechanisms reported in literature (de Jong et al, 1996) were simulated in MatLab™ to obtain the process parameters related to reactor sizing and selectivity.
In addition, the process needs listed above, together with design and operational aspects such as materials of construction, pressure drop and catalyst handling, were also taken into account by means of a criteria matrix, where every process need was scored. Based on the above, the final reactor concept is intended to fulfill the weaknesses of conventional alkylation processes in terms of safety, design and operation, without affecting selectivity and product quality.
It must be noted that the scope of this study is limited to a theoretical approach based on literature, therefore issues related to the hydrodynamics of the reaction system and catalyst manufacturing were not addressed in detail.
2. KINETICS AND MODELLING OF ALKYLATION REACTIONS
To model the kinetic of the alkylation reactions, the model proposed by de Jong et al. (1996) was used. This model considers the following set of reactions:
According to experimental results reported in literature (Zuazo, 2004; van Broekhoven, Hendrikus, Klaver and Nieman, 2012), influence of catalyst deactivation in conversion and selectivity to alkylate is negligible at least during the first 20 hours of operation; therefore assuming operation before catalyst gets deactivated, deactivation was not considered in this model, so reactions described by Equations 3 and 4 are not taken into consideration. In this way, according to the de Jong's model and assuming steady state, the reaction rate expression for each component is given as a function of ki and k2 as follows:
3. METHODS AND PROCEDURE
As stated before, the reaction system selection approach developed by Krishna and Sie (1994) was used as a first instance to obtain the most promissory reaction systems, taking into account different levels from catalyst design to reactor operation. Following, the Krishna and Sie considerations for reaction system selection:
Level I: Catalyst design
- Effect of particle size
- Geometry of catalyst particles
- Pressure drop, reactor productivity and strength of catalyst particles
- Effect of diffusion limitations on selectivity
- Spatial distribution of activity within catalyst particles
- Intraparticle heat effects
Level II: Injection and dispersion strategies
- Injection of reactants
- Degree of mixing
- Removal of energy in situ
Level III: Hydrodynamics and flow regime
- Phase or phases in which reactions take place
- Flow regime
Thereafter, the selection of the most appropriate reaction set up is completed by considering the fulfilment of design, construction and operation needs in a criteria matrix, where every promissory reaction system is evaluated and compared to each other in a relative way. The criteria used in the proposed evaluation matrix are given as follows:
- Scaling up
- Mass and heat transfer
- Catalyst surface area
- Pressure drop
- Mixing
- Catalyst attrition
- Catalyst separation
- Catalyst manufacture
- Catalyst regeneration
- Catalyst addition
- Design and construction
- Operation
Finally, the selected reaction set up is compared to the conventional alkylation processes, in order to determine whether the weaknesses of conventional processes are overcome or not with the proposed concept.
4. RESULTS
After applying the Krishna and Sie approach for reaction systems selection, the following results were found:
Catalyst design
In order to minimize olefin oligomerization, a small particle size is preferred to reduce mass transfer limitations and establish kinetic control of the reaction. This condition is given for a high catalyst effectiveness, which is reached when the Thiele modulus (Φ) is around 1 or lower. As observed in Equation 9 (adapted from Fogler, 2004), a small value of the generalized second order Thiele modulus is obtained with a high surface area to volume ratio.
It must be noted that Equation 9 is shown just as an indication of the relationship between the surface area to volume ratio and the catalyst effectiveness; however, experimental data with actual catalyst must be performed to determine these parameters.
Even though a high surface to volume ratio is obtained by using rings and hollow extrudates, due to the fact that alkylation in liquid phase is preferred over gas phase (Simpson, Wei & Sundaresan, 1996), the hollow space of these catalyst shapes are not desirable for alkylation reactions, since they would be filled with relatively stagnant liquid, promoting oligomerization reactions.
Therefore, the recommended geometries to be used is solid shapes, such as trilobes or quadrilobes in the case of a fixed bed operation, or spheres for slurry or mobbing bed operations. However, extrudates of more sophisticated shapes are less strong, and in case of a fixed bed reactor, pressure drop would be an issue if particles are under attrition. This would be an important limitation, especially if it is considered that having a small Thiele modulus implies the use of a relatively small particle size.
The main problems associated with this kind of catalyst particle would be the high pressure drop if a fixed bed operation is selected, or dealing with an additional solid-liquid separation step to recover the catalyst when working with moving beds or slurry reactors.
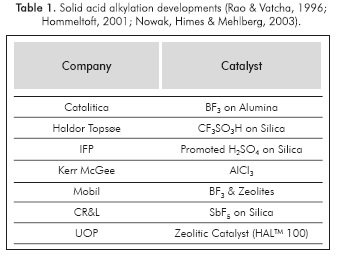
These difficulties are overcome with the application of a structured packing, such as monoliths or beads-on-a-string reactors (BSR). Regarding the catalytic material that can be used in this process concept, several options have been tested in previous studies. Table 1 shows some of the solid acid catalysts that have been tested.
It must be noted that an important characteristic on the catalysts is the acid site density. Other remark is that hydride transfer is the step that determines the product quality and the catalyst lifetime. Several studies concluded that very strong acid sites are necessary to effectively catalyze hydride transfer (Feller, 2003).
Brønsted acid sites catalyze the alkylation reaction, while Lewis acid sites promote the formation of unsaturated compounds (Diaz-Mendoza, Pernett-Bolano & Cardona-Martinez, 1998). In the case of zeolites, studies on ultrastable Y and β zeolites have shown that catalyst performance improves as the ratio of stronger to weaker acid sites increases
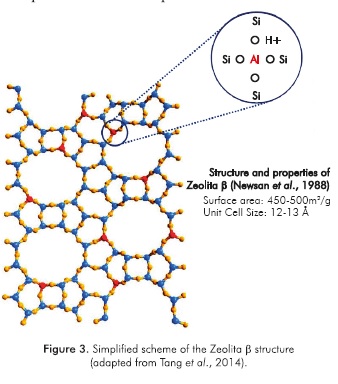
A high concentration of strong acid sites is achieved by reducing the Si/Al ratio (Corma, Martinez & Martinez, 1994; Shanjiao etal., 2007), where the lowest reported value for this variable has been found to be 6 (Yoo & Smirniotis, 2002). On the other hand, zeolite β has been found as one of the catalyst with better experimental results (de Jong et al, 1996), which can be explained based on its open framework structure and high concentration of Bronsted acid sites in comparison with other types of zeolites. A simplified scheme of the zeolite p structure is shown in Figure 3.
Injection and dispersion strategies
According to the mechanism given in Figure 2, formation of dimmers and oligomers is a function of olefins concentration; those are undesired products due to their low octane number and tendency to deactivate the catalyst (de Jong et al., 1996; Hommeltoft et al., 1997). In order to minimize oligomers formation a staged olefins injection is proposed, in this way a high isobutane/olefins ratio is kept and a fast consumption of olefins can be achieved.
On the other hand, given that a low concentration of olefins is preferred for obtaining a high alkylate selectivity and low catalyst deactivation (Taylor & Sherwood Jr., 1997), a well mixing CSTR-like system, where the concentration of olefins is expected to be as low as possible, would be a better option versus a plug flow system (Feller, Guzman, Zuazo & Lercher, 2004).
Regarding heat transfer, alkylation is an exothermic reaction; therefore, in an adiabatic operation mode an increase in temperature is expected. A staged olefins injection also helps to maintain a proper temperature profile, avoiding temperatures run away and low octane product.
In addition, some evaporation of isobutane is expected in an adiabatic operation; nevertheless, this evaporation will help at the same time to minimize the increase of temperature.
Hydrodynamics flow regime and reactor selection
As mentioned, selectivity of alkylate is favored by the reaction in liquid phase; therefore, reactants and products must be in liquid state. On the other hand, considering previous studies by Querini (2000) and Delia Costa & Querini (2010), as well as the book of Matlack (2010), the presence of hydrogen may be helpful to promote a high alkylate selectivity, as well as in preventing quick deactivation of acid sites in the catalyst by formation of oligomers. Considering the foregoing, the chosen reactor concept must be suitable for multiphase operation.
Selection of the reactor concept
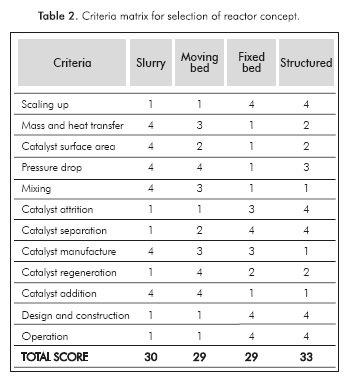
In this study, according to the requirements previously discussed, 4 families of reactors capable to operate in multiphase regime were considered as promissory: Slurry Reactor, Moving Bed Reactor, Fixed Bed Reactor and Structured Reactor. Table 2 summarizes the matrix of criteria used in the reactor concept selection. The number represents the relative suitability of each alternative, where a grade of 4 is the most suitable for a given criterion in comparison with the others.
From Table 2, it can be observed that a structured reactor, without having the best mass and heat transfer performance, gives a trade-off among all the process, design, construction and operation needs. On the other hand, it has to be noted that slurry reactor configuration offers the best alternative for mass and heat transfer, catalyst surface area and mixing; however, such a configuration does not overcome problems such as catalyst regeneration, and must deal with a difficult catalyst separation step.
5. CALCULATIONS AND DISCUSSION
Based on results obtained from Table 2, but keeping in mind the need to overcome mass transfer limitations, an option that combines the advantages of the structured and slurry reactor is presented in this section.
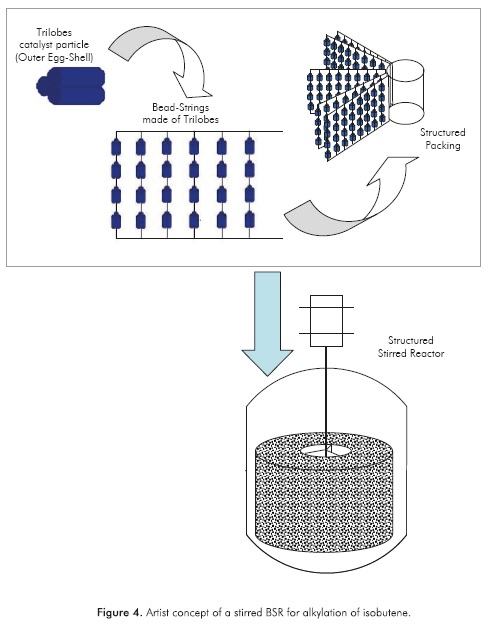
The proposed structured reactor is a beads-on-a-string reactor (BSR), which consists of a dense arrange of multiple beads-on-a-strings, which comprise a kind of packing. A BSR is characterized by conventional particulate catalyst that is fixed on parallel strings or rods, where the catalytic material may consist of commercially available extrudates that are mechanically stringed on wires or stacked on rods (Calis et al, 1998).
As explained in the previous section, the beads are proposed to be trilobes catalyst particles of the smallest practical size, with a uniform distribution of the active sites, since the active phase is a zeolite. The BSR concept was tested for catalytic reduction of NOX by Calis (1995), where a catalyst effectiveness between 0.550.85 was reported for the used extrudates; therefore, an average catalyst effectiveness of 0.7 was considered for the present study.
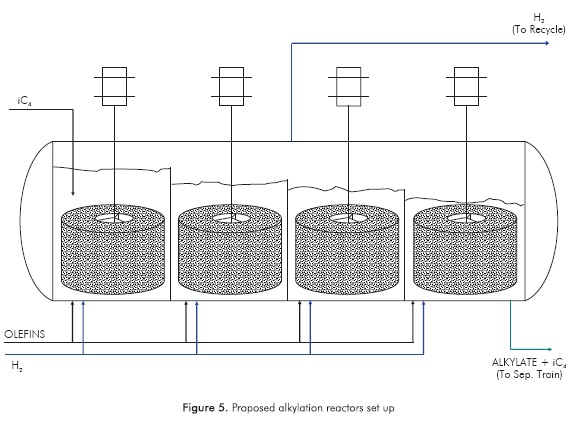
In addition, this BSR is proposed to be used as a mixer in the way of a rotating bed, resembling the set up proposed by Edvinsson-Albers et al (1998), where a catalytic monolith was used as a stirrer at the same time. From the hydrodynamic point of view, the BSR has been proposed as a suitable alternative for multiphase reactions (Calis et al, 1998); however, a detailed analysis of the reactor system hydrodynamics is out of the scope of this study. An illustration of the different scales of the proposed reactor concept is depicted in Figure 4.
A set-up consisting in four alkylation reactors in series (see Figure 5), where the olefins are injected in a staged way reactor by reactor, where the product from each reactor goes to the next by gravity, is proposed. The number of four reactors in series is selected based on an olefins conversion of 99%, according to the alkylation reactions model developed in MatLab™, which was based on the kinetic model described in Section 2. It must be noted that for this model hydrogen is not considered as a reactant, since it is only acting as an agent to prevent dimers and oligomers formation.
Since the expected operation time of the catalyst is at least 20 hours (Zuazo, 2004; van Broekhoven et al. 2012), another identical set of reactors must be placed for a switching operation, while one set is in operation, the other one is in catalyst regeneration. The proposed method to regenerate the catalyst is then to put the catalyst in contact with hydrogen without the presence of hydrocarbons (Querini, 2000; della Costa & Querini, 2010; Matlack, 2010), which is a typical method used in refinery operations to strip out heavy hydrocarbon compounds such as the oligomers that are expected to be formed on the catalyst.
In this aspect, a further research most be conducted to estimate time and process conditions for catalyst regeneration. Nevertheless, it must be noted that some partial pressure of hydrogen is given in the set of reactor in operation, with the purpose of preventing the formation of these oligomers and make the catalyst operation time long enough.
In order to compare the performance of the proposed alkylation reactor with conventional alkylation processes (benchmark), the MatLab™ reaction model previously described was used, which allowed to obtain the data for the expected performance of the proposed concept to produce a given amount of alkylate.
On the other hand, benchmark data was obtained by means of scaling data from actual conventional alkylation plants, considering the same amount of alkylate to be produced as in the proposed concept model. Catalyst regeneration and materials of construction were also considered as a benchmark issue.
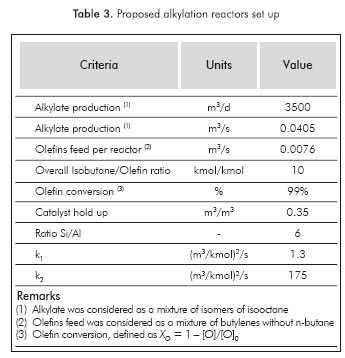
The input data and kinetic parameters used to solve the proposed concept model are given in Table 3.
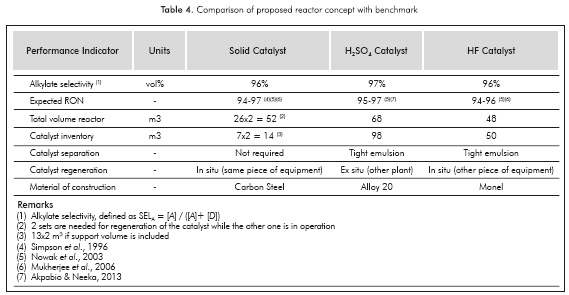
Based on data in Table 3 and model solutions, the results for a set of 4 reactors and the comparison with the selected benchmarks are presented in Table 4:
6. CONCLUSIONS AND NEEDS FOR FURTHER RESEARCH
- A reactor concept for isobutane alkylation was developed, overcoming safety and environmental problems related to the handling of very aggressive liquid acids, such as sulfuric or hydrofluoric acid. In this case, a zeolitic solid acid catalyst was proposed to replace the conventional liquid acid catalysts. According to literature, zeolite β was found to be the catalyst with better experimental results.
- Catalysts with high concentration of Bransted acid sites are more suitable for alkylation of isobutane. For zeolites (including zeolite β), it was found that concentration of Bronsted sites can be increased by lowering the Si/Al ratio. According to the above, a Si/Al ratio of 6 was used in the calculations, based on literature.
- By applying the reaction system selection procedure by Krishna and Sie, a structured catalyst in a CSTR-like configuration was designed, integrating the advantages of both approaches. In this concept, a structured packing made with bead-strings of trilobes is proposed (BSR), which is used as stirrer at the same time. Further experimentation with a lab-scale set-up of this concept is recommended to evaluate the hydrodynamic behavior and mechanical catalyst strength under a rotating regime.
- Two sets of four reactors in series were suggested, so that one set is in operation, while the other is in regeneration, expecting cycles of 20 hours. A staged injection of olefins is proposed to maximize alkylate selectivity and yield over dimmers and oligomers, this strategy increases the isobutane/olefins ratio in every reaction step.
- Further research and experimentation is also recommended to get experimental data to validate the results obtained in this theoretical study, but also to get data related with the operational aspects, such as time and process conditions for catalyst regeneration. Other aspect that must be evaluated is the catalyst manufacturing cost and difficulty in relation with the catalyst shape and bead-strings construction.
ACKNOWLEDGEMENTS
The author wishes to acknowledge Dariana Hernandez from UOP for her contribution to obtain the results of this study.
REFERENCES
Akpabio, E. J. & Neeka, J. B. (2013). Review of petroleum refinery acid catalyzed alkylation processes: a message to Nigerian refineries. Petroleum Tech. Develop. J., 3 (1), 84-97. [ Links ]
Albright, L. F. (2009). Present and future of alkylation processes in refineries. Ind. Eng. Chem. Res., 48: 1409-1413. [ Links ]
Bacha, J. et al. (2007). Diesel Fuels Technical Review. San Ramon, USA: Chevron Products Co. van Broekhoven, E. H., Hendrikus, M., Klaver, G. & Nieman, J. (2012). Alkylation process using a catalyst comprising rare earth containing zeolites and a hydrogenation metal. US Patent No. 8,163,969-B2. [ Links ]
Calis, H. P. (1995). Development of dustproof, low pressure drop reactor with structured catalyst packings: the bead string reactor and the zeolite-covered screen reactor. Ph. D. Thesis, Technische Universiteit Delft, the Netherlands. [ Links ]
Calis, H. P., Takacs, K., Gerritsen, A. W. & van den Bleek, C. M. (1998). Bead-String Reactor in: Cybulski, A. & Moulijn, J. A. Structured catalysts and reactors. New York: Marcel Dekker. [ Links ]
Corma, A., Martinez, A. & Martinez, C. (1994). Isobutane/2-butene alkylation on ultrastable Y-zeolites: Influence of zeolite unit cell size. J. of Cat., 146: 185-192. [ Links ]
Deila Costa, B. O. & Querini, CA. (2010). Isobutane alkylation with solid catalysts based on beta zeolite. Ap. Cat. A: Gen., 385: 144-152. [ Links ]
Diaz-Mendoza, F. A., Pernett-Bolano, L. & Cardona-Martinez, N. (1998). Effect of catalyst deactivation on the acid properties of zeolites used for isobutane/butene alkylation. Thermochim. Acta, 312: 47-61. [ Links ]
Edvinsson-Albers, R. K., Houterman, M. J. J., Vergunst, T., Grolman, E. & Moulijn, J. A. (1998). Novel Monolithic Stirred Reactor. AIChEJ., 44 (11): 2459-2464. [ Links ]
Feller, A. (2003). Reaction mechanism and deactivation pathways in zeolite catalyzed isobutane/2-butene alkylation. Ph. D. Thesis, Technischen Universität München, Germany. [ Links ]
Feller, A., Guzman, A., Zuazo, I. & Lercher, J. A. (2004). On the mechanism of catalyzed isobutane/butene alkylation by zeolites. J. of Cat. 224:80-93. [ Links ]
Fogler, H. C. (2004). Elements of Chemical Reaction Engineering, 3rd ed. Upper Saddle River, USA: Prentice Hall. [ Links ]
Hommeltoft, S. I., Ekelund, O. & Zavilla, J. (1997). Role of ester intermediates isobutane alkylation and its consequence for the choice of catalyst system. Ind. Eng. Chem. Res., 36: 3491-3497. [ Links ]
Hommeltoft, S. I. (2001). Isobutane alkylation: Recent developments and future perspectives. Appl. Cat. A: General, 221:421-428. [ Links ]
De Jong, K. P. et al. (1996). Paraffin alkylation using zeolite catalysts in a slurry reactor: Chemical engineering principles to extend catalyst life-time. Chem. Eng. Sei., 51:2053-2060. [ Links ]
Krishna, R. S. & Sie, T. (1994). Strategies for multiphase reactor selection. Chem. Eng. Sei., 49: 4029-4065. [ Links ]
Matlack, A. S. (2010). Introduction to Green Chemistry, 2nd ed. Boca Raton, USA: CRC. [ Links ]
Meyers, R. A. (2004). Handbook of Petroleum Refining Processes, 3rd ed. New York, USA: McGraw Hill. [ Links ]
Mukherjee, M., Nehlsen, J., Sunderesan, S., Susiu, G. D. & Dixon, J. (2006). Scale-up strategy applied to solid-acid alkylation process. Oil & Gas J., 104 (26) 48-54. [ Links ]
Newsam, J.M., Treacy, M.M.J., Koetsier, W.T. & de Gruyter, C.B. (1988). Structural characterization of zeolite beta. Proc. R. Soc. Lond. A, 420: 375-405. [ Links ]
Nowak, F. M., Hirnes, J. F. & Mehlberg, R. L. (2003). Advances in hydrofluoric (HF) acid catalyzed alkylation. NPRA Annual Meeting, San Antonio, USA. [ Links ]
Querini, CA. (2000). Isobutane/butene alkylation: regeneration of solid acid catalysts. Cat. Today, 62: 135-143. [ Links ]
Rao, P. & Vatcha, S. R. (1996). Solid-acid alkylation process development is at crucial stage. Oil & Gas J., 94 (37) 56-61. [ Links ]
Shanjiao, K., Yanjun, G., Tao, D., Ying, Z. & Yanying, Z. (2007). Preparation and characterization of zeolite beta with low Si02/A1203 ratio. Petroleum Sei., 4 (1) 70-74. [ Links ]
Simpson, M. F, Wei, J. & Sundaresan, S. (1996). Kinetic analysis of isobutane/butene alkylation over ultrastable H-Y zeolite. Ind. Chem. Eng. Res., 35: 3861-3873. [ Links ]
Tang, B., Dai, W., Sun, X., Guan, N, Li, L. & Hunger, M. (2014). Aprocedure for the preparation on Ti-Beta zeolites for catalytic epoxidation with hydrogen peroxide. Green Chem., 16: 2281-2291. [ Links ]
Taylor, R. J. & Sherwood Jr., D. E. (1997). Effects of process parameters on isobutane/2-butene alkylation using a solid acid catalyst. Appl. Cat. A: General, 155: 195-215. [ Links ]
Yoo, K. & Smirniotis, P. G. (2002). The influence of Si/Al ratios of synthesized zeolites for the alkylation of isobutane with 2-butene. Appl. Cat. A: General, 227: 171-179. [ Links ]
Zuazo, I. (2004). Deactivation routes in zeolite catalyzed isobutane/2-butene alkylation and regeneration procedures. Ph. D. Thesis, Technischen Universität München, Germany. [ Links ]
AUTHORS
Hernando Salgado
Affiliation: Ecopetrol, Cartagena Refinery, Department of Process Engineering
Chemical Engineering, Universidad Industrial de Santander
P. D. Eng, Process Design, Delft University of Technology
e-mail: Hernando.Salgado@ecopetrol.com.co