Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383
C.T.F Cienc. Tecnol. Futuro vol.6 no.3 Bucaramanga Jan./June 2016
EVALUATION OF THEOBROMA CACAO POD HUSK EXTRACTS AS CORROSION INHIBITOR FOR CARBON STEEL
EVALUACIÓN DE EXTRACTOS DE CÁSCARA DE CACAO (Theobroma Cacao L.) COMO INHIBIDORES DE CORROSIÓN EN ACERO AL CARBÓN
AVALIAÇÃO DE EXTRATOS DE CASCA DE CACA U (Theobroma Cacao L.) COMO INIBIDORES DE CORROSÃO EM AÇO-CARBONO
Daniel-Enrique Pedroza-Periñán1, Melissa-Andrea Villalobos-Vasquez1, Pedro-Javier Meza-Castellar2, and Isabel-Cristina Paz-Astudillo3*
1 Programa de Ingeniería Química, Facultad de Ingeniería, Universidad de Cartagena, Cartagena, Bolívar, Colombia
2 Programa de Ingeniería de Procesos, Facultad de Ingeniería, Fundación Universitaria Tecnológico Comfenalco, Cartagena, Bolívar, Colombia
3 Facultad de Ingeniería Agronómica, Universidad del Tolima, Ibagué, Tolima, Colombia
e-mail: icpaza@ut.edu.co.
How to cite: Pedroza-Periñán, D. E., Villalobos-Vásquez, M. A., Meza-Castellar, R J. & Paz-Astudillo, I. C. (2016). Evaluation of Theobroma Cacao pod husk extracts as corrosion inhibitor for carbon steel. CT&F - Ciencia, Tecnología y Futuro, 6(3), 147-156.
* To whom correspondence should be addressed
(Received: Jun. 19, 2015; Accepted: Mar. 15,2016)
ABSTRACT
Cacao pod husks were used as raw material to obtain anti-corrosive extracts. The extraction was performed by adding 50 g of grinded husk to 300 mL of solutions of deionized water and ethanol, varying solvent concentration from 0 to 50% v/v, temperature from 28 to 50°C and extraction time from 2 to 5 hours. Then, the extracts were filtered and submitted to characterization tests. The anticorrosive activity was studied by weight loss analysis on A36 steel plates in 1M hydrochloric acid with variations of extract concentration (0, 1, 3, 7 and 10% v/v). A maximum corrosion inhibition efficiency of 91.13% was achieved with an extract concentration of 10% v/v. This extract was obtained with 16.1% v/v ethanol, at 50°C and during 5 hours of extraction time. The results proved that cacao pod husk is a suitable raw material for the production of corrosion inhibitors. Phenols were the predominant components in this extract and mainly account for its anticorrosive activity. The adsorption mechanism of the inhibitor on the metal surface was studied by Langmuir isotherm analysis and the results suggested the occurrence of a physisorption.
Keywords: Cacao crop residue, Corrosion inhibitors, Weight loss method, Phenols, Langmuir isotherm.
RESUMEN
Cáscara de la vaina del cacao se utilizó como materia prima para obtener extractos anticorrosivos. La extracción se realizó adicionando 50 g de cáscara molida a 300 mL de soluciones de etanol en agua desionizada, variando la concentración de solvente entre 0 y 50 %v/v, la temperatura desde 28 hasta 50°C y el tiempo de extracción entre 2 y 5 horas. Luego, los extractos se filtraron y se sometieron a pruebas de caracterización. La actividad anticorrosiva sobre placas de acero A36 sumergidas en ácido clorhídrico 1 M se estudió mediante el método de pérdida de peso, variando la concentración del extracto (0, 1, 3, 7 y 10 %v/v). Se logró una máxima eficiencia de inhibición a la corrosión de 91,13 % con una concentración de extracto de 10 %v/v. Este extracto se obtuvo con 1 6.1 %v/v de etanol, a 50°C y durante 5 horas de tiempo de extracción. Los resultados demostraron que la cáscara de la vaina del cacao es una materia prima adecuada para la producción de inhibidores de corrosión. Los fenoles son los componentes predominantes en este extracto; razón por la cual, la actividad anticorrosiva se le atribuye principalmente a ellos. El mecanismo de adsorción del inhibidor sobre la superficie metálica se estudió mediante un análisis con isoterma de Langmuir, los resultados indicaron la ocurrencia de una fisisorción.
Palabras clave: Cascarilla de cacao, Inhibidores de corrosión, Método de pérdida de peso, Fenoles; Isoterma de Langmuir.
RESUMO
Acasca da semente do cacau foi utilizada como matéria-prima na obtenção de extratos anticorrosivos. A extração foi feita pela adição de 50 g de casca moída a 300 mL de soluções de etanol em água desionizada, variando a concentração de solvente entre 0 e 50% v/v, a temperatura de 28 até 50°C e o tempo de extração entre 2 e 5 horas. Logo depois, os extratos foram filtrados e submetidos a testes de caracterização. A atividade anticorrosivo sobre chapas de aço A36 submergidas em ácido clorídrico 1 M foi analizada mediante o método de perda de peso, variando a concentração do extrato (0, 1, 3, 7 e 10 %v/v). Assim foi atingida uma máxima eficiência de inibição à corrosão de 91,13% com uma concentração de extrato de 10 %v/v. Esse extrato foi obtido com 16.1 %v/v de etanol, a 50°C e durante 5 horas de tempo de extração. Os resultados demonstraram que a casca da semente do cacau é uma matéria-prima adequada para a produção de inibidores de corrosão. Os fenóis são os componentes predominantes neste extrato; é por isso que atividade anticorrosivo é atribuída principalmente a esse componente. O mecanismo de adsorção do inibidor sobre a superfície metálica foi estudado mediante uma análise com isoterma de Langmuir, os resultados indicaram a ocorrência de uma fisisorção.
Palavras-chave: Casca de cacau, Inibidores de corrosão, Método de perda de peso, Fenóis; Isoterma de Langmuir.
1. INTRODUCTION
Carbon steel is one of the most widely used construction materials in water distribution, power production and chemical industries due to its cost effectiveness and mechanical properties (Fouda et al., 2013). Nevertheless, it has a low corrosion resistance in acidic media, which represents a major drawback for its applications since many industrial processes use acid solutions, e.g.: pickling, cleaning, descaling, oil wet cleaning (Kalaiselvi et al., 2010). Therefore, corrosion inhibitors are used to prevent the degradation of this material,. This commonly results in tremendous economic savings regarding equipment maintenance and safe operating procedures (Mourya, Banerjee & Singh, 2014). However, conventional inhibitors like chromates and nitrates have proved to exert toxic effects on the environment and human health (Abdel-Gaber et al., 2011). Thus, attention is being redirected to develop eco-friendly inhibitors commonly obtained from plant extracts and other natural products (Oguzie et al, 2010). These materials have many benefits such as low cost, high availability, renewability, non-toxicity, and their phytochemical composition, which presents a number of compounds frequently related to anticorrosive activity (Vrsalovic, Kliskicand & Gudic, 2009). Among these substances, organic compounds containing N, O or S atoms, electronegative functional groups and Π electrons in triple or conjugated double bonds are of special interest. Specific inhibition mechanisms are related with the interaction of such compounds with the metal surface via an adsorption process (Ikpi et al., 2012).
Phenols, saponins, tannins, oils and fats are some of the substances reported as corrosion inhibitors on steel, aluminum, zinc and other metals (Rajalakshmi, Prithiba & Leelavathi, 2012). These compounds can be found in the chemical structure of many plants and other natural resources (Yoo et al., 2012). Current research deals with the use of plant extracts as a source of green corrosion inhibitors that could efficiently replace and eliminate the negative impact caused by conventional anticorrosive products (Faustin et al, 2015).
Many of the aforementioned compounds are present in the composition of cocoa (Theobroma Cacao) (Yapo et al., 2013). A study conducted by Umoru, Fawehinmi, and Fasasi (2006) about the inhibitive effect of extracts from cocoa (Theobroma Cacao) and kolanut (Cola Acuminata) leaves on the corrosion of mild steel showed that the extracts from these plants are potential inhibitors of mild steel corrosion in seawater and marine environment. The highest inhibition efficiency was obtained when the concentration of the inhibitors was increased up to the optimum level (4% of each of the extracts).
The pod husk is a byproduct of cocoa crop, commonly disposed to rot on the plantations, causing foul odors and the spread of crop diseases such as black pod rot (Vriesmann, Amboni & De Oliveira, 2011). Around 39 million tons of pod husk are produced annually (International Cocoa Organization, 2015), therefore disposal alternatives need to be implemented in order to avoid environmental damages.
With the purpose of offering solutions for agricultural and industrial sectors, the main objective of this work was to obtain extracts from Theobromona cacao pod husk of the Colombian Caribbean Region and assess their potential corrosion inhibition activity on A3 6 steel in an acidic medium. This study was also intended to identify the active substances with anticorrosion properties present in extracts; establish the best extraction conditions that guarantee an extract with the highest efficiency, and define the adsorption mechanism of the inhibitor over materials.
Although, the properties of Theobroma cacao peel extracts as corrosion inhibitor have been studied before, the experimental conditions and methodology were different from those used in this work. The inhibition and adsorption properties of Theobroma Cacao peel polar extract (TCPE) on corrosion inhibition efficiency of 0.3%C mild steel in 1.5 M HC1 solution for various exposure times, TCPE concentrations and operation temperatures were investigated by Yetri et al. (2014). Their results support the hypothesis of this work, which states that the addition of cacao peel extract in acidic media is effective to minimize corrosion effects on carbon steel. Similarly, the need to conduct more research on this topic also became evident in order to provide proper solutions for the industrial sector.
2. THEORY AND CALCULATIONS
Corrosion Inhibition Efficiency
Gravimetric calculations regarding the effectiveness of a corrosion inhibitor are based on the weight loss that the material undergoes when it comes in contact with the corrosive medium. A piece of the material is weighted before being exposed to degradation in an environment where no corrosion inhibitor has been added, and weight measurements are taken after a specified period of time. The same procedure is performed with samples containing different concentrations of the inhibitor. Then, Corrosion Inhibition Efficiency (CIE) is calculated by comparing the weight loss that was experienced by the protected materials with the one observed in the sample with no inhibitor (blank), as observed in Equation 1:
where ΔW0 and ΔWt are the weight losses underwent by the blank and the inhibited material, respectively (initial weight - final weight). It should be noted that this efficiency depends on a specific period of time which has to be clearly stated when reporting the result. Twenty-four hours is the most frequently used timeframe for these calculations (Mu & Li, 2005).
Adsorption Mechanism and Langmuir Isotherm
The organic compounds inhibit corrosion via adsorption on the area of the material exposed to the inhibitor (Bentiss et al., 1999). Commonly, this process takes place by two main mechanisms: a blocking in the reaction (corrosion) sites or the formation of a barrier that reduces the diffusion rate of corrosive species to the material surface. Many factors such as concentration of the inhibitor, its chemical structure, chemistry of the solution, nature and surface charge of the material, temperature and electrochemical potential have a bear on the adsorption process (Abbasov et al., 2013).
Langmuir adsorption isotherm expression (Equation 2) is one of the methodologies commonly used to obtain further information about this specific phenomenon of mass transfer. It relates to the surface coverage θ (which is defined as corrosion inhibition efficiency/100) and the inhibitor concentration C, allowing the calculation of the adsorption equilibrium constant K for different temperatures.
This constant can then be used to give an insight between the two possible types of adsorption that could occur; physisorption or chemisorption, by calculating the standard heat of adsorption, according to van't Hoff Equation 3:
where R is the universal gas constant, T is the temperature at which the corrosion-inhibition reaction took place, and C is the molar concentration of water at standard conditions. Physisorption takes place at ΔH values lower than 48.2 kJ/mol, while chemisorption is attributed to ΔH values approaching 100 kJ/mol (Martinez & Stern, 2002).
3. METHODOLOGY
Reagents and Materials
Cacao pods from Criollo variety were grown and harvested after 4 months at a local farm in Magangue town of Bolivar (Colombia). 96% v/v ethanol and 37% v/v HC1 from Sigma Aldrich were used. Deionized water was prepared at the Unit Operations Lab of the Chemical Engineering Department from the University of Cartagena. A36 steel plates (4 cm long, 3 cm wide, 4 mm thick) were provided by a local junkyard.
Preparation of Anticorrosive Extracts
The cacao pod husks were washed out with water and cut open to remove all the cocoa beans and mucilage from their interior. Then, the remaining husks were milled. The extracts were obtained adding 50 g of pod husk milled and undried (moisture content 84% w/w) to 300 mL of solvent, at constant temperature during a specific time. Then, the solutions were filtered. A complex experimental design of 23 was used, working with solvent concentration, temperature and extraction time as factors. The levels of these factors were set as follows: solvent concentration between 0% v/v (pure deionized water) and 50 %v/v ethanol; temperature between 28°C (room temperature) and 50°C; extraction time from 2 to 5 hours. Sixteen extracts were obtained in this stage. Table 1 shows the factors and levels of the experimental design, which were obtained using the software STATGRAPHICS® Centurion XVI. It was set as a complex 22 factorial design with central points and star points.
Characterization of the Extracts
50 mL of each extract were characterized with the purpose of determining the presence of specific substances commonly related to anti-corrosive activity. Analyses were conducted by the Laboratory of Pharmaceutical Chemistry of the University of Cartagena. The methods applied were the AOAC 30.184 to determine tannins, Folin Ciocalteau reactive for phenolic compounds, AOAC 920.39C for oils and fats and visible spectrophotometry technique at 528 nm for saponins. Sixty four characterizations were performed in this stage.
Evaluation of the Corrosion Inhibition Activity
In order to test the corrosion inhibition properties of the extracts, analyses of weight loss were performed on A3 6 steel plates using 1M hydrochloric acid (HQ) solution as corrosive medium. Initially, the plates (4 cm x 3 cm x 4 mm) were polished using an electric polisher until the surface was smooth, without scrap metal, rust or dirt, so that no passivation (shielding of surface by a previous oxide layer) could occur. Then, the samples were immersed in 75 mL of the corrosive media with and without addition of the extract. The plate remained fixed vertically in the liquid, inside a closed container with tight lid. The inhibition extract was added directly to the HC1 solution, and its concentration in the solution was varied (1, 3, 7 and 10% v/v). Once the corrosion process ran for 24 hours, each sample was lifted from the corrosion medium. The plate was washed carefully with distilled water and dried at room temperature. Finally, the weight of each sample was measured using analytical balance (Sensitivity 1 mg). This procedure was performed in spans of 24 hours during 14 days. The corrosion inhibition efficiency was determined using the Equation 1. The statistical analysis of data was performed with STATGRAPHICS® Centurion, and the extraction conditions rendering the highest corrosion inhibition efficiency were predicted.
This prediction was subsequently tested in an additional experiment. A Langmuir isotherm analysis was performed on the plate that exhibited the highest inhibition efficiency with the purpose of determining the adsorption mechanism through which the inhibitor molecules adhere to the surface of the metal. Sixty-nine samples were submitted to corrosion test in this stage.
4. RESULTS AND DISCUSSION
Characterization of the Extracts
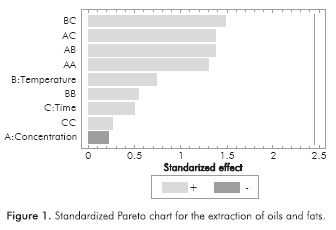
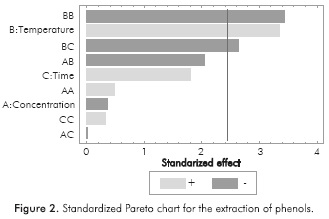
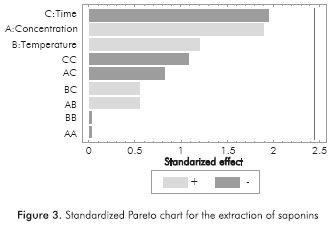
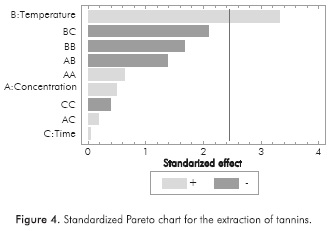
Characterization tests proved the presence of tannins, phenols, oils and saponins in the extracts obtained. Table 2 shows the concentration of each chemical species according to the different extraction conditions. Due to the number of experiments performed, it is difficult to identify any trends in design; therefore, a Pareto chart analysis was developed using STATGRAPHICS® Centurion XVI (See Figures 1 to 4). Thus, the effect of extraction variables on concentration of chemical species in the extracts was assessed. Also, a quadratic regression model with 10 coefficients was considered.
As shown in Table 2, all of the target substances were present in the extracts. It is expected that a synergistic action among these compounds occurs when the corrosion on the plates is inhibited, according to the reported literature on this matter. The presence of oxygen heteroatoms, aromatic rings and electronegative functional groups in the structure of these substances makes it possible to hypothesize that cacao pod husk extracts could exert the desired anticorrosive activity (Zarrokeia/.,2011).
Standardized Pareto charts indicated that only the temperature exerted a significant effect on the extraction of phenols and tannins. Likewise, the interaction between temperature and the extraction time has a slightly lower negative effect in the case of phenols, so that high temperatures during prolonged extraction time lead to low concentrations of phenols in the extract. According to the statistical analysis, no factor influenced the extraction of saponins, oils and fats. This is attributed to the range of the extraction conditions, which were established on the basis of an environmentally friendly and inexpensive methodology. A wider range of temperatures, ethanol concentrations and extraction times perhaps could evidence additional effects on the response variable.
Evaluation of the Corrosion Inhibition Activity
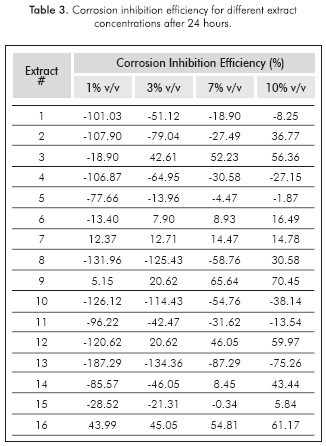
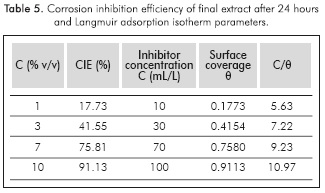
Corrosion inhibition efficiency was calculated using the values of weight loss in each plate after 24 hours, according to Equation 1. The results are shown in Table 3.
Many negative values of efficiency were obtained, which means that some of the extracts did not act as corrosion inhibitors, but rather accelerated the degradation process of the plates. This can be attributed to a possible decomposition of the organic compounds, which leads to the formation of acetic acid. This additional compound may induce a synergistic corrosive action along with the hydrochloric acid, accelerating the degradation rate of the plates (Amri, Gulbrandsen & Nogueria, 2008). Nevertheless, all of the extracts were proven to have higher efficiencies as their concentration increased in the corrosive medium. The highest inhibition (70.45%) was achieved by 10% v/v of extract # 9, which was prepared at 10% v/v of ethanol, 46°C and 4.3 hours of extraction time. Phenols and tannins were the substances with higher yield in this sample.
Data of corrosion inhibition efficiency were analyzed with the purpose of predicting the extraction conditions that would provide the highest corrosion inhibition efficiency. The response surface methodology was applied using STATGRAPHICS® Centurion XVI. The prediction was performed based on a quadratic regression model with ten coefficients. As a result, the following conditions were established: ethanol concentration: 16.1% v/v, operation temperature: 50.4°C; extraction time: 4.9 hours.
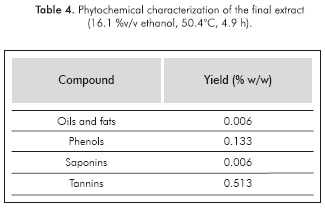
A final extract was prepared under these conditions. It was submitted to characterization tests (see Table 4) and its inhibition efficiency was assessed with the same methodology described above (See Table 5). Also, in order to determine the adsorption mechanism that describes the interaction between this organic compounds and the metal surface, an analysis by Langmuir isotherm of adsorption was carried out using the information of Table 5.
Both phenols and tannins were obtained around their highest proportions, while the yields of saponins, oils and fats remained at relatively low-intermediate values.
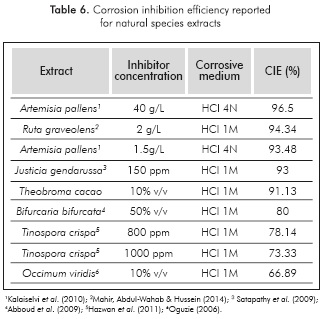
As predicted, higher corrosion inhibition efficiencies were attained with the final extract. The highest efficiency was achieved with the highest extract concentration. The anticorrosive activity is mainly attributed to the presence of phenols and tannins (Rajalakshmi et al., 2012), since these were the chemical species with the highest yield in the extract (see Table 4). Table 6 presents the corrosion inhibition efficiency reported for extracts of different natural species. The results indicated that cacao pod husk extracts present a high corrosion inhibition efficiency when compared to similar natural products, and therefore, the use of this agricultural residue as raw material for producing corrosion bio-inhibitors could be a viable alternative.
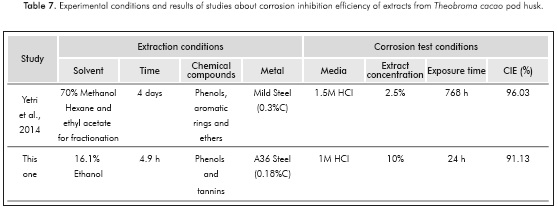
Likewise, the inhibition efficiency attained in this study was similar to the efficiency of Theobromona cacao peel polar extract obtained by Yetri et al. (2014). Although the extraction conditions and the metal subject to analysis in both studies were different. Table 7 summarizes the experimental conditions of each study.
Yetri et al. (2014) obtained a polar extract using methanol as solvent and other organic compounds to fractionate it, generating a solution rich in phenolic, aromatic rings and ether compounds, while the methodology applied in this study only uses an aqueous solution of ethanol, which renders this procedure more environmentally friendly and cheaper. On the other hand, although the metal used in this study is more prone to oxidation, the extract appears to be less stable as the achieved inhibition corrosion efficiency proved to be lower than the polar extract for a shorter exposure time. This is an assumption that could be tested later through electrochemical impedance spectroscopy.
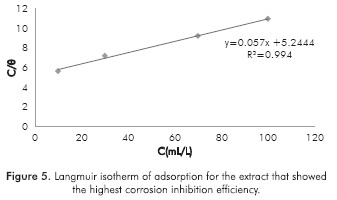
Figure 5 shows the lineal regression of the Langmuir isotherm, obtained from the data of Table 5. An adsorption equilibrium constant K of 0.1906 L/mL was calculated according to Equation 2. Likewise, a standard heat of adsorption of -13.1680 kJ/mol was obtained with van't Hoff Equation 3. This value suggests that the inhibitor adheres to the metal surface by physisorption, which matches the results obtained for Yetri et al. (2014).
5. CONCLUSIONS
-
Green corrosion inhibitors can be obtained from cacao pod husk by means of an extraction with etha-nol and relatively low temperatures and extraction times. This represents an advantage regarding the implementation of an economic, environmentally friendly process, since neither expensive materials, nor toxic substances were required or produced. The anticorrosive activity of the extracts is related to the presence of antioxidants and substances of natural origin in the chemical composition of husks. Phenols and tannins were the predominant compounds in the extract that exerted the highest inhibition efficiency: thus, inhibitive action can be mostly attributed to these components. Analysis by Langmuir isotherm suggests that the inhibitor molecules adhered to the metal surface through physisorption.
-
This work is one of the few studies conducted in the world about extracts from Theobromona cacao pod husk and their use as corrosion inhibitors. It represents a significant contribution as it provides scientific information related to the behavior of extract on A3 6 steel plates. Moreover, optimal operation conditions were determined based on a relation between the extraction conditions and corrosion inhibitor efficiency. However, more studies on this topic are required in order to settle the technical and economic viability of this alternative.
ACKNOWLEDGEMENTS
The authors thank the Chemical Engineering Program of the University of Cartagena for providing us with access to use the Materials Science Lab.
REFERENCES
Abbasov, V. M., Abd El-Lateef, H. M., Aliyeva, L. I., Qasimov, E. E., Ismayilov, I. T. & Khalaf, M. M. (2013). A study of the corrosion inhibition of mild steel C1018 in C02-saturated brine using some novel surfactants based on corn oil. Egypt J. Petrol., 22(4), 451-470. [ Links ]
Abboud, Y., Abourriche, A., Ainane, T., Charrouf, M., Bennamara, A., Tanane, O. & Hammouti, B. (2009). Corrosion inhibition of carbon steel in acidic media by bifurcaria bifurcata extract. Chem.Eng. Commun., 196(7), 788-800. [ Links ]
Abdel-Gaber, A. M., Abd-El-Nabey, B. A., Khamis, E. & Abd-El-Khalek, D. E. (2011). A natural extract as scale and corrosion inhibitor for steel surface in brine solution. Desalination, 278(1-3), 337-342. [ Links ]
Amri, J., Gulbrandsen, E. & Nogueira, R. P. (2008). The effect of acetic acid on the pit propagation in C02 corrosion of carbon steel. Electrochem. Commun., 10(2), 200-203. [ Links ]
Bentiss, F., Traisnel, M., Gengembre, L. & Lagrenee, M. (1999). A new triazole derivative as inhibitor of the acid corrosion of mild steel: electrochemical studies, weight loss determination, SEM and XPS. Appl. Surf. Sei., 152(3-4), 237-249. [ Links ]
Faustin, M., Maciuk, A., Salvin, P., Roos, C. & Lebrini, M. (2015). Corrosion inhibition of C38 steel by alkaloids extract of Geissospermum leave in 1 M hydrochloric acid: Electrochemical and phytochemical studies. Corros. Sei., 92: 287-300. [ Links ]
Fouda, A. S., Eldesoky, A. M., Elmorsi, M. A., Fayed, T.A. & Aria, M. F. (2013). New eco-friendly corrosion inhibitors based on phenolic derivatives for protection mild steel corrosion. Int. J. Electrochem. Sei., 8:10219-10238. [ Links ]
Hazwan, M., Jain, M., Razali, N., Dahon, N. & Nasshorudin, D. (2011). The effect of Tinospora crispa extracts as a natural mild steel corrosion inhibitor in 1M HCl solution. Arab. J. Chem. In Press. doi:10.1016/j.arabjc.2011.07.002 [ Links ]
Ikpi, M. E., Udoh, I.I., Okafor, P. C, Ekpe, U. J. & Ebenso, E. E. (2012). Corrosion inhibition and adsorption behavior of extracts from piper guineensis on mild steel corrosion in acid media. Int. J. Electrochem. Sei., 7: 12193-12206. [ Links ]
International Cocoa Organization. (2015). Quarterly bulletin of cocoa statistics, XLI (1) - Cocoa year 2014/15. [ Links ]
Kalaiselvi, P., Chellammal, S., Palanichamy, S. & Subramanian, G. (2010). Artemisia pollens as corrosion inhibitor for mild steel in HCl medium. Mater. Chem. Phys., 120(2-3), 643-648. [ Links ]
Mahir, M. Abdul-Wahab, S. & Hussein, A. (2014). Corrosion inhibition of carbon steel in IM HCl solution by Ruta graveoleons extract. J. Chem. Pharm. Res., 6(5), 996-1001. [ Links ]
Martinez, S. & Stern, I. (2002). Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in the low carbon steel/mimosa termin/sulfuric acid system. Appl. Surf. Set, 199(1-4), 83-89. [ Links ]
Mourya, P., Banerjee, S. & Singh, M. M. (2014). Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor. Corros. Sci., 85: 352-363. [ Links ]
Mu, G. & Li, X. (2005). Inhibition of cold rolled steel corrosion by Tween-20 in sulfuric acid: Weight loss, electrochemical and AFM approaches. J. Colloid. Interf. Sci., 289(1), 184-192. [ Links ]
Oguzie, E. E. (2006). Studies on the inhibitive effect of Occimum viridis extract on the acid corrosion of mild steel. Mat. Chem. Phys., 99(2-3), 441-446. [ Links ]
Oguzie, E. E., Enenebeaku, C. K., Akalezi, C. O., Okoro, S. C, Ayuk, A. A. & Ejike, E. N. (2010). Adsorption and corrosion-inhibiting effect of Dacryodis edulis extract on low-carbon-steel corrosion in acidic media. J. Colloid. Interf. Sci., 349(1), 283-292. [ Links ]
Rajalakshmi, R., Prithiba, A. & Leelavathi, S. (2012). An overview of emerging scenario in the frontiers of eco-friendly corrosion inhibitors of plant origin for Mild Steel. J. Chem. Acta, 1(1), 6-13. [ Links ]
Satapathy, A. K., Gunasekaran, G., Sahoo, S. C, Amit, K. & Rodrigues, P. V. (2009). Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros. Sci., 51(12), 2848-2856. [ Links ]
Umoru, L. E., Fawehinmi, I. A. & Fasasi, A. Y. (2006). Investigation of the inhibitive influence of Theobroma cacao and Cola acuminata leaves extracts on the corrosion of a mild steel in sea water. J. Appl. Sci. Res., 2(4), 200-204. [ Links ]
Vriesmann, L. C, Amboni, R. D. M. C. & De Oliveira, C. L. (2011). Cacao pod husks (Theobroma cacao L.): Composition and hot-water-soluble pectins. Ind. Crop. Prod., 34(1), 1173-1181. [ Links ]
Vrsalovic, L., Kliskicand, M. & Gudic, S. (2009). Application of phenolic acids in the corrosion protection of Al-0.8Mg alloy in chloride solution. Int. J. Electrochem. Sci., 4: 1568-1582. [ Links ]
Yetri, Y., Emriadi, Jamarun, N. & Gunawarman. (2014). Corrosion inhibition efficiency of mild steel in hydrocloric acid by adding Theobroma Cacao peel extract. International Conference on Biological, Chemical and Environmental Sciences (BCES-2014), Penang, Malaysia. [ Links ]
Yapo, B. M., Besson, V., Koubala, B. B. & Koffi, K. L. (2013). Adding value to cacao pod husks as a potential antioxidant-dietary fiber source. Am. J. Food Nutr., 1(3), 38-46. [ Links ]
Yoo, S. H., Kim, Y. W., Chung, K., Baik, S. Y. & Kim, J. S. (2012). Synthesis and corrosion inhibition behavior of imidazoline derivatives based on vegetable oil. Corros. Sci., 59:42-54. [ Links ]
Zarrok, H., Oudda, H., Zarrouk, A., Salghi, R., Hammouti, B. & Bouachrine, M. (2011). Weight loss measurement and theoretical study of new pyridazine compound as corrosion inhibitor for C38 steel in hydrochloric acid solution. Der Pharma Chem., 3(6), 576-590. [ Links ]
AUTHORS
Daniel-Enrique Pedroza-Perífián
Affiliation: Universidad de Cartagena
Chemical Engineer, Universidad de Cartagena
e-mail: dpedrozap@unicartagena.edu.co
Melissa-Andrea Villalobos-Vasquez
Affiliation: Universidad de Cartagena
Chemical Engineer, Universidad de Cartagena
e-mail: mvillalobosv@unicartagena.edu.co
Pedro Javier Meza-Castellar
Affiliation: Fundación Universitaria Tecnológico Comfenalco
Chemical Engineer, Universidad Nacional de Colombia
M. Sc. in Environmental Engineering, Universidad de Cartagena
e-mail: pmeza@tecnocomfenalco.edu.co
Isabel-Cristina Paz-Astudillo
Affiliation: Universidad del Tolima
Chemical Engineer, Universidad Nacional de Colombia
M. Sc. in Chemical Engineering, Universidad del Valle
Ph. D. in Automatic Engineering, Universidad Nacional de Colombia
e-mail: icpaza@ut.edu.co