1. INTRODUCTION
The characterization of asphaltenes has been a mandatory subject for researchers, since the composition of this oil fraction strongly affects almost the entire value chain for Petroleum companies. Particularly the treatment of water-in-oil (W/O) emulsions represents high operational costs that companies would like to reduce or avoid. With the advances in high resolution mass spectrometry (FTICR-MS) and fractionation methods, it has been possible to get deeper glimpse into asphaltenes [1]-[4]. For example, today there is no doubt about their average molecular weight (usually not above 1400 Da) [5] while this was the subject of extensive debates two decades ago [6,7], Moreover, through FTICR-MS, the tremendous complexity of asphaltenes has been explored, since more than twenty different functional groups represented in classes such as HC, Nn, NnOo, (n=l, 2, 3 and o=l, 2, 3) have been detected [1]. Finding the relationship between molecular composition and properties is the main goal behind all the efforts to characterize asphaltenes. For instance, the stability of W/O emulsions might be explained by the presence of surface active materials naturally present in crude oil [8]-[12] These naturalsurface active species can be either naphthenic acids, or polar compounds that form part of resins or asphaltenes, since they have an amphiphilic character according to the Bancroft's rule [13]. Particularly, asphaltenes have been widely recognized in the Literature as petroleum's "natural surfactants," being considered as the most relevant emulsion stabilizers [14,15], Different authors have reported that asphaltenes are adsorbed at the oil-water nterface, thus the high stability of W/O emulsions could be a result of strong interfacial films formed by these compounds [16]-[22].
Although their composition has been widely studied, asphaltenes have not been properly defined on a molecular basis. Even nowadays, the definition of asphaltenes is based on solubility, such as the fraction of crude oil insoluble in aliphatic solvents (such as heptane) and soluble in aromatic solvents (such as toluene) [23], From a molecular level, the understanding is that the main characteristic is their high aromaticity. They may be represented by compounds between five to ten fused aromatic rings, bearing heteroatoms such as N, 0 and S [24]-[26], The aromatic fused rings have the capacity to auto-associate and form supra-molecular aggregates, which can additionally trap compounds with different structural characteristics that are not necessarily insoluble in aliphatic solvents [27], Fourier-transform ion cyclotron resonance mass spectrometry (FTICR MS) has made it possible to establish that asphaltenes have a wide compositional variability [28], Based on these outcomes, a necessary question arises: What does fraction of asphaltenes grants interfacial activity to such compounds? It has been clarified that crude oil asphaltene content itself is not correlated with W/O emulsion stability. Czarnecki et a I. have shown that the entire asphaltenes fraction is not responsible for emulsion stability [29,30], Conversely it has been suggested that "only a sub-fraction" of asphaltenes is nvolved in emulsion stabilization [31]-[36].
2. STATE OF THE ART
Several works have shown that asphaltene aggregates display greater interfacial activity within the W/O interface than the solvated (free) asphaltenes that, in fact, are not structurally able to be absorbed over this surface [2,37], This implies that the key to understanding this behavior is in the nature of the compounds that interact with asphaltenes and can co precipitate with them (occluded compounds). It has been demonstrated that a fraction of the asphaltenes corresponds to occluded compounds such as saturated biomarkers and alkyl aromatic compounds containing heteroatoms in their structure [2,38,39].
Based on the ideas set out above, the composition of the n heptane iinsoluble fraction obtained from six Colombian crude oils was studied through FTICR MS to make a step forward in answering questions regarding the role of asphaltenes in emulsion stabilization.
3. EXPERIMENTAL DEVELOPMENT
SAMPLES PREPARATION
Six asphaltenes were precipitated from their respective crude oils following the ASTM standard method D6560-12. Quickly, 1.0 g of crude oil was mixed with 40 ml_ of n C7 and heated for 40 minutes under reflux. The mixture was stored in the dark overnight and the precipitated raw asphaltenes were collected by filtration. Asphaltene cleaning was performed using Soxhlet apparatus with n-C7 as a recycling solvent, until the solvent was clear. The precipitates were recovered by dissolution in hot toluene which was subsequently rotovapored to produce solid asphaltenes. The identity and the characterization of the crude oils employed are displayed in Table 1.
FTICR MS ANALYSIS
FTICR MS analyses were performed using a Bruker SolariX FTICR mass spectrometer equipped with an actively shielded 15 T superconducting magnet. For (+) APPI toluene solutions with a concentration between 0.05 and 0.2 mg/mL were used. Samples were injected with a syringe pump at a 200 μL/h rate trough an Apollo II APPI source. The time-of-flight was adjusted to 0.9 ms to transfer the ions to the ICR cell throughout an electrostatic focus. The ICR cell was operated within 200-1200 Da mass range. The spectra were the result of 100 scans.
Negative mode ESI spectra were acquired using an Apollo I electrospray source. Ammonium hydroxide (20 μL to 1 ml_ of sample solution) was added to solutions of 0.05 to 0.2 mg/mL in toluene: methanol 50:50. Samples were infused at a flow rate of 450 μL/h using a syringe pump. Source optics were operated with - 220 V for capillary column end and - 60 V for skimmerl voltages. Ions were accumulated for 0.050 s in an octapole with a 5 MHz frequency and 350 Vpp of radiofrequency (rf) amplitude. The time-of-flight (TOF) was set to 0.7 ms to transfer the ions to an ICR cell. 270-1100 Da mass range, and 4 M acquired data size was employed. The time domain data sets were co-added from 100 data acquisitions.
MASS CALIBRATION AND ASSIGNATION
APPI FT-ICR mass spectra were calibrated internally using DataAnalysis from Bruker, employing a homologous series of HC class of DBE 29 for CU, and S1-DBE 21 for the rest of the samples. Peaks with relative abundance greater than 5 times the signal-to-noise ratio were exported to a database in order to assign the spectra in Composer64 Version 1.5.4. The peaks were assigned with a match tolerance of 0.5 ppm in a range from 200 to 1100 Da. ESI FT-ICR mass spectra were calibrated internally using Data Analysis (Bruker Daltonics) with nitrogen class N1 (DBE 20-25) homologous series, using peaks of relative abundance greater than 5 times the signal to-noise ratio.
EMULSION STABILITY EVALUATION
From a 5000 mg/L solution of asphaltenes (5.0 mg of n-C7 insoluble fraction dissolved in 1.0 ml_ dichloromethane (DCM)), 2 ml_ of a 1000 mg/L asphaltenes solution was prepared by dilution in an atmospheric distillate (299-350°C) as organic phase. pH was adjusted to 8-9 using 20 μL of NH4OH, and the resulting mixture was added over 2.0 mL of brine (0.680 g/L NaCl, 0.067 g/L KCl, 0.330 g/L NaHC03 and 0.044 g/L NaS04). The mixture was vigorously agitated and sonicated for 5 min. The emulsion braking progress was registered as a function of time (0,5, 24, 48 h and 10 days) and compared to a blank (2 mL atmospheric distillate + 2 mL brine + 20 μL NH4OH).
4. RESULTS ANALYSIS
As observed in Table 1, there is significant variability in the samples studied. The raw crude oils have API gravities from 42.0 to 9.4, thus spanning light to heavy oils. The sulfur content and the TAN values also show a wide range. Interestingly, TK oil is a sample with low API gravity (12.1), low amount of asphaltenes (2.5%) and the highest determined TAN for the set of samples studied.
(+) APPI ANALYSIS
The main classes detected (RA>0.5%) by positive mode APPI FTICR MS are listed in Table 2. These data show hydrocarbons, as well as nitrogen containing, sulfur containing, oxygen containing compounds and porphyrins. Interestingly, the class N4OVwasthe most abundant in asphaltenes CH with 18 % relative abundance, followed by the nitrogen containing classes NO, N, NOS and NS. The class N40V was detected for four of the samples studied, and their relative abundances might be correlated with the vanadium content listed in Table 1. As has been reported, the greater the relative abundance for the N4OV class, the greater the vanadium content observed in crude oil [31,32, which corroborates that the vanadium forms part of the asphaltene fraction. On the other hand, asphaltene TK and CH had almost twice as much N as the HC class, meanwhile the S was higher than the 0 class for almost all asphaltenes. This implies that the relative abundances varied for all classes determined, in such a way that it would not be possible to define asphaltenes in terms of the concentration of specific functional groups.
Table 2 Relative Abundance for the classes detected by (+) APPI.
| Class | CU | F | R C | TK | CH | |
| HC | 28.1 | 19.2 | 15.4 | 15.8 | 14.7 | 6.2 |
| N | 16.2 | 12.0 | 11.8 | 11.4 | 26.2 | 9.8 |
| S | 3.2 | 12.5 | 12.2 | 10.3 | 10.5 | 8.6 |
| 0 | 15.7 | 7.1 | 13.4 | 5.7 | 3.6 | 4.0 |
| NO | 8.8 | 8.0 | 8.2 | 9.9 | 11.3 | 10.8 |
| NS | 1.8 | 6.4 | 6.2 | 7.6 | 6.1 | 9.2 |
| OS | ND | 3.7 | 8.1 | 2.8 | ND | 4.6 |
| s2 | 9.6 | 3.5 | 4.6 | 4.6 | ND | 5.3 |
| N2 | ND | 3.0 | 1.9 | 2.4 | 4.8 | 2.2 |
| 02 | 5.5 | 7.6 | 5.7 | 2.7 | 2.0 | ND |
| N02 | 2.6 | 3.7 | 2.1 | 1.4 | 1.9 | 1.5 |
| N20 | ND | 2.5 | ND | 3.4 | ND | 5.3 |
| 02S | ND | 1.9 | 1.7 | ND | ND | 0.0 |
| OS2 | ND | ND | 2.6 | 1.1 | ND | 1.9 |
| NS2 | ND | 1.1 | ND | 1.8 | ND | 2.3 |
| NOS | ND | 4.3 | 4.4 | 6.2 | 2.5 | 9.7 |
| N4OV | ND | 1.1 | 0.5 | 8.6 | ND | 17.9 |
| HC [H]* | 2.7 | 1.9 | 1.2 | 0.6 | 3.1 | ND |
| S2 [Hf] | 3.5 | ND | ND | ND | ND | ND |
| 02 [H]* | ND | ND | ND | 3.5 | 8.9 | ND |
Source: Authors * Protonated families.
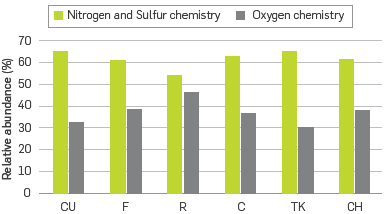
Alternatively, the detected classes can be classified as oxygen containing and non oxygen containing compounds. By grouping these classes, it will be possible to evaluate chemical effects of the compounds comprising the asphaltenes fraction over emulsification tendencies. Figure 1 shows the summed relative abundances of two groups of classes termed as: HC, HC[H], N, S, NS, S2, S2[H], N2, NS2, and N4OV mainly governed by nitrogen and sulfur chemistry; and 0, NO, OS, 02, 02[H], N02, 02S, OS2, N20, and NOS, mainly governed by oxygen chemistry. In the case of porphyrins, despite of having an oxygen molecule, the four nitrogen arranged in fused aromatic rings make the chemistry of these molecules closer to the first group.
Grouping the data in such a way opens up the possibility of visualizing ntermolecular interactions via n-n stacking by their aromatics cores, polar forces throughout heteroatoms such as N and S, and hydrogen bonding throughout -OH groups. Samples CU and TK had very similar profiles with around 65% of nitrogen-sulfur and 30 % of oxygen species, and sample R had the highest abundance in oxygen compounds with around 46%. This means that the molecules in R would have greater possibilities to interact via hydrogen bonding.
To gain a glimpse into the aromaticity and carbon number distributions, the contour plots for double bond equivalent (DBE) vs, carbon number (CN) for the HC, S and S2 classes are presented in Figure 2. The appearance of HC and S plots is consistent with some reports, where the asphaltenes are related to highly condensed aromatic cores with 510 rings, bearing about 1015 aliphatic carbons, distributed as methyl substituents and short aliphatic moieties [2,4]. In the case of asphaltenes CU, the most intense signals were between DBE 25 and 40, which implies 7 to 13 aromatic rings, and a carbon number of 30 to 60, interestingly the lightest crude oil (42° API). For asphaltenes F and C a second distribution below DBE 15 was detected, presumably due to a poor cleaning process during the ASTM D6560-12 procedure. S class contour plots show very similar distributions for all samples having DBE values between 20 and 30 and carbon numbers between 30 and 40. Nevertheless, the S2 class showed an atypical result for asphaltenes CU, where the compounds detected were under DBE 16 (dashed red line). These compounds clearly cannot be associated with highly condensed aromatic cores and hence do not directly associate with asphaltenes. This outcome might demonstrate that highly aliphatic compounds can be occluded in the asphaltenes fraction [40].
(-) ESI ANALYSIS
Table 3 presents the relative abundance percentage for the classes detected by (-) ESI. Twenty-one classes were identified and, as in positive APPI, none of them showed themselves to be predominant.
Table 3 Relative abundances for the classes detected by (-) ESI.
| Class | CU | F | R | C | TK | CH |
| N | 4.1 | 16.6 | 26.3 | 16.5 | 2.9 | 13.5 |
| N2 | 1.1 | 11.4 | 8.3 | 9.5 | 0.9 | 7.4 |
| N2S | 0 | 1.9 | 1.6 | 2.9 | ND | 2.8 |
| NS | 0.6 | 9.2 | 13.8 | 12.2 | 0.6 | 12.7 |
| N20 | 3.2 | 4.7 | 5.8 | 11.2 | 3.4 | 15.2 |
| N202 | 1.9 | ND | 0.7 | 1 | 7.7 | 2.2 |
| N2OS | 0 | ND | ND | 2.5 | ND | 4.2 |
| NO | 13.7 | 6.6 | 17.2 | 7.8 | 4.3 | 8.3 |
| N02 | 14.5 | 4.9 | 5.6 | 4 | 24.6 | 4 |
| N02S | 0 | ND | 0.9 | 0.8 | 5.1 | 1.8 |
| N03 | 8.4 | ND | 0.8 | ND | 10.3 | ND |
| N03S | 0 | ND | ND | ND | 1.5 | ND |
| N04 | 0 | ND | ND | ND | 2.4 | ND |
| NOS | 1.6 | 2.3 | 7 | 4.6 | 0.8 | 7.4 |
| 0 | 5.8 | 1.2 | ND | 1.9 | 1.1 | 0.6 |
| 02 * | 21.3 | 24.7 | 0.8 | 12.4 | 5.3 | 5.2 |
| 02S | 0 | 1.8 | ND | 1.8 | 2.4 | 0.6 |
| 03 | 13.3 | 4.5 | ND | 1 | 2.2 | 0.5 |
| 03s* | 0.7 | 1.1 | 2 | 2.8 | 2.7 | 1.4 |
| 04 | 3.8 | 0.7 | ND | ND | 9.3 | ND |
| 04S | 0 | 3.2 | 3 | 2.3 | 1.5 | 4.8 |
Source: Authors, * These classes are commonly understood as sulfonates ¡o3s) and carboxylic acids (02).
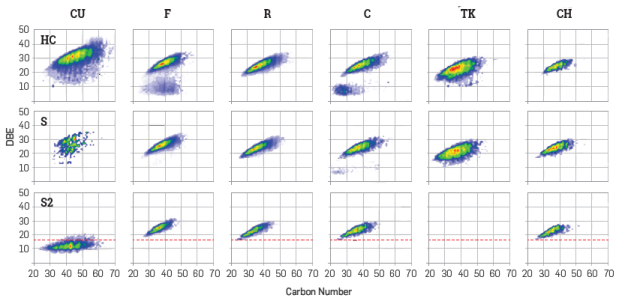
Figure 2 summed DBE Vs CN plots for the classes HC, S, and S2 detected by (+) APPI for the asphaltenes studied
For instance, the class O2 (generally associated to carboxylic acids) was the most abundant for samples CU and F, while the N class was the main for samples R and C. In the case of the samples TK and CH, the classes NO2 and N2O were predominant, respectively. Structural details must be considered since classes such as O2 can be related either to polyaromatic oxygen, containing compounds such as phenols when DBE>10, or carboxylic acids when DBE<10, as has been previously reported [41].
Different authors have discussed the surface-active character of oils to combined effects of the presence of carboxylic acids and asphaltenes in crude oils. Accordingly, these carboxylic acids and the asphaltenes perform a synergic effect, resulting in emulsion stabilization [19,42]. Thus, one possibility is that the aggregates formed between these species are the emulsion stabilizing materials. These supra-molecular entities formed as a natural mechanism to preserve asphaltene molecules dispersed in crude oil, behaving as emulsifiers/surfactants when water is present and, as a consequence of the addition of n heptane (or sudden changes in pressure or temperature) they can precipitate as a whole. Therefore, it is worth considering that, depending on the proportion between of acids sulfonates and polycondensed aromatic species, it is possible to produce an appropriate amount of surface-active aggregates; in other words, entities able to interact at the water-oil interphase and stabilize an emulsion. This is consistent with the emulsion stabilization phenomena explained from a "colloidal state" point of view, as was proposed by Stanford et. al, [37] and not by individual molecular entities with a low molecular weight, as stated in different research works [14,43].
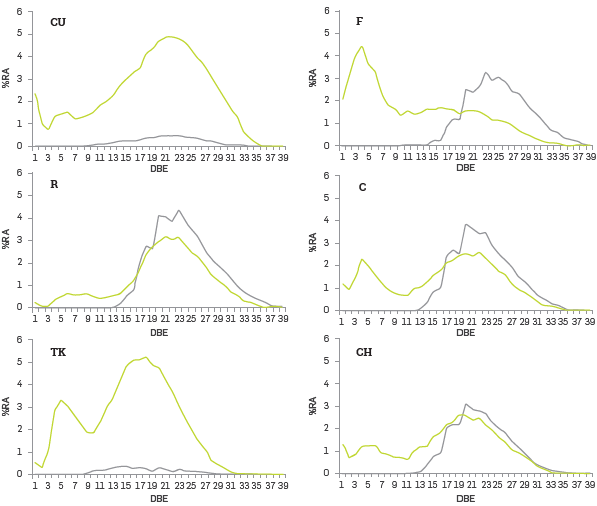
Figure 3 Distribution of DBE grouped as oxygen (NxOy+NxOySz+Oy+OySz) in blue and non oxygen containing classes.
According to the results discussed so far, it is possible to infer that asphaltenes, as a whole, might display amphiphilic character as their structures are dominated by polyaromatic polarizable nuclei bearing functionalities that are able to form hydrogen bonding nteractions with different polar-headed long chained entities (acids and/or resins), thus providing a pathway for the formation of supramolecular surface-active species. Additional evidence on these inferences arose from analyzing oxygen containing classes in particular. Figure 3 presents the distribution of DBE re-grouped as oxygen containing (NxOy+NxOySz+Oy+OySz) and non-oxygen containing classes (NX+NXSZ) from negative ESI.
It is possible to observe an overlapping between these two groups for DBE values ranging from 13 to 33 in four of the studied asphaltenes (F, R, C and CH). In the case of CU and TK, this overlapping was not observed. Based on this observation, the following hypothesis stands up. Lipophilic graphene-like asphaltenes trap natural surfactants (such as naphthenic acids or petroleum sulfonates) which in some way provide the hydrophilic emulsion stabilizing functions of the aggregates formed.
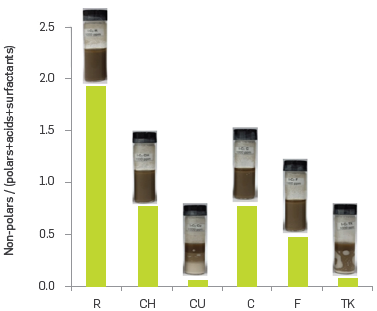
Figure 4 was created taking into account the ideas set out above. By considering the sum of the relative abundances for the classes Nx and NxSy, it is possible to observe two different groups of samples. For CU and TK, these values were below 10 RA %, while F, R, C and CH showed RA % above 30. Regarding the classes NxOy + NxOySz, the asphaltenes CU, TK and CH presented a comparatively high RA %, while asphaltenes R and CH showed the lower RA % for compounds OxSz and Ox.
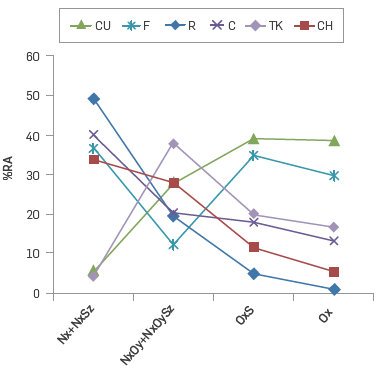
Figure 4 Relative abundances for classes Nx and NxSy, classes NxOy + NxOySz, the asphaltenes studied.
Now, it is possible to consider that the oxygen containing compounds detected via ESI (-) FTICR MS within asphaltenes F, R, C and CH present a higher affinity for the non oxygenated compounds in comparison with asphaltenes CU and TK.
EMULSION STABILITY ANALYSIS
We addressed the evaluation of the emulsion stabilizing properties of the n-C7 insoluble fractions being studied. Figure 5 presents systems before agitation (a), and after 30 minutes (b) and 24 hours (c).
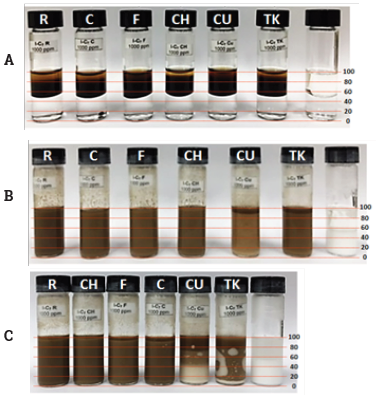
Figure 5 Emulsion stabilization test, (a) before agitation, (b) after 30 minutes and (c) after 24 hours.
Before agitation, two phases were distinguished for all the samples, i.e. the bottom aqueous phase (50%) and the oily phase above (50%). After 30 minutes, samples R, C, F and CH showed one phase, meanwhile for CU and TK two phases were present where the oily phase represented around 10% of the system. After 24 hours, R and C remained as one phase, F and CH started to break the emulsion with an oily phase of around 5%, meanwhile CU and TK showed three phases. This means that samples CU and TK showed a lower emulsion stabilization capacity over time, and by comparing the first four samples, F and C showed a subtle difference with respect to R and CH. Precisely, samples CU and TK had the lowest concentration of oxygenated compounds detected by APPI (see Figure 1).
Finally, a stability coefficient based on the relative abundance of some classes detected via (-) ESI was proposed to explain the observed behavior. The proposed parameter can be considered as a relative distribution of polar compounds. It is calculated as a relationship between the relative abundances of classes Nx+NxSz (usually with DBE>15) and oxygen containing classes NxOy+NxOySz+Oy+OySz, which can be alternatively understood as polar (NxOy+NxOySz), acid (Oy) and surfactant (OySz) classes. Figure 6 contains the stability coefficients calculated for each n C7 insoluble fraction, together with the appearance of the emulsions after 24 h. From this diagram, it is possible to differentiate a region where high emulsion stability is observed, which corresponds to stability coefficients from 0.5 to 2.0.
This can be understood as the interval where the non oxygen containing (Nx+NxSz) and the oxygen-containing compounds (NxOy+NxOySz+Oy+OySz) are in a proportion at which the supra-surfactant can be efficiently formed. These results suggest that occluded compounds and asphaltenes form molecular aggregates that are naturally dispersed in crude oil, and demonstrate that these aggregates achieve a surface-active character at the oil-water interface. Therefore, such natural surfactants must be considered as the actual emulsion stabilizing agents in crude oil/water systems.
CONCLUSIONS
Six n-heptane insoluble fractions (asphaltenes) were analyzed by (+) APPI and ( ) ESI FTICR MS. By sorting classes as nitrogen sulfur and oxygen containing compounds through (+) APPI, it was possible to provide an overview of the total composition of samples and distinguish the asphaltenes compositionally. Some asphaltenes had relatively more oxygenated compounds, implying that such crude oils might have relatively more polarizable aromatic compounds, with the ability to perform intermolecular interactions with different molecules via n-n stacking and hydrogen bonding. Supporting this idea, grouping the classes detected by (-) ESI as oxygen (NxOy+NxOySz+Oy+OySz) and non oxygen containing classes (Nx+NxSz) it was noticed that a sub fraction of asphaltenes with a noticeably lower aromaticity, as well as different functional groups (occluded carboxylic acids and sulfonates) are "trapped" by the asphaltene's polarizable aromatic moieties. This may imply that these two groups of compounds should be in a proportion at which surface-active aggregates are formed (these are naturally dispersed in crude oil), and that such aggregates perform a surfactant role at the oil-water interface. Therefore, these supra surfactant entities must be considered as the actual emulsion-stabilizing agents in crude oil water systems. Based on these outcomes, an emulsion stability coefficient was proposed based on FTICR MS data, showing that the proportion of this sub-fraction (the relative abundance of occluded oxygen containing and non oxygen containing compounds) is related to the surface-active character of a n-C7 insoluble fraction and thus, to the stable emulsion formation tendency of a crude oil.