1. INTRODUCTION
Ionic liquids (LIs) represent a good alternative within the known "new materials"; they are formed by an organic cation and an inorganic or organic anion; as a consequence of their broad charge distribution and big size, a melting point below 100°C could be brought for most of them. Additional properties include: negligible vapor pressure and the fact that they can be tailored for specific applications by combining different cations and anions. [1]
There have been several investigations reported, stating that ionic liquids have good properties for extracting organic and inorganic species in competition with traditional volatile organic compounds (VOC's) in liquid-liquid extraction processes. One specific application is related to the dehydration of bioethanol, using ILs as entrainers to break the azeotrope in an extractive distillation process. [2]-[5]. Suitable entrainers are introduced to the mixture and must cause a significant change in the relative volatility of the azeotrope components with high selectivity and high solvent capacity for the components to be separated. The use of ionic liquids as entrainers is attractive when compared to traditional entrainers used (the low vapor pressure of the ionic liquids, regeneration and reuse); however, ILs viscosity is a key factor for industrial applications and the anion seems to have a strong influence on this property [6]; in most of the room temperature ionic liquids have viscosities > 30 cP. So talking about low viscosity ionic liquids is a relative term [7] because these high viscosities are useful for some applications for extraction processes; these high values contribute to poor penetration of porous solid materials and restrict mass transfer at solvent interfaces [8]; in addition, ionic liquids are still too expensive for large scale industrial applications. In order to establish processes with lower energy consumption and simpler downstream processing, alternative technologies still using ionic liquids are under investigation. Supported Ionic Liquid Membranes (SILM) is one of them; in these materials, porous membranes impregnated with selective Ionic Liquids have been widely used in many industrial processes, one of them for breaking azeotropes. In this application, ionic liquids act as a transmembrane medium that facilitates selective transportation of the separated components [9]. Another approach used has been the preparation of hollow fiber membrane contactors with ionic liquids, creating a barrier between the feed and the extracting solving, providing a large mass transfer area. Other results showed that from a 0.17M ethanol solution, a 3.43M solution could be obtained after elution through the membrane system with an extraction efficiency of 20,18%. A pervaporation separation of a ternary mix (ethyl acetate/ethanol/water) has been also investigated, using a buck paper supported ionic liquid membrane; temperature and downstream pressure conditions on the pervaporation performance were evaluated. The membrane exhibited good pervaporation performance, suggesting great potential due to a good balance in the trade-off between the permeation flux and the separation factor [10]. The use of impregnated membranes with Ionic Liquids solves problems such as loss of liquid through vaporization or dissolution though still affected by rapid fouling. Another investigation branch pointed toward the anchorage of the ionic liquids on different matrixes including: zeolites, silica, cellulose, polymers, among others [11]-[13]. Although most of the applications in this area are in heterogeneous catalysis. These composites are solid hybrid materials which make them easy to handle and cheaper, in addition to the fact that these could be recovered and regenerated [14]-[15].
In recent years, researchers have reported ternary VLE data for systems containing water, ethanol and an IL; most of the IL cations studied were [BMIM] or [EMIM], and the anions included: [BF4]-, [Cl]-, [N(CN)2]-, and [OAc]-.[16]-[18]. In this research we are reporting a comparative study of two different investigations with ILs technology. In the first study, extractive distillations were used to evaluate: [EMIM][Cl], [EMIM][OAc] and [BMIM][Cl] with the purpose of measuring its effect in the enhancement of the relative volatility of the bioethanol-water mixture, and establishing a correlation between the structure of the ILs and its efficiency to break the azeotrope. Based on this first study stablishing that the [EMIM][Cl] has the largest effect on the enhancement of the relative volatility, this IL was covalent bonded to silica -an inorganic structure with excellent mechanical properties- with the purpose to evaluate its absorption capacity of water for bioethanol dehydration. Results of the comparative study are reported and showed that immobilized functional ionic liquids in extraction processes combine advantages such as selectivity, less time consuming, recyclability, and significantly reducing (84%) the requirement for the amount of the IL, while retaining or even improving the extractive efficiency.
2. EXPERIMENTAL DEVELOPMENT
MATERIALS
Ionic liquids [EMIM][Cl] from Merck® and [BMIM][BFJ from Sigma-Aldrich were used without further purification; [BMIM][Cl] and [EMIM][OAc] were prepared by ion Exchange on a glass column packed with 96.85 g ion exchange resin (Amberjet® 4200 with a matrix of divinyl benzene styrene copolymer from Merck) strongly basic.
For [EMIM][OAc] preparation, a KOAc 0,5 M solution was eluted through the column pumped upwards with a peristaltic Masterflex L/S pump, in order to activate the column. Once activated, a 0.5 M solution of [EMIM][Cl] was eluted at a continuous flow of 1mL/ min in order to obtain the anion exchange. The IL [BMIM][Cl] was prepared using the same methodology for [BMIM][BF4] procedure, but activating the resin with a 0.5M KCl solution. All elution was done upwards at room temperature and 90.66 kPa [19].
The infrared spectrum for all the ionic liquids was obtained with a FT-IR Bruker Tensor 27 spectrometer with KBr cells for liquids. Likewise, the ionic liquids were characterized by 1H-NMR dissolved in deuterated water in a NMR Bruker Avance III 400 MHz Ultrashield.
DEHYDRATION OF BIOETHANOL EMPLOYING AN EXTRACTIVE DISTILLER WITH THE ILS [EMIM][CL], [BMIM][CL] AND [EMIM][OAC] AS ENTRAINERS.
A cold finger distiller was used for the extractive distillations, using [EMIM][Cl], [EMIM][OAc], and [BMIM][Cl] ionic liquids as entrainers.
In this system, also known as short path distillation apparatus, the distillate travels a short distance, often just a few centimeters from one glass bulb to another by using a vertical condenser (cold finger). The mix of ionic liquid/bioethanol (93.45 % v/v) at different mass ratios from 0.1 to 0.6 was set in the first glass bulb with a stirrer on the stirring hot plate (at 80°C); hot vapors reaching the cold finger (at 10°C), are condensed and drop into the second glass bulb and are collected for later analysis.
The water content of the ternary mix was measured three-fold before and after the extractive distillation for titration with a coulometer Karl Fischer 831 KF Metrohm.
DEHYDRATION OF BIOETHANOL EMPLOYING A COMPOSITE MATERIAL LI-SI02 ON A FIX BED REACTOR SYNTHESIS OF l-METHYL-3-METHOXYSILYL PROPYL IMIDAZOLIUM CHLORIDE
Stoichiometric amounts of (3-chloropropyl) trimethoxy sylane (Merck S.A) and 1-methyl imidazolium (Merck, S.A.) were mixed and heated at 78°C under inert atmosphere and reflux. The produced l-ethyl-3-methoxysilyl propyl imidazolium was rotoevaporated, washed and dried under vacuum. A yellow viscous material was obtained with 78% yield.
SYNTHESIS OF MESOPOROUS SILICA
Three main types of interactions are involved for this synthesis, according to the nature of the surfactant and the pH of the synthesis media. In alkaline media, where the siliceous species bear negative charges (I-), the interactions with cationic surfactant (S+) are electrostatic (SI") [20] non-ionic surfactants (S°) used in neutral media, allowing hydrogen bonds and Van der Waals interactions (S°l°) [21]. In acidic media, the interaction between the silica and the surfactant is mediated by X" anions as described in S+X~l+ [22]; in this case glycerol was used as a porogenic agent (a renewable, non-toxic, highly available and cheap material) [23] and TEOS (tetraethoxysilane) (Merck S.A) used as a siliceous source. In the first step, hybrid micelles were formed by interaction between the glycerol and TEOS under controlled pH. In the second step, the condensation reaction occurred by the addition of NaF, which is both the starter of the poly-condensation reaction and the precursor of the material with meso- structural properties.
After the evaluation of different reaction parameters it was determined that using CH3COOH at pH 2 as a reaction media, molar ratios TEOS - Glycerol and NaF:TEOS of 1:6 y 1:10, respectively and dried at 90°C for 12 hours- allow to synthesize Si02 with 85% yield.
SYNTHESIS OF l-METHYL-3-METHOXYSILYL PROPYL IMIDAZOLIUM CHLORIDE - MESOPOROUS SILICA COMPOSITE.
A mix of Ionic liquid/silica with a molar ratio of 0,2 was heated at 90°C for 24 hours; the produced composite was filtered and then washed with methanol.
DEHYDRATION OF BIOETHANOL EMPLOYING THE IL-SILICA COMPOSITE MATERIAL.
First tests were performed with the azeotrope mixture in liquid phase throughout 0.5g of composite set in a quartz column at flow rates of 0.1, 0.14, 0.2 and 0.26 mL/min. Results showed that there is no dehydration under those conditions; the explanation is that longer contacting times are required for the hydrogen bond formation between ionic liquid and water molecules.
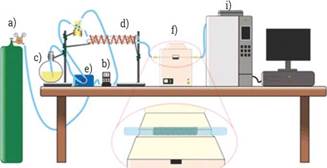
With the results obtained from the azeotrope mixture in liquid phase, the next step was to vaporize it. Figure 1 shows an experimental setup used in order to determine the dehydration percentage of bioethanol (93.45 % v/v); tests were performed based on an experimental design of multilevel factorial type. For the experiments, the biocomposite (0,15 g) was set in a quartz reactor with an internal diameter of 4 mm. The reactor temperatures evaluated were 60, 70 y 80°C, with feed flows of bioethanol of 0.1, 0.2, 0.3 y 0.4 mL/min and -N2- as carrier gas at 130,100 y 80 mL/ min. This system was coupled to a GO system (Shimadzu GC-2014) with FID detector.
MATERIALS CHARACTERIZATION
FTIR and Raman spectra were recorded on a Tensor 27 Bruker with an ATR cell and a Raman confocal microscope LabRam High Resolution (HR) from Horiba, respectively. X-ray diffraction (XRD) patterns were recorded on a BRUKER model D8 ADVANCE equipped with a graphite monochromator powder diffraction system using Cu Kal radiation of 0.15406 nm wavelength operated at 40 kV y 30 mA. The BET (Brunauer-Emmett-Teller) specific surface areas were calculated using absorption data in a relative pressure range of P/P0 = 0.05-0.25. The pore size distribution curve was calculated from the absorption branch using the BJH (Barrett-Joyner-Halenda) method. The total pore volumes were estimated from the amounts absorbed at a relative pressure (P/P0) of 0.99. A quantitative analysis was performed by XRF on a dispersive wavelength instrument 4KW BRUKER model S8 TIGER. A scanning electron microscopy (SEM) was performed using a FE-SEM QUANTA FEG 650 (FEI) system with a LFD detector, at 7 kV equipped with EDX. NMR spectra was recorded on a Bruker Advance III 400 MHz Ultrashield. A mass spectra was recorded using LDI (Laser Desorption Ionization) and MALDI with CHCA as a matrix using a Bruker Ultraflextreme MALDI TOF-TOF (Bruker Daltonics, BiLLerica, MA).
3. RESULTS AND ANALYSIS
EXTRACTIVE DISTILLATION OF BIOETHANOL
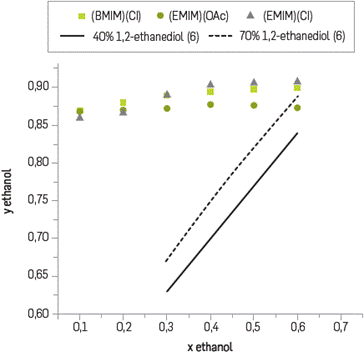
Figure 2 shows the effect of the selected IL on the VLE of the azeotropic system bioethanoL-water; in the same figure simulation data for 40% and 70% of 1,2-ethanedioL used traditionally as entrainer reported by [16], have been included for comparison purposes. From the figure it can be concluded that the studied ILs are able to break ethanol-water azeotrope with high selectivity and capacity. Our results are in agreement with [16] and [24] who reported that imidazolium based-IL exhibit the best entrainer performance with respect to traditional organic compounds used and hyperbranch polymers.
Selectivity was deduced as a function of the composition of liquid (X) and vapor (Y) phases, and activity coefficients through the next equation:
Where 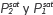 (saturation pressures) were determined by using the Antoine equation [25].
(saturation pressures) were determined by using the Antoine equation [25].
Table 1 shows selectivity values of bioethanol with respect to water after the addition of the IL at specific mass ratios. From the table it is clear that a ratio above of 0.3 selectivity of [EMIM] [Cl] showed the bigger effect. Once it was determined that [EMIM] [Cl] has the Largest effect on enhancement of the relative volatility the next experiment encompassed three consecutive stages of extraction by using a mass ratio of 0.55 and measuring the water content on the vapor phase used by the Karl Fischer method. The time for each extractive distillation was 9 hours until equilibrium. Results showed that starting with bioethanol of 93.45% purity and by using the IL [EMIM] [Cl] it is possible to obtain bioethanol of 99.29% purity after three stages of extraction.
Table 1 Selectivity of bioethanol with respect to water over the mass ratios of IL/bioetanol/wáter studied.
| w | Selectivity of bioethanol with respect to water (S12) employing different ILs. | ||
|---|---|---|---|
| [BMIM](C1) | [EMIM][OAc] | [EMIM](C1) | |
| 0.1 | 0.500 | 0.470 | 0.462 |
| 0.2 | 0.554 | 0.499 | 0.486 |
| 0.3 | 0.605 | 0.524 | 0.609 |
| 0.4 | 0.636 | 0.561 | 0.704 |
| 0.5 | 0.661 | 0.553 | 0.724 |
| 0.6 | 0.671 | 0.538 | 0.739 |
Source: Authors.
DEHYDRATION OF BIOETHANOL BY USING THE COMPOSITE WATER ABSORBENT ON FIX BED REACTOR
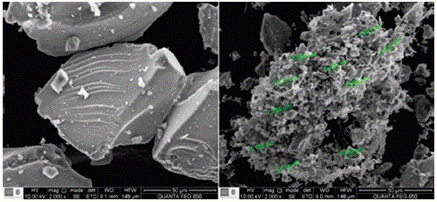
As a result of the synthesis of the composite, a white powder was obtained. The left hand side of Figure 3 shows a SEM image of synthesized silica where amorphous particles with average sizes between 90 y 120um are observed; there is no presence of aggregates evidencing a good dry process; additionally BET analysis allowed to calculate a surface area of 104.02 m2/g and an average porous size of 28 nm. On the right hand side the SEM image of the composite can be seeing; a non-homogeneous micrometric size aggregates with the ionic liquid covering the silica surface can be observed. As expected the calculated surface area for the composite is lower (71.23 m2/g) as well as its porous size (8.74 nm). A detailed description of the composite characterization by FTIR, Raman, 1H and Si-NMR, MS-LDI, XRF and MALDI TOF-TOF has been reported somewhere else [26. A characterization of l-methyl-3-methoxysilyl propyl imidazolium chloride, detected formation of a dimer with one ionic liquid molecule containing the 3-methoxysilyl group, in agreement with calculations reported by Mathews [27 related to ionic liquid dimers formation; in this case the dimer seems to be highly stable and could have middle or diagonal geometry as shown in Figure 4. The characterization allowed confirming that there is a covalent bond between the matrix and the IL with no modification of the solid support.
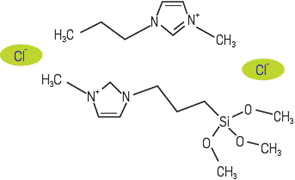
Figure 4 Scheme showing the middle phase structure of synthesized 1-methyl-3-methoxysilyl propyl imidazolium chloride dimer. Based on calculations published by Mathews [27].
Using the setup explained in Figure 1, the bioethanol (93.45%) was vaporized and pumped up to the reactor containing the composite using N2 as carrier gas. The percentage of water extracted from the bioethanol in the dehydration process was calculated using 6.55% as the initial water content in the bioethanol, which was 100% of the content water to be removed under working conditions.
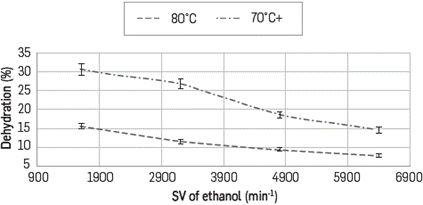
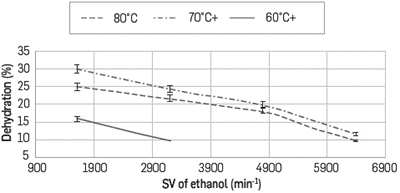
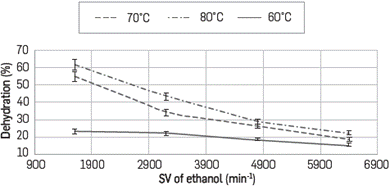
Dehydration profiles as a function of space velocity of ethanol (0.1, 0.2, 0.3 and 0.4 mL/min) for every N2 flow tested (130, 100 y 80 mL/min) are shown in Figures 5, 6 and 7, respectively. In all cases it is observed that to obtaing the higher percentages of removal, it is required to use the lower possible feeding flow.
At a N2 flow of 130 mL/min at 60°C dehydration is not observed at any ethanol flow, and this is explained by noticing that at this high flow there is not enough interaction time between the azeotrope and the composite. Additionally, if the reactor is at 60°C and the azeotrope was evaporated at 80°C, condensation could occur when it reaches the reactor. Under these considerations at 130 mL/min, the maximum extraction percentage was 31% having the reactor at 80°C and at ethanol flow of 0.1 mL/min.
At lower values of space velocity (SV), higher percentages of dehydration are observed when the temperature increases and the N2 flow rate decreases. For example at N2 flow rate of 80 mL/min it reaches dehydration percentages of up to 61% at 80°C and around 55% at 70°C; however it diminishes down to 30% by lowering the reactor temperature to 60°C as shown in Figures 6 and 7. This behavior is based on the type of interactions occurring between Ethanol - LI and water - LI where the formation of H-bonds between the ionic liquid and water is the main interaction. Calculations have been established to the acceptor ability (p-value) of halide anions - Cl- in this case- and this is explained experimentally based on its absorption capacity and hygroscopic behavior. Cl- anion prefers to form in-plane H-bonds. The force driving the absorption is related to internal order, especially when structures with very low energy are favored and the lifetime of the hydrogen bonds is associated to the librational movement of the water and the anion rotation [28].
There is an absorption process thermodynamically favored toward the interaction water-IL; so by increasing the temperature, all molecules of azeotrope are in vapor phase (azeotrope boiling point is 78°C) which occurs partially at 70°C and the dehydration diminishes. By lowering the flow rate from 130 to 80 ml/min there is more residence time with the ionic liquid in the composite with more interaction between the species.
The tendency of [BMIM][Cl] and [EMIM][Cl] to H-bonding with water has been studied with theoretical calculations showing that water is attracted to the neighboring Cl- where the anion prefers to form in-plane H-bonds.
Additionally, it has been demonstrated through calculations that the water forms different kind of complexities where both protons form H-bonds with two anions, and the anion has low coordination, as in this case: CL-H-O-H-CL.
In summary, what happens is an absorption process thermodynamically favored toward water molecules due to favorable energy requirements. As the temperature increases, more molecules are in vapor phase which increases the dehydration process's efficiency. Comparing the two dehydration processes evaluated, it was found that by using pure ionic liquid as entrainer it is required to use 0.45 g for removing 46.79% of water from 15 ml of ethanol (93.45%), energy is required as the extractive distillation is performed at 80°C, but there is an additional energy required to homogenize the bioethanol and the entrainer. On the other hand, when dehydration is performed by using the composite, there are additional costs for the composite preparation and, again, additional energy is required as the dehydration is performed at 80°C; however, the process is performed in just few minutes and the amount of ionic Liquid required is only 0.0724 g to remove the 78,17% of water from 15 ml_ of ethanol (93.45%).
An additional issue to be addressed was the possibility to regenerate and to reuse the ionic liquids which were evaluated for both the extractive distillation and the composite. The ionic liquids used as entrainers were regenerated by drying them at 110°C after being reused between 10 and 13 times; in all cases their efficiency did not drop below 15%. The composite material was also generated after three cycles of dehydration by drying it at 110°C and its efficiency did not diminish to more than 2%. Additional advantages of the composite include the fact that is solid material which can easily handle and the extraction process is less time consuming.
CONCLUSIONS
In summary the work herein shows that imidazolium ionic liquids can be used as entrainer for breaking the azeotrope ethanol: water and that those ionic liquids can be immobilized on porous solid material and used in extraction processes, combining advantages such as selectivity, less time consumption, recyclability and significantly lowers (84%) the requirement for the amount of the IL while retaining or even improving the extractive efficiency.