Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Universitas Scientiarum
Print version ISSN 0122-7483
Univ. Sci. vol.15 no.2 Bogotá May/Aug. 2010
Habitat preference of Zoantharia genera depends on host sponge morphology
La preferencia de hábitat de géneros de Zoantharia depende de la morfología de la esponja
A preferência de habitat de gêneros do Zoantharia depende da morfologia da esponja
Pontificia Universidad Javeriana, Bogotá, D.C. Colombia
montenegroj@javeriana.edu.co; laacosta@javeriana.edu.co
Received: 12-06-2010; Accepted: 14-08-2010
Abstract
Objective. Studies about sponge-zoanthid symbioses have been focused on understanding the specificity of the association, rather than testing what are the characteristics that make the host suitable to be colonized. For the first time it is investigated whether the Zoantharia Parazoanthus and Epizoanthus preference is related to the host sponge morphology (shape and mechanical resistance). Materials and methods. Sponges were categorized according to their shape and mechanical resistance. The presence/absence of zoanthids was recorded in 1,068 sponges at San Andres Island, and their habitat preference was evaluated using indices and confidence intervals. Results. 85 Parazoanthus colonies (78% of the total associations) and 24 Epizoanthus colonies (22%) were associated to sponges (10.2% in total). Parazoanthus uses branched and compressible sponges although prefers encrusting and fragile sponges, while Epizoanthus showes the opposite pattern, it can inhabit encrusting and fragile sponges but prefers branched and compressible sponges. Conclusion. These results indicated that sponge morphology is an important trait in zoanthid habitat selection. On the other hand, the similarity in the habitat used by zoanthids suggests the possibility of inter-generic competition if common resources are limited in time and space, while the differential habitat preference allows the competitive coexistence of both genera.
Key words: Epizoanthus, host, Parazoanthus, symbiont, sponge, morphology.
Resumen
Objetivo. Los estudios sobre la simbiosis entre zoantídeos y esponjas se han centrado en la comprensión de la especificidad de la asociación, en lugar de explorar cuáles son las características que hacen que un huésped sea adecuado para ser colonizado. Por primera vez se investiga si en los Zoantharia Parazoanthus y Epizoanthus la preferencia está relacionada con la morfología de la esponja hospedera (forma y resistencia mecánica). Materiales y métodos. Las esponjas fueron categorizadas según su forma y resistencia mecánica. La presencia/ausencia de zoantídeos se registró en 1.068 esponjas en la Isla de San Andrés y la preferencia de hábitat se evaluó utilizando diferentes índices e intervalos de confianza. Resultados. 85 colonias de Parazoanthus (78% del total de asociaciones) y 24 colonias de Epizoanthus (22%) se encontraron asociadas a esponjas (10,2% en total). Parazoanthus usó esponjas ramificadas y compresibles, pero prefirió las incrustantes y frágiles; mientras que Epizoanthus demostró el patrón contrario, habitando en las esponjas incrustantes y frágiles, pero prefiriendo las esponjas ramificadas y compresibles. Conclusión. Estos resultados indican que la morfología de las esponjas es un aspecto importante para la selección de hábitat por parte del zoantídeo. Por otra parte, la similitud en el hábitat usado por los zoantídea sugiere la posibilidad de competencia entre géneros si los recursos que comparten llegan a ser limitantes en tiempo y espacio; mientras que, la diferencia en la preferencia de hábitat permitiría la coexistencia competitiva de ambos géneros.
Palabras clave: Epizoanthus, hospedero, Parazoanthus, simbiontes, esponja, morfología.
Resumo
Objetivo. O estudo sobre a simbiose entre zoantídeos e esponjas têm-se centrado na compreensão da especificidade da associação, em vez de explorar quais são as características que tornam ao hospedeiro adequado a ser colonizado. Pela primeira vez se pesquisa nos Zoantharia, Parazoanthus e Epizoanthus, se a preferência está relacionada com a morfologia da esponja hospedeira (forma e resistência mecânica). Materiais e métodos. As esponjas foram classificadas de acordo com a sua forma e resistência mecânica. A presença/ausência de zoantídeos foi registrada em 1.068 esponjas na ilha de San Andres e a preferência de habitat foi avaliada através de diferentes índices e intervalos de confiança. Resultados. 85 Parazoanthus (78% do total das associações) e 24 Epizoanthus (22%) foram encontradas associadas com esponjas (10,2% do total). Parazoanthus utilizou esponjas ramificadas e compressíveis, mas preferiu as incrustantes e frágeis; enquanto Epizoanthus demonstrou o padrão oposto, habitando nas esponjas incrustantes e frágeis, mas preferindo as esponjas ramificadas e compressíveis. Conclusão. Estes resultados indicam que a morfologia das esponjas é um aspecto importante na seleção do habitat pelos zoantídeos. Além disso, a semelhança do habitat utilizado pelos zoantídeos sugere a possibilidade de competição inter- gêneros se os recursos compartilhados são limitantes no tempo e no espaço; enquanto que, a diferença na preferência de habitat permitiria a coexistência competitiva de ambos os gêneros.
Palavras-Chave: Epizoanthus, hospedeiro, Parazoanthus, simbiontes, esponja, morfologia.
Introduction
Habitat preference is a subject of great importance that allows researchers to infer the ecological requirements of a given organism, and to explain its abundance and spatial distribution (1). It is also important to understand ecological (competition, local extinction, coexistence) and evolutionary processes (adaptations, niche separation, and speciation) in a changing world. Habitat is defined as the place that has all the resources (biotic and abiotic) and conditions for survival, reproduction and the establishment of local populations (2, 3), or the place (spatially limited) where density or other population parameters are different from those of other localities or contiguous patches (4). For example, in symbiotic (host and guest) relationships, the host will be the space and habitat for the guest, and different host types (patches) will define the symbiont population density. However, the space, as part of the organism's habitat, can be available or not, depending on the presence or absence of limiting factors (physical and biological) that prevent the establishment, survival or reproduction of any given organism (host or symbiont). According to Brinkman et al. (5) the use of an available habitat is linked to its abundance in the system, but it does not mean that all the available habitats will be used. On the other hand, habitat preference is understood as a consequence of selection or asymmetrical use of some resources over others by each individual in the population (in a nonrandom way), from all the available habitats (6, 4), and must be evaluated at the population level (7). According to Litvaitis et al. (8) and Matthiopoulus (9) habitat preference can only be inferred, or indirectly evaluated, through the use that the organisms make of physical and biological resources in the available habitats. However, results may be misinterpreted because the preference for some particular resources is commonly extrapolated as the organism's habitat preference (all of the resources and conditions), when only a part of its ecological requirements has been evaluated (10).
The asymmetric use of any resource depends on the organism's requirements, with criteria that vary in time and space in relation to life cycle stages or even within a particular stage when the resources change. But it also depends on the quality, quantity and availability of resources in a particular patch (6). Some researchers have proposed that habitat preference must be evaluated as the difference between the relative proportions of all used habitat and its availability (potential use). This statement implies selecting a habitat and establishing realistic categories depending on the organisms life history to measure the habitat categories that the organisms use, do not use, select (asymmetric use), and prefer at the population level.
Considering the arduous and complex fieldwork required to assess all the resources used by an animal, and in order to build up new theory, we propose that habitat preference may be easily evaluated by examining symbiotic interactions such as those that involve guest and host (11). In particular, sessile organisms could be used as an example for habitat preference assessment because once the selection for a particular substrate (habitat) has been done by larvae, it will not change through the remaining life cycle of the organism, and the host used as habitat (easily delimited) should provide all or the primary resources required for the symbiont survival, growth, and reproduction. In consequence, symbiont habitat preference could be inferred based on few resources and with less sampling effort.
In the marine environment, sponges (sessile, filter feeding organisms with large morphological plasticity) are an important component of reef ecosystems and are well known for having a large number of symbiotic associations (12).
Chavarro et al. (13) explain the benefits that sponges provide to their symbionts, such as substrate, microhabitat, shelter and food, as it happens with the Anthozoa, Gastropoda, Bivalvia, Polychaeta, Pycnogonida, Maxillopoda, Malacostraca, Ofiuroidea, Zoantharia and Asteroidea (14). The sponge-zoanthid association (Cnidaria, Anthozoa, Zoantharia, suborder Macrocnemina) is widely distributed in tropical and subtropical waters (i.e. Pacific, Mediterranean; 15), from the intertidal zone down to the deep sea. These symbioses are common also in the Caribbean region, where two genera, Parazoanthus (4 species) and Epizoanthus (2 species), are associated to 14% of the total described sponges (close to 92 species; 16).
Studies related to sponge-zoanthid symbioses have been focused mainly on specificity rather than on preference. Specificity is defined by Lincoln et al. (17) as the state of being restricted to a given species. Other definitions indicate that it is the taxonomic range of hosts that can be used by a specific symbiont; the quality of being specific to a particular organism (on which or in which it lives) rather than organisms in general, or the host to symbiont ratios (18). Studies about specificity on sponge-zoanthid associations began with West (19), who quantified the relationship of Parazoanthus and Epizoanthus with their most common host sponges. Later, Crocker and Reiswig (20) inferred that Parazoanthus and Epizoanthus substrate specificity was "relatively high" (50% of zoanthids species are restricted to a single or a few host sponges). In a more recent, intensive and extensive study done in the Caribbean, Swain and Wulff (16) did not agree with the last statement after examining the pattern of specificity at multiple taxonomic levels. Most Parazoanthus and Epizoanthus appeared to have a low degree of specificity and were able to successfully associate themselves with many species of sponges, whereas sponges were highly specific to zoanthids (species accepted as partners); most of the sponge species are exclusively associated with a single zoanthid species and none of the hosts with more than two.
Currently it is known that the sponge-zoanthid association goes from diffuse to specialized. In the Caribbean region zoanthids colonize several different sponge orders and use many sponge species: 46 sponge species are colonized by Parazoanthus swiftii, 22 by P. parasiticus, 13 by P. catenularis, 8 by P. puertoricense, and 5 by Epizoanthus cutressi (16). Epizoanthus sp. nov. sensu Crocker and Reiswig (20) is the most specialized species, colonizing a single sponge genus (three Plakortis species). It is also known that new species combinations in the spongezoanthid symbioses may occur in nature depending on the island, depth, habitat studied and sampling effort (16). This fact may increase the known habitat amplitude for a particular zoanthid species, affecting the evaluation of any used resource (i.e. substratum) since more categories will have to be considered.
None of the previous authors have objectively evaluated preferences as the difference between the relative proportions of all used resources, and their availability (potential use) has not been measured. Mathematical indices, graphical methods, and statistical procedures (confidence intervals) have not been applied, nor has a particular variable of the host sponge been fully evaluated. In consequence, the following question remains: is there a zoanthid preference for any particular resource provided by the sponges? In our study, by using an approach different to the traditional specificity approach, we tried to test whether sponge morphology is an important trait in zoanthid habitat preference. That is based on Crocker and Reiswig's (20) hypothesis that sponge morphology and structure (spicules) may be a characteristic favouring zoanthid larvae colonization. We assume that if zoanthid preference really exists in nature, this preference will become evident even working at higher taxonomic levels (genera) as it has happened in other studies on biological patterns.
Parazoanthus and Epizoanthus differ in a variety of ecological characteristics: 1. The degree of intimacy with the host; while adult colonies of Parazoanthus live on or beneath the host sponge surface, in Epizoanthus the colony is embedded in the sponge pinacoderm (only tentacles remain exposed). The degree of intimacy has been negatively correlated with the number of host sponge species, being lower for Epizoanthus (16). 2. The volume and size of expanded polyps as well as the tentacle number are lower in Epizoanthus than in Parazoanthus. Those variations in characteristics could be mechanisms to avoid competition via niche differentiation (different host species) as suggested by Swain and Wulff (16). Testing preference by using a particular and important resource such as sponge morphology will give us new clues related to this hypothesis, and can be useful in revealing the underlying ecological and evolutionary factors in the symbiosis.
Materials and methods
The quantification was carried out at the oceanic island of San Andres, which is part of an extensive archipelago in the Colombian Caribbean (13° 19'- 13° 31' N, 81° 20' - 81° 25' W; Zea 1987; Díaz et al. 1995). "Blue Wall" and "La Piscinita" sites were selected in order to maximize the chances of finding sponge-zoanthid associations and to cover the highest possible number of habitats (windward and leeward sides, respectively) and microhabitats within areas containing a high coral reef development based on richness and coverage within a depth range of 5 to 30 m (Figure 1).
The geomorphologic profile of Blue Wall is characterized by a reef flat starting at 5 m in depth and composed of dispersed coral colonies down to 10 m; this terrace ends in a deep slope at 300 m off the SE coast, dominated by corals (Diploria sp., Millepora sp., and Porites sp.) and sponges (21, 22). In contrast "La Piscinita" or "Poxhole" is a well developed fringing reef, with a fossil coral reef terrace at 4 m in depth, and a high coral diversity and cover down to 20 m in depth, where the slope starts (dominated by Montastraea sp. and Agaricia sp.) (21). In San Andres, 89 sponges (22) and five zoanthids have been reported (23), with the exception of Epizoanthus sp. nov.
Data Sampling
In each study area, 12 haphazard transects (50 x 1 m) were set with a rope in the most diverse sites. Two transects per depth were quantified, parallel to the coast, separated 10 m away. The sampling was done every five meters between 5 to 30 m depth. Total diving effort was of approximately 1,968 min (five trained divers). All of the sponges and the presence/absence of Zoantharia (Parazoanthus and Epizoanthus) within transects (50 m2) were recorded. Sponges were recorded and classified according to their shape, following categories described in Bell and Barnes (24) with some minor modifications, shown here in parentheses: cup, tubular, encrusting (0-1 cm in thickness), globular (including massive types), and branching (including arborescent types). Sponge taxonomy was not studied in this project, but some interactions were recorded in a videotape.
The cup-shaped sponges were identified by an inverted cone shape, a terminal oscule with a diameter greater than its base, and a single vertical projection. Tubular sponges have one or more cylindrical elongations with a diameter smaller or equal to the oscule located in the apical region, and projected from a common base. The encrusting sponges cover the substrate without a defined shape (25). Globular sponges have wide bases, soft texture, thickness, compact consistence, and can have a defined shape. Branching sponges have elongated forms, a small base, and branch diameter relatively constant (25), (Figure 2).
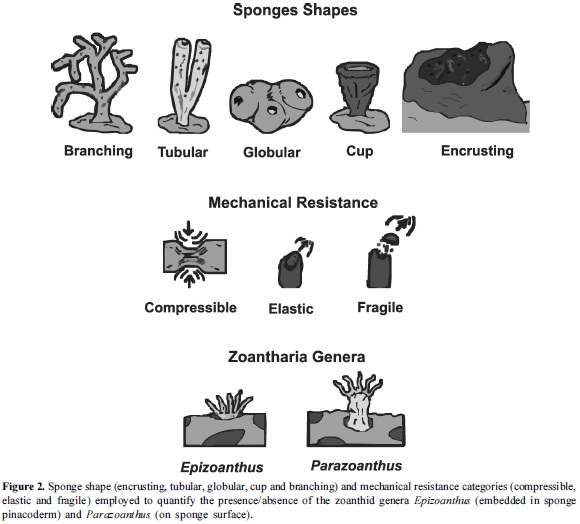
Sponges were also classified according to their mechanical resistance into compressible, elastic and fragile. We deduced these categories from the study by Chanas and Pawlik (26) on sponge mechanical strength. Compressible sponges were defined as those that return to their initial state after removing the pressure force applied in opposite directions by finger compression without suffering structural damage (27), (Figure 2). Elastic ones are able to recover their original shape after a tension force has been applied pulling away any of the sponge edges with two fingers (27). Fragile sponges are susceptible to fragmentation when pressure and tension forces are applied simultaneously (27). In this case a small portion at the sponge's edge was compressed and pulled simultaneously to record whether it would fragment easily (Figure 2). External forces were applied using the thumb and index fingers.
Parazoanthus and Epizoanthus genera were identified in situ using descriptions made by Crocker (28) and West (19), and a taxonomic key developed for San Andres Island (30). In the Caribbean Region there are four species of Parazoanthus reported: P. parasiticus, P. puertoricense, P. swiftii, and P. catenularis; and two species of Epizoanthus: E. cutressi and Epizoanthus sp. nov. (16), the latter being the only one not reported previously in the study area.
Data analysis
Data on habitat preference were analyzed for each genus independently using HaviStat© v. 1.0, a computer application designed to carry out all the procedures indicated below (30). The χ2 and G tests were used to test the null hypothesis that resource use (host sponge shape) occurs in accordance to their availability considering all habitats simultaneously (sponges).
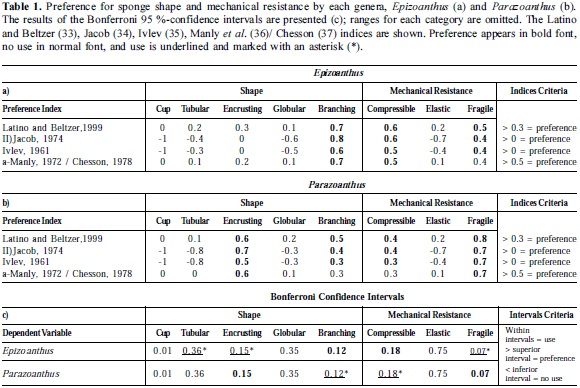
The niche amplitude indices of Colwell and Futuyma (31) and Hurlbert (32) were applied to infer whether the sponges were used asymmetrically. To determine which sponge shape is preferred by zoanthids, the indices of Latino and Beltzer (33), Jacob (34), Ivlev (35), Manly et al. (36)/ Chesson (37) were applied ("/" meaning similar indices). To identify which types of sponges were used by zoanthids and to corroborate preferences, the Bonferroni 95%-confidence intervals were used (38). It is important to clarify that these indices were unable to show habitat use while confidence intervals did. In accordance to Hall et al. (39) a resource or habitat is considered unused when the studied organism is not found often or frequently, or when the computed index value falls below an arbitrary criterion established by the author. A graphical analysis to contrast the sponge availability (total sponges sampled) with zoanthid use (category frequency) was done following Yu and Lee (40).
Results
From the total 1,068 sponges investigated, 109 were associated with Zoantharia (10.2 %). Out of those 109 sponges with the association, 85 were associated with Parazoanthus (78 % of the total symbioses) and 24 with Epizoanthus (22 %).
Parazoanthus and Epizoanthus frequencies were dependent on both sponge shape (Parazoanthus χ2 = 110.6, df = 4, p <0.001, n = 85; G = 96.7, df = 4, p <0.001, n = 85; Epizoanthus χ2 = 36.4, df = 4, p <0.001, n = 24; G = 24.0, df = 4, p <0.001, n = 24) and mechanical resistance (Parazoanthus χ2 = 64.8, df = 2, p <0.001, n = 48; G = 43.4, df = 2, p <0.001, n = 48; Epizoanthus χ2 = 33.3, df = 2, p <0.001, n = 38; G = 27.6, df = 2, p < 0.001, n = 38). According to the four indices, Parazoanthus preferred encrusting and branching sponges, while Epizoanthus only preferred branching sponges. Both genera preferred fragile and compressible sponges. However, the Bonferroni confidence intervals indicated that Parazoanthus used branching and compressible sponges, but preferred encrusting and fragile ones, while Epizoanthus showed an inverse pattern; it used encrusting and fragile sponges but preferred branching and compressible sponges (Table 1).
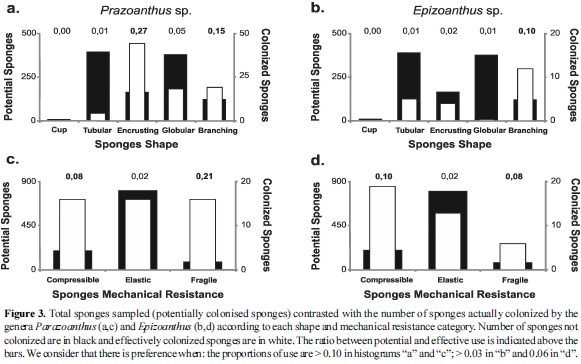
When the total number of sponges sampled (available or potentially colonised by zoanthids) were independently contrasted with the Parazoanthus and Epizoanthus colony frequencies by using graphs, Parazoanthus showed preference for encrusting and branching shapes, while Epizoanthus only for branching; both genera preferred compressible and fragile sponges (Figure 3). The niche breadth indices revealed that both shape and mechanical resistance were not exploited or uniformly used by zoanthids.
Discussion
The results indicate that both zoanthid genera use the sponge shape and mechanical resistance categories asymmetrically; Parazoanthus prefers encrusting and fragile sponges and Epizoanthus branching and compressible ones.
Shape
The differences between genera in sponge shape preference could be explained by the possible feeding strategies used by each genus according to its location on the host (41) or intimacy level (16). Parazoanthus species are passive filter feeders and autotrophic, while Epizoanthus could be considered an active filter feeders that take advantage of the host filtration system without generating their own water flow.
Species of the genus Parazoanthus live on top or beneath the host sponge surface and prefer encrusting sponges, a sponge shape that allows covering more surface area in comparison with other sponge shapes, and thus maximizing the sponge organic matter uptake through gravitational sedimentation. Rubenstein and Koehl (43) indicated that some zoanthids could be considered as passive filter feeders and consequently Parazoanthus may benefit from the sponge shape. They are unable to generate water flow by themselves as it has been observed in other marine species, depending largely on the natural water currents to bring exogenous food within the range of their tentacles. It is possible that large polyps and higher tentacle numbers in Parazoanthus (16) besides the strategy of covering 75% to 100% of the host sponge surface (P. swiftii, 16) are adaptations to maximize rainfall capture, predation, and at the same time light uptake.
It is also well documented that encrusting shapes of benthic organisms maximize the obtainment of light. Parazoanthus parasiticus, Parazoanthus catenularis and Epizoanthus cutressi have zooxanthellae (Symbiodinium spp., 19) but it is unknown if they are obtained from the parental colony (incorporated via the gametes or larvae formation) or from the water column. Swain and Wulff (16) found that sponges that host zooxanthellae are nearly exclusively associated with zoanthids species that host endosymbiont zooxanthellae too, suggesting a shared strategy for maximizing exposure to sunlight (16). As a consequence, the encrusting shape (maximum superficial area covered) of host sponges will improve the photosynthetic activity of the zoanthid symbiotic algae and the capture of organic matter. Although most encrusting sponge species are often overtopped and shaded, the most abundant genus in the study area - Cliona (Table 2) is frequently found under high light exposure conditions and associated to P. parasiticus. In the sea anemones Bunodeopsis globulifera and Bunodeopsis antilliensis the superficial area and accessory structures are good examples of how to maximize the uptake of both precipitated organic matter and light (44, 45).
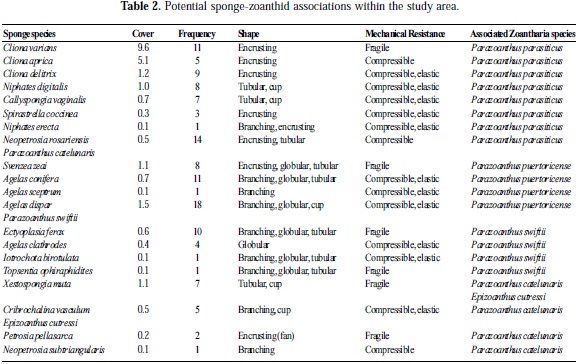
The listed associations are based on: the sponge morphologies reported and described by Zea (22) for San Andres Island, the relative cover of sponges recorded in 56 sampling stations during 1992-1997 (Zea S., Pers. Com.), unpublished sponge frequency data (sponge presence/ location; Zea S.,Pers. Com.), the zoanthids reported by Acosta et al. (23) in San Andres Island (Colombian Caribbean), and the sponge-zoanthid association reported in the Caribbean by Swain and Wulff (16).
On the other hand, Epizoanthus polyps which are embedded in the sponge pinacoderm (19) may take advantage of the water flow generated by the sponge (13), by intercepting transported organic matter (by direct impact of particles; 43, 41), as has been proposed for some symbiotic filter feeding polychaetes (Sabellidae sp.).
Kaandorp (46) suggested that the branching shape in any organism will increase the surface area in contact with the water column, partly explaining the relative higher nutrient uptake. In consequence for branching sponges, a higher number of incurrent channels in relation with their environment will maximize the filtration rate, particularly if the nutrient diffusion coefficient is dependent on water flow (47). The hypothesis that Epizoanthus may benefit from the host flow due to its higher degree of intimacy could also help to explain the low number of host sponge species associated to this genus, found by Swain and Wulff (16).
Mechanical resistance
Mechanical resistance, as well as other physical characteristics of sponges (shape and color) result from the influence of several environmental conditions (48) such as current, turbidity (organic matter and light), and settlement substrate type. Resistance is also provided by spicules and proteins, which depending on their composition and concentration determine the sponge matrix structure and its tensile strength.
The preference for fragile and compressible sponges is difficult to explain due to the lack of studies quantifying mechanical resistance and explaining how a particular matrix structure can be modified due to the presence of a symbiont. For example, histological studies have shown the building up of a thick network of spongin (by lophocytes) when cirripedes settled in the osculum of Ircinia fasciculate, as well as a massive proliferation of microscleres and an increase of fibrogenesis in Geodia cydonium colonized by polychaetes (49, 50). In the spongezoanthid associations, the sponge Calcifibrospongia actinostromarioides is able to physically react to zoanthids by reorganizing its own skeletal elements to form a cyst (50).
The sponge matrix structure could be an important factor for the selective settlement (larvae chemotaxis) and the development of the zoanthids. Two mechanisms explain the actual establishment of zoanthid-sponge associations, through larval settlement and direct contact (grafting), where portions of a zoanthid colony already infesting a sponge migrate between adjacent hosts, as in the case of P. swiftii (28). It has been assumed that sponge-zoanthid interactions could start, as in other benthic cnidarians, with: 1. the planktonic larvae guided to potential substrates by chemical signals (51), 2. the selection and establishment on an adequate substrate (in the case of zoanthids with obligate symbiosis with sponges) (16), 3. the metamorphosis to polyp stage (52), and finally, 4. the colony growth through asexual reproduction (budding; 51, 50). However, these processes have never been observed (53), or even worse, the planktonic larvae which have not been well described for Parazoanthus and Epizoanthus might not be identical.
According to Crocker and Reiswig (20) the megascleras silica spicules that project on the surface of Iotrochota birotulata penetrate the polyp mesoglea of Parazoanthus swiftii and could be used as anchoring structures in the colonization process. After reviewing the work of Zea (22) who described 89 Colombian Caribbean sponges in detail, we conclude that the higher megaescleras spicule frequency is found on fragile sponges (28 %; including also most encrusting shapes), followed by compressible (26 %) species, and finally in elastic (19 %) sponges. This trait besides the three-dimensional spicule organization could explain the selective colonization of Parazoanthus on fragile sponges and of Epizoanthus on compressible sponges. The importance of the sponge matrix structure (spicule composition, concentration, and organization) for zoanthid larvae colonization is an open research field that deserves further investigation in order to understand the underlying mechanism of the symbioses and the co-evolutionary race of both groups (finding a suitable sponge to colonize vs. avoiding or facilitating colonization).
Considering indirect evidence as the general sponge dominance (habitat availability) in San Andres Island (Table 2) and the sponge-zoanthid associations known for the Caribbean, it is possible to infer that P. parasiticus and E. cutressi are the most common zoanthids in the study area. This hypothesis was validated recently (November 2009) at the same study sites (San Andres) where sampling of both genera revealed relative frequencies of 86.6% and 75% of P. parasiticus and E. cutressi, respectively. The dominant sponge, Cliona varians, is one of the few having the shape and mechanical resistance (encrusting and fragile, respectively) appropriate for P. parasiticus (Table 2), and consequently Cliona should be preferentially colonized by this zoanthid that uses a wide range of sponges with different morphologies (Table 2). Also, according to Crocker and Reiswig (20), Cliona was one of the sponges most frequently colonized by P. parasiticus in Barbados.
On the other hand, the cover of Cribrochalina vasculum in the study area is small, although this small cover could offer the morphological characteristic required by E. cutressi (branching and compressible). As claimed by Crocker and Reiswig (20), this zoanthid is apparently restricted to a few primary host sponge species as an acceptable settling surface. Epizoanthus sp. nov., however, had not been reported previously in San Andres, and its three single hosts (Plakortis spp.; 20) seem to be rare within the study area.
Given that Parazoanthus and Epizoanthus are using similar resources (encrusting and branching sponges, with fragile and compressible mechanical resistance), and recognizing that these taxa as other sessile organisms are substrate-limited, there is the possibility of past interspecific competition under limiting host sponges conditions. The first evidence of competition was presented by West (19) for Parazoanthus catenularis and Epizoanthus cutressi, which colonize and compete for space on the same host sponge, Xestospnogia muta. Later, Swain and Wulff (16) showed that these two species can also colonize Cribrochalina dura and C. vasculatum, that P. catelunaris and P. parasiticus colonize Neopetrosia proxima and Xestospongia rosariensis, and P. puertoricense and P. swiftii colonize Agelas dispar and A. sventres, respectively. Only in Xestospongia muta, a simultaneous colonization of both genera has been reported. The current consequences of such past competition could be the divergence on host selection, settlement location and growth of the two zoanthid genera, and the differential strategies of food and energy obtainment. Our findings suggest that the preferences observed in shape and mechanical resistance could be a possible outcome of competition processes in the past. The persistence of their differences until present would allow the coexistence of both genera. According to Crocker and Reiswig (20) the difference in substrate preference could be an adaptation that contributes to the coexistences of both genera.
Further research to test habitat preference and to infer that sponge morphology influences the 'choice' of the zooanthids larvae will imply: 1. taxonomic information at the species level for sponges and zoanthids (difficult to achieve since at least 700 named sponge species are known), in order to distinguish patterns resulting from shared evolutionary history from those resulting from morphology per se; 2. to measure sponge cover instead of abundance (real habitat available to be colonized by larvae); 3. to quantify zoanthid larvae mortality (pre and post settlement); 4. to test the assumptions of potential sponge-zoanthids associations within the study area; 5. to verify the symbiotic associations; and 6. to analyze the relationships between shape, mechanical resistance and the sponge skeletal properties (matrix structure) to find out which is the factor that most influences the association patterns.
Conclusions
In conclusion, results suggest that sponge morphology, that is shape and mechanical resistance, is an important factor in habitat selection by Parazoanthus and Epizoanthus. The use of similar resources by both genera raises the possibility of an inter-generic competition occurring in the past (niche differentiation and evolution); while differential habitat preference may be allowing the coexistence of both genera in the present. These results are a general approach to explain the nature and functionality of the sponge-zoanthid association and the first step to clarify which are the resources that drive the association. Future studies must investigate these patterns of morphological preferences at the species level for both sponges and zoanthids.
Acknowledgments
Thanks to Dr. Diego Cabrera, Romana Miles, Alfonso Cifuentes and to the anonymous reviewers for their generous help with the English version, scientific comments and corrections to the manuscript; also to the students of the Coral Reef Ecology course delivered in 2005, 2006 and 2009 at the Pontificia Universidad Javeriana for their assistance in the field work.
Finantial support
This research was funded by the authors and supported by the Department of Biological Sciences at the Pontificia Universidad Javeriana.
Conflict of interest
The authors state that results presented in this article do not involve conflict of interests.
Referencias
1. Manly B, McDonald L, Thomas D. Resource Selection by Animals. Statistical Design and Analysis for Field Studies. Chapman & Hall, London, UK. 1993, 220p. [ Links ]
2. Batzli GO, Lesieutre C. The influence of high quality food on habitat use by arctic microtine rodents. Oikos 1991; 60, 299-306. [ Links ]
3. Lubin Y, Ellner S, Kotzman M. Web relocation and habitat selection in a desert widow spider. Ecology 1993; 75, 2456-2459. [ Links ]
4. Morris DW. Toward an ecological synthesis: a case for habitat selection. Oecologia 2003; 136, 1-13. [ Links ]
5. Brinkman AG, Dankers N, van Stralen M. An analysis of mussel bed habitats in the Dutch Wadden Sea. Helgoland Marine Research 2002; 56, 59-75. [ Links ]
6. Krausman RP. Some Basic Principles of Habitat Use. Grazing Behavior of Livestock and Wildlife. University of Idaho. Idaho Forest, Wildlife, and Range Experiment Station Bulletin 1999; 70, 85-90. [ Links ]
7. Meager JJ, Utne-Palm AC. Effect of turbidity on habitat preference of juvenile Atlantic cod, Gadus morhua. Environmental Biology of Fishes 2007; 81, 149-155. [ Links ]
8. Litvaitis JA, Tittus K, Anderson EM. Measuring vertebrates use of terrestrial habitats and foods. 1994; 254-274. In Koeln GT, Cowardin LM, Strong LL. Research and management techniques for wildlife and habitats. 5th ed. Wildlife Society Bethesda, Maryland. 1994. [ Links ]
9. Matthiopoulus J. The use of space by animals as a function of accessibility and preference. Ecological Modelling and Systems Ecology 2003; 159, 239-268. [ Links ]
10. Underwood AJ, Chapman MG, Crowe TP. Identifying and understanding ecological preferences for habitat or prey. Journal of Experimental Marine Biology and Ecology 2004; 300, 161-187. [ Links ]
11. Thrall PH, Hochberg ME, Burdon JJ, Bever JD. Coevolution of symbiotic mutualists and parasites in a community context. Trends in Ecology and Evolution 2006; 22, 120-126. [ Links ]
12. Clavico EEG, Muricy G, da Gama BAP, Batista D, Ventura CRR, Pereira RC. Ecological roles of natural products from the marine sponge Geodia corticostylifera. Marine Biology 2006; 148, 479-488. [ Links ]
13. Chavarro SB, Zea S, Diaz JM. Esponjas y otros Microhabitat de ofiuros (Ofiuroidea: Echinodermata) en ambientes arrecifales del archipiélago de San Bernardo (Caribe Colombiano). Boletín de Investigaciones Marinas y Costeras 2004; 33, 29-47. [ Links ]
14. Betancourt LM, González FF, González AB, García G, Rolando BZ. Variation of antimicrobial activity of the sponge Aplysina fistularis (Pallas, 1766) and its relation to associated fauna. Journal of Experimental Marine Biology and Ecology 1998; 223, 1-18. [ Links ]
15. Reimer JD, Sinniger F, Hickman CPJr. Zoanthid diversity (Anthozoa: Hexacorallia) in the Galapagos Island: a molecular examination. Coral Reefs 2008; 27, 641-654. [ Links ]
16. Swain TD, Wulff JL. Diversity and specificity of Caribbean sponge–zoanthid symbioses: a foundation for understanding the adaptive significance of symbioses and generating hypotheses about higher-order systematics. Biological Journal of the Linnean Society 2007; 92, 695-711. [ Links ]
17. Lincoln R, Boxshall G, Clark P. A dictionary of ecology, evolution and systematic. Cambridge University Press, New York, USA. 1998, 371p. [ Links ]
18. Zhou D, Hyde KD. Host-specificity, host-exclusivity, and host-recurrence in saprobic fungi. Mycological Research 2001; 105, 1449-1457. [ Links ]
19. West DA. Symbiotic zoanthids (Anthozoa:Cnidaria) of Puerto Rico. Bulletin of Marine Science 1979; 29, 253-271. [ Links ]
20. Crocker LA, Reiswig HM. Host Specificity in Sponge-Encrusting Zoanthidea (Anthozoa: Zoantharia) of Barbados, West Indies. Marine Biology 1981; 65, 231-236. [ Links ]
21. Geister J, Díaz JM. A field guide to the oceanic barrier reefs and atolls of the southwestern Caribbean (Archipelago of San Andres and Providencia, Colombia). Proceedings of the 8th International Coral Reef Symposium 1997; 1, 235-262. [ Links ]
22. Zea S. Esponjas del Caribe Colombiano. Primera Edición. Catálogo Científico. Instituto de Investigaciones Marinas de Punta Betín-INVEMAR. Santa Marta, Colombia. 1987, 286p. [ Links ]
23. Acosta A, Casas M, Vargas CA, Camacho JE. Lista de Zoantharia (Cnidaria: Anthozoa) del Caribe y Colombia. Biota Colombiana 2005; 6, 147-162. [ Links ]
24. Bell JJ, Barnes DK. Sponge morphological diversity: a qualitative predictor of Species Diversity?. Aquatic Conservation 2001; 11, 109-121. [ Links ]
25. Wulff JL. Resistance vs recovery: morphological strategies of coral reef sponges. Functional Ecology 2006; 20, 699-708. [ Links ]
26. Chanas B, Pawlik RJ. Defenses of Caribbean sponges against predatory reef fish. II. Spicules, tissue toughness, and nutritional quality. Marine Ecology Progress Series 1995; 127, 195-211. [ Links ]
27. Hibbeler RC. Mechanics of materials. 5th Edition. Prentice Hall, New Jersey, USA. 2003, 864p. [ Links ]
28. Crocker LL. The ecology of the Zoanthid-Sponge symbiosis in Barbados. M.Sc. Thesis. Biological Sciences Faculty, McGill University, Montreal, 1978, 98 p. [ Links ]
29. Vargas C. Zoanthideos (Anthozoa:Hexacorallia) del complejo arrecifal y litoral rocoso de la isla de San Andrés, Caribe Colombiano. Pontificia Universidad Javeriana, Bogotá D.C. Colombia. 2002, 49p. [ Links ]
30. Montenegro J, Acosta A. Programa innovador para evaluar uso y preferencia de hábitat. Universitas Scientiarum 2008; 13, 115-124. [ Links ]
31. Colwell RK, Futuyma DJ. On the measurement of niche breadth and overlap. Ecology 1971; 52, 567-576. [ Links ]
32. Hurlbert SH. The measurement of niche overlap and some relatives. Ecology 1978; 59, 67-77. [ Links ]
33. Latino S, Beltzer A. Ecología trófica del benteveo Pitangus sulphuratus (aves: Tyrannidae) en el valle de inundación del río Paraná, Argentina. Organismes i Sistemes 1999; 14, 69-78. [ Links ]
34. Jacob J. Quantitative measurement of food selection. A modification of foraging ratio and Ivlev's electivity index. Oecologia 1974; 14, 413-417. [ Links ]
35. Ivlev VS. Experimental ecology of the feeding of fishes. Yale University Press. New Haven. 1961. In Cock MJW. The Assessment of Preference. Journal of Animal Ecology 1978; 47, 805-816. [ Links ]
36. Manly BFJ, Miller P, Cook LM. Analysis of a selective predation experiment. American Naturalist 1972; 106, 719-136. [ Links ]
37. Chesson J. Measuring preference in selective predation. Ecology 1978; 59, 211-215. [ Links ]
38. Cherry S. A Comparison of Confidence Interval Methods for Habitat Use-Availability Studies. Journal of Wildlife Management 1996; 60, 653-658. [ Links ]
39. Hall LS, Krausman PR, Morrison ML. The habitat concept and a plea for standard terminology. Wildlife Society Bulletin 1997; 25, 173-182. [ Links ]
40. Yu SL, Lee TW. Habitat preference of the stream fish, Sinogastromyzon puliensis (Homalopteridae). Zoological Studies 2002; 4, 183-187. [ Links ]
41. Ilan M, Ben-Eliahu MN, Galil BS. Three deep water sponges from the eastern Mediterranean and their associated fauna. Ophelia 1994; 39, 45-54 [ Links ]
42. Klitgaard AB. The fauna associated with outer shelf and upper slope sponges (Porifera: Demospongiae) at Faroe Islands, northeastern Atlantic. Sarsia 1998; 80, 1-22. [ Links ]
43. Rubenstein DI, Koehl MAR. The Mechanisms of Filter Feeding: Some Theoretical Considerations. American Naturalist 1977; 111, 981-994. [ Links ]
44. Sebens KP, DeRiemer K. Diel cycles of expansion and contraction in coral reef anthozoans. Marine Biology 1977; 43, 247-256. [ Links ]
45. Day RJ. Algal symbiosis in Bunodeopsis: sea anemones with 'auxiliary' structures. Biological Bulletin 1994; 186, 182-194. [ Links ]
46. Kaandorp JA. Morphological analysis of growth forms of branching marine sessile organisms along environmental gradients. Marine Biology 1999; 134, 295- 306. [ Links ]
47. Kaandorp JA, Lowe CP, Frenkel D, Sloot PMA. Effect of Nutrient Diffusion and Flow on Coral Morphology. Physical Review Letters 1996; 77, 2328-2331. [ Links ]
48. Manconi R, Pronzato R. Life cycle of Spongilla lacustris (Porifera, Spongillidae): a cue for environment-dependent phenotype. Hydrobiologia 1991; 220, 155-160. [ Links ]
49. Connes R, Patis J, Sube J. Réactions tissulaires de quelques démosponges vis-a-vis de leurs commensaux et parasítes. Naturaliste Canadien 1971; 98, 923-935. [ Links ]
50. Willenz P, Hartman WD. Skeletal reaction of the Caribbean coralline sponge Calcifibrospongia actinostromarioides Hartman toward an epizoic zoanthidea. In: van Soest RWM, van Kempen TMG, Braekman JC(ed) Sponges in space and time. Biology, Chemistry, Paleontology. Balkema, Rotterdam: 1994; 1, 279-288. [ Links ]
51. Lewis SM. Sponge-zoanthid associations: Functional interactions. Smithsonian Contributions to the Marine Sciences 1982; 12, 465-474. [ Links ]
52. Rodriguez SR, Ojeda FP, Inestrosa NC. Settlement of benthic marine invertebrates. Marine Ecology Progress Series 1993; 97, 193-207. [ Links ]
53. Babcock RC, Ryland JS. Larval development of a tropical zoanthid (Protopalythoa sp.). Invertebrate Reproduction and Development 1990; 17, 229-236. [ Links ]
54. López M, Zea S. Current trends of space occupation by encrusting excavating sponges on Colombian coral reefs. Marine Ecology 2005; 26, 33-41. [ Links ]














