Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Universitas Scientiarum
Print version ISSN 0122-7483
Univ. Sci. vol.17 no.1 Bogotá Jan./Apr. 2012
Simultaneous decolorization and detoxification of black reactive 5 using TiO2 deposited over borosilicate glass
Decoloración simultánea y la desintoxicación de reactivo negro 5 con TiO2 depositadas sobre el vidrio de borosilicato por la técnica de sedimentación.
Descoloração simultânea e a desintoxicação do preto reativo 5 com TiO2 depositadas em vidro de borosilicato pela técnica de sedimentação.
Johana Puentes-Cárdenas1, Alex Florido-Cuellar2, Jairo Cardona-Bedoya2, Paola Bohorquez-Echeverry1, Claudia Campos-Pinilla1, Viviana Gutiérrez-Romero1, Aura Pedroza-Rodríguez1*
1Grupo de Biotecnología Ambiental e Industrial (GBAI). Departamento de Microbiología. Facultad de Ciencias. Pontificia Universidad Javeriana. Bogotá, D.C., Colombia.
2Grupo de Investigación en Materiales Semiconductores y Superiónicos. Departamento de Física. Facultad de Ciencias. Universidad del Tolima. Alto de Santa Helena. Ibagué. Colombia.
Received: 16-12-2011; Accepted: 24-03-2012
Abstract
Objective. Removal and detoxification of azo dye by photocatalysis with TiO2. Materials and methods. TiO2 films were prepared by sedimentation at pH 1.3, using as support a borosilicate glass, annealed for 1 hour at 450 °C. Physical characterization was performed by Scanning Electron Microscopy, X-ray diffraction and UV/VIS spectrometry. Dye Black Reactive 5 removal was carried-out in a quartz photo-reactor. Results. Optical characterization revealed the films displayed evident TiO2 spherical particles of various irregular sizes, porous, and without fractures. The average crystal size was 77.5 nm and 77.7 nm for 50 °C (dried temperature) and 450 °C (annealed temperature) respectively. The energy of the band gap (GAP) was 3.02 and 2.68 eV respectively. Maximum concentration of dye that negatively affected color removal was 80 mg/L (17%). At lower dye's concentrations (10, 50 and 70mg/L) decolorization was greater than 80%. TiO2 films were reused for five consecutive cycles of 6 hours at 10 mg/L (>80%), and three cycles of 10 hours at 70 mg/L (> 80%). Toxicity results demonstrate that Daphnia magna was more sensitive than Lactuca sativa. Conclusion. TiO2 films obtained by sedimentation demonstrated a high reactive black 5 decolorization and COD removal (86% and 100%), as well as toxicity reduction.
Key words: photocatalysis with TiO2, sedimentation techniques, reactive black 5, Lactuca sativa and Daphnia magna.
Resumen
Objetivo. Decolorización y disminución de la toxicidad de un azo colorante usando fotocatálisis con TiO2. Materiales y métodos. Las películas de TiO2 fueron crecidas por sedimentación a pH 1,3 empleando vidrio de borosilicato como sustrato. La caracterización física se realizó por medio de microscopía electrónica de barrido, difracción de rayos X y espectrometría de absorción UV/vis. Los estudios de remoción del colorante Negro Reactivo 5, se realizaron en un fotoreactor de cuarzo. Resultados. De acuerdo con la caracterización óptica, las películas de TiO2 presentaron partículas esféricas, con diferentes tamaños, irregulares y sin fracturas. El tamaño del cristal fue 77,5 nm y 77,7 nm para 50 y 450° C y la energía de banda prohibida fue 3,02 y 2,68 eV, respectivamente. La concentración máxima del colorante que afectó negativamente la remoción de color fue 80 mg/L (17%). A concentraciones más bajas de NR5 (10, 50 y 70 mg/L) la decoloración fue superior al 80%. Las películas de TiO2 se reutilizaron por 5 ciclos de 6 horas a 10 mg/L y 3 ciclos de 10 h a 70 mg/L. Los estudios de toxicidad en Lactuca sativa y Daphnia magna demostraron que D. magna fue más sensible que L. sativa. Conclusión. Las películas de TiO2 crecidas por sedimentación decoloraron el colorante negro reactivo 5 en un 86% y removieron la demanda química de oxígeno en un y 100%, igualmente redujeron la toxicidad.
Palabras clave: fotocatálisis con TiO2, técnica de sedimentación, negro reactivo 5, Lactuca sativa and Daphnia magna.
Resumo
Objetivo. Descolorar e diminuir a toxicidade de um corante azo utilizando fotocatálise com TiO2. Materiais e métodos. Os filmes de TiO2 foram obtidos por sedimentação a pH 1,3 usando vidro de borosilicato como substrato. A caracterização física foi realizada por microscopia eletrônica de varredura, difração de raios X e espectrometria de absorção UV/vis. Os estudos de remoção de corante Preto Reativo 5, foram realizados num fotorreator de quartzo. Resultados. De acordo com a caracterização óptica, os filmes de TiO2 presentearão partículas esféricas com tamanhos diferentes, irregulares e sem fracturas. O tamanho do cristal foi de 77,5 nm e 77,7 nm para 50 e 450°C e a energia de banda proibida foi 3,02 e 2,68 eV, respectivamente. A concentração máxima do corante que afetou negativamente a remoção de cor foi de 80 mg/L (17%), em concentrações mais baixas (70, 50 e 10 mg/L) a descoloração foi superior a 80%. Os filmes TiO2 foram reutilizados em 5 ciclos de 6 horas a 10 mg/L e 3 ciclos de 10 horas a 70 mg/L. Os estudos de toxicidade com Lactuca sativa e Hydra attenuata demonstraram que H. attenuata foi mais sensível que L. sativa. Conclusão. Os filmes de TiO2 obtidos por sedimentação descoloram o corante Preto Reativo 5 e removeram a demanda química de oxigénio em 86% e 100%, respectivamente; do mesmo modo reduziram a toxicidade.
Palavras-chave: fotocatálise com TiO2 técnica de sedimentação, Preto Reativo 5, Lactuca sativa e Daphnia magna.
Introduction
Reactive black 5 ((tetrasodium-4-amino-5-hydroxy-3, 6 (bis(4-(2-(sulfonatooxi) ethylsulfonyl) phenyl) azo)-naphthalene-2, 7-disulfonate)) is a common dye used in the textile industry. It is an azo-dye, consisting of two aromatic rings linked by a nitrogen double bond. This complex structure is considered a chromophore group that is associated with auxochromic amino or hydroxyl groups. This property determines the dye's absorption wavelength, and fiber affinity. Additionally, the dye contains sulphate sodium (Na+ SO3), which acts as a solubilizing agent. Biological degradation implementing traditional treatment systems (activated sludge reactor, upflow anaerobic reactors and bio optional) is slow and may form an intermediate compound, which is more persistent than the original. These intermediates are not detected by spectrometry visible, because they absorb in the ultraviolet range between 200 and 400 nm) (1). For this reason they generate a variable environmental impact related to photosynthesis inhibition, decreased dissolved oxygen concentration, alteration of some populations at different trophic levels, and aquatic ecosystem persistence (2, 3).
The pressure exerted by institutions that regulate national and international environmental policies and the need companies have to implement cleaner production systems have generated concern to evaluate and implement other types of treatments to ensure color removal, chemical oxygen demand (COD), and reduced toxicity. An alternative presented by The Green Chemistry and Solid State Physics is the implementation of heterogeneous photocatalysis with titanium dioxide using natural or artificial light. In this process there are basically two photo-chemical reactions that take place on the surface of TiO2 when irradiated with ultraviolet light (UV): i) photo-induced redox reactions of adsorbed substances and ii) TiO2 induced hydrophilic photo-conversion. TiO2 semiconductor has become, by the combination of these two types of reactions, a potential application in waste-water treatment. The partial transformation or mineralization of the pollutants by the action of a semiconductor, is based on the generation of electron-hole pairs and subsequent recombination processes of interfacial charge transfer (oxidative or reductive) that promote the oxidation of a variety of pollutants and inactivation of microorganism in short periods of time (4,5).
Generally in azo dyes, photocatalytic oxidation near to the azo bond is the attacked site and the aromatic intermediates are released (6). These phenolic compounds can be transformed into organic acid by naphthalene ring opening to form aliphatic intermediates. Under these conditions mineralization is possible with subsequent CO2 formation. However, it has been reported for some compounds the generation of fragments more toxic than the parent compound. Thus, implementation of bioassay with different types of organisms is critical to perform toxicity measurements. Daphnia magna is often used for evaluation of acute, as well as chronic toxicity, in industrial waste-water and dyes. Different countries employ this organism because of its fast-growing properties, ease of maintenance, high sensitivity towards chemical pollutants, and very reproducible results (7). Several authors report D. magna as the best organism for evaluating the effect of semiconductor oxides and effluent post-treatment with an oxidation processes (7-9). Different studies have reported the use of another model, the vegetable Lactuca sativa. With lettuce the aim is to evaluate if the root can germinate or if its growth is inhibited by the action of different materials or intermediates of the photocatalytic treatment. Some reports have described both positive and negative effects on root elongation (9,10). Hence, it can be utilized as a complementary toxicity assay to evaluate an alteration in plant physiology; in particular if water is used in agricultural practice or if its re-used.
An industrial and textile industry scalable and economically viable pilot for a photocatalyst should take into account: substrate type, semiconductor adhesion to the substrate, high UV light photo-oxidation resistance, mechanical stability, and low cost, among others (4). Borosilicate glass or borosilicate is such substrate. Its chemical composition is approximately: silicon dioxide (SiO2:80.4%), aluminum oxide (Al2O3: 2.4%) boric oxide (B2O3: 13%), and sodium hydroxide (Na2O: 3.9%) (11). Due to silicon oxide's (SiO2) modifications, it yields a vitreous material with mechanical stability, low expansion coefficient, high thermal shock resistance, and higher proportion of B2O3 to alkali. Substitution decreases cation migration to the surface of the semiconductor, acting as an obstruction to the photocatalytic process. This is also known as semiconductor poisoning (4). On the other hand, selection of the growth method should also be considered. A variety of methods have been reported, but some are expensive, require special equipment, and the development of large-scale films is difficult to implement (4,5). A good option for film growth is the sedimentation method under the action of gravitational forces. Among the main parameters that affect growth by sedimentation are: i) material size, ii) mono dispersity, iii) density of particles in the solvent, iv) surface charge density of the spheres, v) rate of evaporation of the solvent, and vi) settling velocity of the particles (12,13).
Consequently, this work presents a simple and rapid method to prepare TiO2 films. We employed an economic catalyst material used as an additive in the pharmaceutical industry. To our knowledge its potential to discolor and reduce RB5 dye toxicity has not been reported in the literature. Additionally, the maximum concentration of dye that inhibits the process of photocatalysis was evaluated. Furthermore, we verified the numbers of cycles that TiO2 films can be re-used at a laboratory scale.
Materials and methods
Preparation of TiO2 solutions
The titanium dioxide (TiO2) used for this study was obtained from a commercial product employed as an additive in makeup elaboration applications with the follow characteristics: A white non-porous power, with an average particle size between 45 and 250 pm. The powder has a density of 4.2 g/cc and 0.27% calcination residue. It is soluble in water (0.07%) and acid (0.10%). One gram TiO2 was ultrasonically dispersed in 100 mL distilled water for 10 minutes (pH 6.4). The solution was brought to a pH of 1.3 by adding 500 pL of HNO3.
Preparation of TiO2 films
Borosilicate glass was used as the substrate (3.8 cm x 5 cm). Glass was cleaned before the deposition process. Substrates were successively sonicated for 15 min 15 mL of each: Milli-Q water, ethanol, and acetone, and dried at 50 °C. After washing, substrates were immersed in a TiO2 solution (1% m/v) and placed horizontally at 50 °C for 4 h to dry. This is known as the growth process. Thereafter, substrates were annealed at 450 °C for 1 h. this final product is known as the film. The methodology was repeated twice (12).
TiO2 film characterization
Film surface morphological features were observed using scanning electron microscopy. TiO2 film crystallization behavior was analyzed by X-ray diffraction (XRD) using a X-Ray diffractometer (SIEMENS D-5000, Germany). Grazing incidence technique was used at 1.5 degrees, with a step size of 0.02 degrees and step time of 0.6 seg. TiO2 film grain size before and after calcination treatment was calculated using the Scherer formula (d = 0.94X/ B(2Q) cos 8, where X is the x-ray wavelength, B(2Q) is the full width at half maximum intensity (FWHM), and 8 is the diffraction angle.
An UV/VISs spectrophotometer was used to measure TiO2 film absorption spectra. To determine the TiO2 film's energy band gap (Eg), a direct band gap for TiO2 was assumed. Based on this assumption absorption coefficient (a) is proportional to the square root of the photon energy (hv). The Eg was determined by ploting (ahv J2 versus (hv) and extrapolating in the linear range at a zero absorption.
Quantity of TiO2 deposited (mg) on substrate (borosilicate mg) was determined by calculating the weight difference before and after the growth process, considering total titanium dioxide area recovered.
Kinetics removal and RB5 (IMCRB5) inhibitory minimum concentration
Experiments were carried out in a 200 mL quartz photoreactor (15 cm height and 5.5 cm diameter) with 120 mL dye solution at various concentrations (10, 50, 70, 80 and 100 mg/L). Two films (18.5 ± 1.4 mg TiO2/mg borosilicate) were introduced into each dye solution and were radiated with 2 UV lamps (254 nm) for 10 h. Lamps were placed at 5 cm distance parallel to the glass. A horizontal shaker stirring at 100 rpm was maintained during all experiments. The analytical determinations for all experiments according to APHA (2005) were decolorization in percentage (%), pH, and chemical oxygen demand (COD) (14).
Dye photocatalytic transformation was followed using a spectrophotometric method which recorded the UV/VIS spectrum in every sample (0, 7 and 14 h) in the wavelength range 200-800 nm.
Sequencing batch experiments or reuse of TiO2 flms
To determine the number of cycles capable of supporting TiO2 films, the photocatalytic reactor was inoculated with the same number of TiO2 films and new RB5 dye solutions at 10 and 70 mg/L were added. The process was monitored for 10 h with samples collected at 0, 6 and 10 h. Afterwards, the effluent was removed and the system was loaded with a new batch of RB5. These experiments were carried out for seven cycles with three repetitions. The parameters evaluated were decolorizarion (%) and COD removal (mg/L).
Test organisms
Four different treatments were used to evaluate TiO2 film's photocatalysis dye treatment effects on two organisms Lactuca sativa and Daphnia magna. The treatments were as follow: T1: untreated dye solutions in distilled water at 70 mg/L, T2: photocatalytic treatment without TiO2 removal (the effluent had TiO2 detached from the films during kinetic removal), T3: photocatalytic treatment with TiO2 removal by centrifugation (8,000 g for 10 min), and T4: TiO2 solutions at 1% (m/v) prepared with distilled water in the dark.
Vegetable model (Lactuca sativa)
A static acute toxicity test (120 h exposure) with Lactuca sativa Var. Great Lake Batavia was performed. For this study four treatments were evaluated (Table 1) with five dilutions per sample (D = 12.5%, D2= 25% and D3= 50%, D4= 75% and D5= 100%). Twenty five seeds with similar size, shape, and color were placed on a Whatman No. 3 filter paper impregnated with 4 mL of sample in a Petri Dish and incubated at 22 ± 2 °C for 5 days in the dark. As a positive control Zn+2 at a concentration of 18 mg/L was used. For negative controls hard water was used. After incubation, the average length of roots per sample was estimated and recorded per concentration. Finally, we estimated the concentration that produced 50% inhibition on root elongation (IC50), using the Probit method with a significance level of p < 0.05 (16).
Animal model (Daphnia magna)
Static acute toxicity bioassay (48 h of exposure) was carried-out with Daphnia magna. For this study 25 mL solution was evaluated in five dilutions per sample as previously discussed: T1 - T4 (D1= 12.5%, D2= 25% and D3= 50%, D4= 75% and D5= 100%). As a positive control we used 0.13 mg/L Cr+6, hard water was used as a negative control. Three replicates were performed for each control and dilution: Each sample contained ten 24 h old neonates. Neonates were observed after incubation at 21 ± 1 °C for 24 h and 48 h, with a 16 h light/8 h dark photoperiod, and light intensity of 800 lux. The number of dead organisms was recorded, and the lethal concentration 50 (LC50) was calculated at 48 h, using the Probit method with a significance level of p < 0.05 (17).
Results and discussion
Preparation of TiO2 films
Sedimentation allows particles in suspension present in an aqueous solution to settle based on the effect of gravity. According to this concept, TiO2 suspensions could present agglomeration and sedimentation behavior depending on particle characteristics. Such features include concentration, polydispersity, density, sedimentation rate, specific surface charge and pH (18, 19).
For this study, a 1% (m/v) TiO2 solution was used. The solution prepared probably determined a settling area or hindered settling, due to high solid concentration. This probably caused collision between aggregates producing a massive deposit over the substrate, most likely due to the solution's acidity. In addition, TiO2 is an amphoteric molecule with an isoelectric point of 6.4. In the presence of acidic distilled water the particles were positively charged by proton absorption, producing an electrostatic attraction resulting in sedimentation.
TiO2 films after drying at 50 °C contained 9.86 ± 0.67 mg of TiO2/mg borosilicate. After calcination the amount decreased to 9.41 ± 0.78 mg TiO2/mg borosilicate. Change in TiO2/mg borosilicate quantity could be attributed to evaporation of physically adsorbed water on the film's surface. A similar result was reported by Ao et al. (2007), with weight loss under different thermal dry conditions of TiO2 films prepared by sol-gel methodology (20).
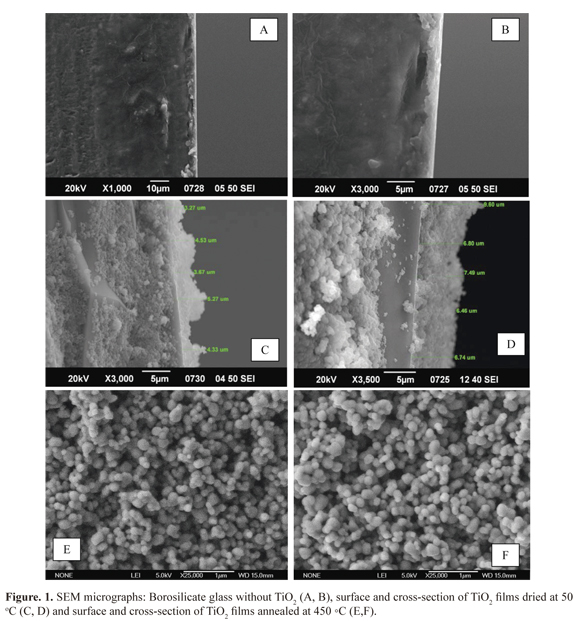
Borosilicate glass without deposit evidenced that front and side of the substrate had a completely smooth surface. This feature might affect the film quality in relation to its homogeneity. In addition, the accelerated sedimentation caused by the pH resulted in a non-homogenous surface (Figure 1 a, b).
On the other hand, films dried at 50 °C and calcined at 450 °C had a thickness of 4.21 ± 0.77 pm and 7.41 ± 1.2 pm respectively (Figure 1 c, d). Formation of TiO2 spherical particles on both films was observed with an irregular and porous surface, with polydisperse aggregates ranging between 200 nm and 70 nm (21). Additionally, films were not cracked by the calcination process. Instead the calcination process helped attach the substrate to the semiconductor oxide (Figure 1 e, f). In addition calcinated films had good mechanical stability, as demonstrated by placing the films under physical stress (sonication). Only 5% detachment was observed. In contrast, films dried at 50 °C had a detachment greater than 80%. The stability was associated with agglomeration and compactation among TiO2 particles. Moreover, double cycle growth could have also promoted attachment where the first layer acted as cement for the second one, increasing the resistance to the ultrasonic wave effect. An evident association between particle size and morphology was observed when comparing commercial TiO2 films and Ti02 Degussa P-25. Conclusively, commercial Ti02 can be used as an easily acquired substitute in Colombia, promoting economic viability for the process (22, 23).
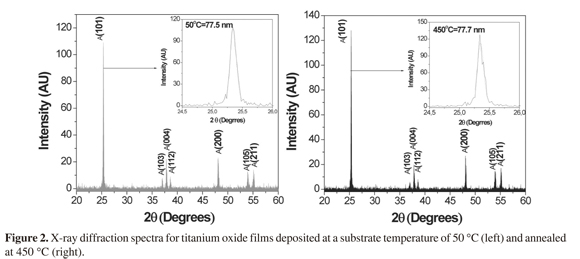
Crystallographic analysis (XRD) is depicted in Figure 2 and demonstrated crystalline films . It was predominately in the anatase phase with planes (101), (103), (004), (112), (200), (105), and (211). In addition, thermal treatment did not induce phase-changes, but increased intensity of some peaks at the following planes: (101), (200), (105) and (211).
These results demonstrate commercial Ti02 stability at high temperatures. Furthermore, increasing temperatures led to a possible semiconductor oxide compaction with an average crystal size of 77.8 nm and 77.3 nm for 50 °C and 450 °C respectively. These results could be associated with a decrease in surface area by a possible collapse between the porous and agglomeration structure from TiO2 and a sintering film phenomenon (23). Furthermore, the average crystal size was similar to that obtained by Verma, 2007 (24), which demonstrated that after three hour heat treatment at 480 °C 40 nm crystals for anatase phase 101 were obtained.
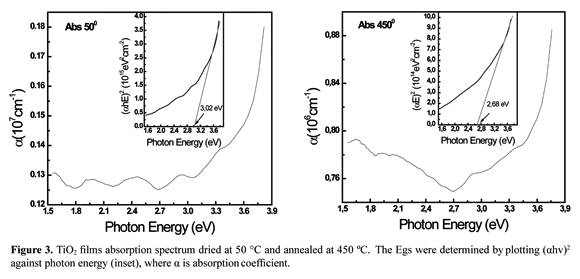
TiO2 films absorption spectrum dried at 50 °C and annealed at 450 °C are illustrated in Figure 3 (a b). Experimental values for Eg were obtained by extrapolation. A linear relationship was established for the curves at the point where absorption was zero. The band gap energies determined were 3.02 eV for the film dried at 50 °C left and right and 2.68 eV for the TiO2 annealing at 450 °C. GAP values were similar to those reported in the literature for crystalline and powder materials such as TiO2 Degussa P-25 used as a reference semiconductor. From these results, it was established that for photocatalysis experiments a radiation source generating a photon with energy equal or greater than the GAP value to induce photooxidation properties and formation of electron-hole pairs is required. This was ensured by using an UV lamp with a maximum emission of 254 nm, approximately equivalent to an energy of 4.8 eV.
Kinetics removal and RB5 (IMCRB5) minimum concentration inhibition
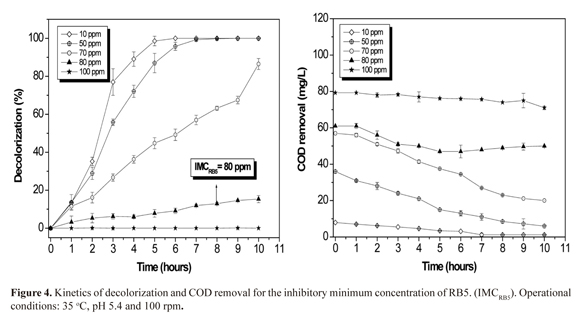
According to decolorizing results, it was determined that minimum concentration of dye (IMCRB5) that inhibits or affects the photocatalytic process (assuming a loss greater than 80% removal) was 80 mg/L (17% at 10 h), followed by 87 and 100% for 70, 50 and 10 mg/L with retention times of 10, 9 and 6 h (Figure 4). Decolorization by TiO2 adsorption in the dark did not exceed 10% for all treatments (data not shown), determining that decolorization was conducted primarily by the photocatalytic process. Hydroxyl radical generated by UV light interaction and catalysis (18.5 ± 1.4 mg TiO2/mg borosilicate) possibly attacked N=N and C-N bonds modifying conjugated to system linked to the dye producing a great structural change. These events resulted in rapid discoloration at the following concentrations: 10, 50, and 70 mg/L (3).
Two factors accounted for decolorization obtained at concentrations of 10, 50 and 70 mg/L: concentration effect of the dye on the photocatalytic process and primary dye adsorption to the semiconductor's surface during stabilization in the dark (30 min). For the first factor, it has been reported that for high dye concentrations UV light's pathway is unable to reach TiO2 films, generating photocatalytic efficiency loss. Regarding the second factor, BR5 dye is anionic, having a sulphonic group generating an electrostatic interaction with the positively charged TiO2 surface. Low concentrations possibly promote this interaction. When the saturation's constant of semiconductor oxide is exceed, the effect is adverse. Active sites of TiO2 are blocked by excess dye, as observed at concentrations of 80 and 100 mg/L (25, 26).
In regards to discoloration percentages our results are consistent with those reported in literature in particular to those documented by Damodar and You, 2010 (3). Although they employed the same dye at high concentrations (100 mg/L) and 0.5 g/L of TiO2 in a 3 L tank,with decolorization greater than 95% after approximately 3.6 h, it is noteworthy that we used a TiO2 concentration 27 fold lower than that employed by Damonar and You (500 mg/L vs. 18 mg/L) In addition, Bergamini et al. 2009 reported a 97% BR5 (70 mg/L) discoloration at 5 minutes after treatment using 4000 mg/L of TiO2 in suspension, a value 200 times higher than that reported in our study (27). Additionally, we observed desorption of TiO2 from the support did not exceed 5% (0.90 ± 0.01 mg of Ti02). This is a low value considering that the sedimentation method and thermal treatment were responsible for attaching semiconductor oxide to borosilicate glass.
Values of pH were measured at the beginning and at the end of the process for each concentration of dye evaluated. At high dye concentration the values ranged between 5.0 ± 0.8 and 4.5 ± 0.3. and low dye concentration pHs were 3.92, 3.03 and 3.00 for 70 mg/L, 50 mg/L and 10 mg/L respectively. This change could possibly be attributed to the formation of acids such as dioxide sulphonic acid, dihidro-dihydronaphthalene, oxalic acid, among others. These products have been reported in the literature as possible intermediates of the photocatalytic degradation (3, 28).
Similarly, COD removal had a comparable behavior to decolorization at high concentrations with removal of 18 and 10% for 80 and 100 mg/L from dye at 10 h (50 and 71 mg/L COD). This demonstrates that TiO2 is affected by the presence of conjugated aromatic structures in high proportion at this concentration. These effects could be due to a hindrance of UV light pathway or a competition between the wavelength and TiO2 for the semiconductor photoexcitation (Figure 4). Contrary, at low concentrations of dye the removal increases with time reaching 85% and 83% at 10 and 50 mg/L at 7 h respectively, compared with 65% at 70 mg/L in 10 h. COD removal was slower than decolorization. Aromatic rings such as anthracene and benzene are likely more difficult to oxidize than N=N and C-N bonds which are responsible for dye chromophore group formation (29).
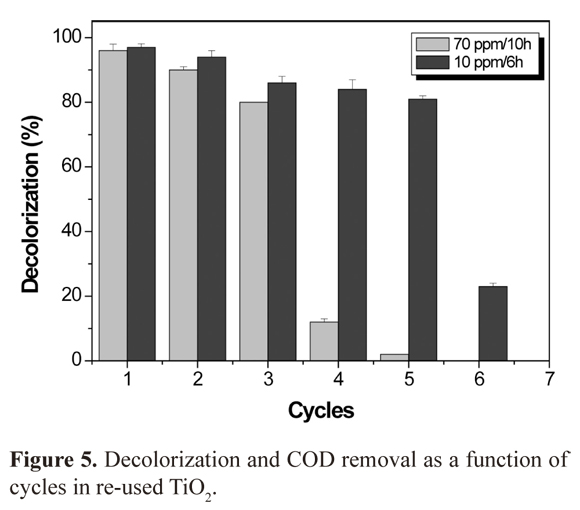
Sequential cycles of removal and re-use of TiO2
A new batch of TiO2 films were produced for this experiment using the same dimensions previously mentioned with approximately 19.12 ± 0.56 mg of catalyst deposited. Figure 5 shows removal cycles results of 70 mg/L (6 cycles of 10 h) and 10 mg/L (6 cycles of 6 h). For the 70 mg/L BR5, 6 cycles of decolorization were carried-out for 60 consecutive hours. We obtained more that 85% decolorization in the three first cyles. A decolorization under 12% was obtained for the rest of the cycles. For 10 ppm (360 h continuously) decolorization remained above 85% for 5 cycles during 30 h. The percentage decreased to 20% afterwards. COD concentration behavior was similar to decolorization on re-used TiO2 cycle number. However, removal was lower compared to decolorization. Again, this demonstrates that the dye has an structure with more complex fractions to oxidize than the chromophores of the dye. Sequential loss in fading could be related to certain progressive adsorption to the surface of TiO2, blocking active sites or the release of small amounts of catalyst. According to studies by other authors it is known that if black reagent 5 is transformed to sulfate (SO42-) from sulphonic groups, and other intermediates such as ammonium (NH4+), nitrite (NO2-) and nitrate (NO3 -) are realased from azo bonds. These ions are adsorbed strongly to TiO2 under acidic conditions and could produce an adverse effect on surface accumulation.
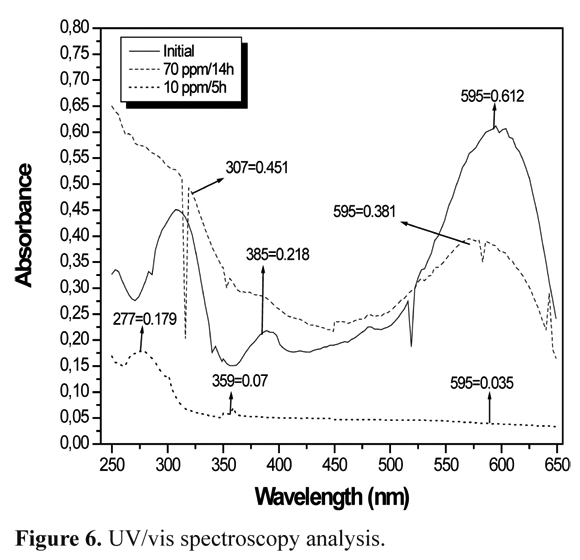
UV/VIS spectra change during treatments
BR5 before photocatalytic treatment (Figure 6) had characteristic peaks as previously reported in the literature (3, 29). A597 nm peak corresponding to the N=N chromophore group containing a conjugated n system was observed. A second peak detected at 310 nm coincides with that of naphthalene. Finally a third peak recognized at 254 nm agrees with that of benzene structures. After 10 h and 6 h photocatalytic dye treatment at 70 mg/L and 10 mg/L concentrations showed a decrease in visible light absorption. This resulted from double bond N=N cleavage, which was more evident at low concentrations. In relation to the 310 nm peak at 70 mg/L and 10 mg/L, a marked decrease was evidenced. The 254 nm peak increased slightly with both concentrations, demonstrating that naphthalene could be transforming into benzene. Other intermediates that may also be present are sulphonic acid-dihydronaphthalene, phenol, oxalic acid, among others (3, 29).
Toxicity study with Lactuca sativa and Daphnia magna
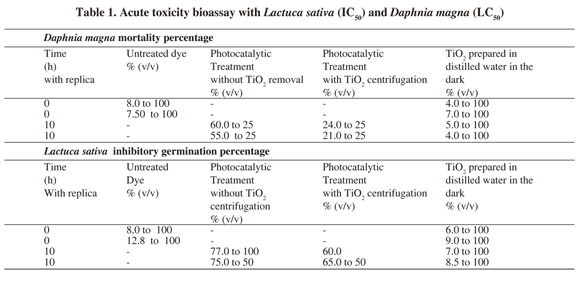
Toxicity studies with Lactuca sativa and Daphnia manga were carried out before and after BR5 decolorization. Results demonstrate that D. magna was more sensitive than L. sativa, with a mortality percentage of 8.0 - 7.5% at 100% (v/v) for T1, 60 - 55% at 25% (v/v) for T2, 24 - 21% at 25% (v/v) for T3, and 5 - 4% at 100% (v/v) for T4. At the same time L. sativa assay displayed an inhibitory germination percentage of 10 - 12% at 100% (v/v), 75 - 77% at 50% (v/v), 60 - 65% at 50% (v/v) and 7.0 - 8.5% at 100% (v/v) for the same order of treatments.
Oxidation processes were very efficient for color removal and COD. However, treated water may retain intermediaries un-absorbable in the visible light spectrum. Because they are refractory they could be generating an adverse effect on the aquatic ecosystem. Due to this precedent, animal and plant tests were performed.
From our results we were unable to perform Lethal dose 50 (LC50) for D. magna nor inhibitory concentrations for L. sativa (IC50). Hence, all values were expressed as percentage (v/v). Dye at 70 mg/L without treatment presented median toxicity for the two organisms tested at 100% (v/v). At lower dilutions no effects were presented. A similar behavior was evidenced for a TiO2 at 1% (m/v) solution prepared in distilled water. This specific treatment aimed to assay TiO2 toxicity responsible for D. magna 's mortality and inhibition of seed germination in L. sativa. Moderate adverse effects presented in D. magna for treatments 2 and 3 could be due to intermediaries formed during the photocatalytic process at 10 h of treatment. Nevertheless, a decrease on acute toxicity was observed, because T2 and T3 for D. magna presented the highest percentages of toxicity but at lower dilution (25%) compared to T1 y T4. L. sativa displayed a higher toxicity percentage compared to D. magna at a 50% dilution. This suggests that the animal model is more sensitive than the vegetal assay (Table 1).
On the other hand, the presence of residual TiO2 in post treatment water (T2) and treatment with removal after centrifugation (T3) could contribute to the adverse effect observed. However, it could be considered that some dye degradation intermediariesmight be adsorbed on the TiO2 surface. In addition to TiO2 we propose that other factors could be contributing to the toxic effect, because of low toxicity values for semiconductor oxide (T4). A possible cause in D. magna's sensitivity relates to TiO2 accumulation in the digestive tract, leading to death within 48 hours.
The mortality percentages obtained in this study were lower: 4% - 5% at 100% (v/v), than those reported by other authors (35% at 100 %) at 48 h. These differences might be related to the material's characteristics, such as particle size, route of synthesis, and concentration evaluated. Crystal mean size could have also contributed to toxicity loss for conditions T2, T3 and T4, with an average of 77.7 nm. This value was higher compared to those reported in different studies. For D. magna lower values (10-20 nm) are strongly related with acute toxicity, since this organism is capable of incorporating and accumulating particles (31).
In addition, TiO2 removal by centrifugation or filtration decreased effluent toxicity, when desorbed TiO2 was removed by centrifugation T2 and T3 treatments lowered mortality and inhibition percentages. Lovern and Klaper (2006) demonstrated that TiO2 recovered by filtration removed particles smaller than 30 nm, resulting in greater toxicity for D. magna (32).
Reduced effects of toxicity on root germination in L. sativa could be related to different aspects: i) plant's cell wall chemical composition is more resistant than cell membranes in D. magna, constituting a physical barrier that reduces TiO2 access. ii) presence of aromatic intermediaries. iii) Formation of nitrite and sulfate ions by mineralization during the process that affect germination at high concentrations. In all, the results obtained by this work lead us to propose for future studies the quantification of intermediaries to verify possible mechanism for dye degradation consider the establishment of phytotoxicity assays.
The results from this work suggest that commercial grade semiconductor oxide could become a substitute material for TiO2 Dagussa for the following reasons: commercial TiO2 has a greater anatase phase proportion, frequently associated with the photocatalytic process. The crystal size is similar to Dagussa's. The commercial source satisfactorily responds to the process of photoexcitation with ultraviolet lamps. Finally it has a reduced cost compared to that of Dagussa at two cents (US dollars) per gram.
Conclusions
Commercial TiO2 films grown by sedimentation process using borosilicate glass as a support, showed high decolorization of RB5 dye and COD removal (86% and 100%), respectively. In addition, toxicity was reduced. Furthermore, when evaluating animal vs. vegetable model, the former was more sensitive. This work demonstrated that photocatalysis with pharmaceutical grade TiO2 provides an easy, low-cost, and reproducible method for treating dyes, allowing its re-use for several consecutive cycles of treatment.
Acknowledgements
We thank engineers Ana Bertha Soto Guzmán and Marcela Guerrero at the Departament of Physics, Research Center
and Advanced Studies - National Polytechnical Institute México D.F. México for physical analysis of TiO2 films, and engineer Dery Esmeralda Ramírez for SEM studies at the Electron Scanning Microscopy Laboratory Universidad de los Andes. Bogotá, Colombia. We thank Maria Lucia Gutierrez for English editing the manuscript.
Financial support
This work was supported by project No. 2730 Pontificia Universidad Javeriana, and COLCIENCIAS grant 2737 Young Research Program, and the cooperation between Pontificia Universidad Javeriana and Universidad del Tolima.
Conflict of interest
There is no conflict of interest on the type of devices or procedures described in this manuscript. Our work is a product of the cooperation between Pontificia Universidad Javeriana (Bogotá) and Universidad del Tolima (Ibagué).
References
1. Satapanajaru T., Chompuchan C., Suntornchot P., Pengthamkeerati P. Enhancing decolorization of Reactive Black 5 and Reactive Red 198 during nano zerovalent iron treatment. Desalination 2011; 266: 218-230. [ Links ]
2. Mahvi A.H., Ghanbarian M., Nasseri S., Khairi A. Mineralization and discoloration of textile wastewater by TiO2 nanoparticles. Desalination 2009; 239: 309-316. [ Links ]
3. Damodar R.A., Sheng-Jie Y. Performance of an integrated membrane photocatalytic reactor for the removal of Reactive Black 5. Separation Purification Technology 2010; 71: 44-19. [ Links ]
4. Minabe T., Tryk D.A. Sawunyama P., Kikuchi Y., Hashimoto K., Fujishima A.. TiO2-mediated photodegradation of liquid and solid organic compounds. Journal Photochemistry and Photobiology A: Chemistry 2000; 137(1): 53-62 [ Links ]
5. Pelton R., Geng X., Brook M. Photocatalytic paper from colloid TiO2 fact or fantasy. Advances in Colloid and Interface Science 2006; 127. 43-53. [ Links ]
6. Konstantinou I. K., Albanis T. A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations A review. Applied Catalisys B: Environmental 2004; 49:1-14. [ Links ]
7. Arslan-Alaton I. Degradation of a commercial textile biocide with advances oxidation processes and ozone. Journal Environmental Management 2007; 82:145- 154. [ Links ]
8. Rizzo L., Meric S., Kassinos D., Guida M., Russo F., Belgiorno V. Degradation of diclofenac by TiO2 photocatalysis: UV absorbance kinetics and process evaluation through a set of toxicity bioassays. Water Research. 2009; 43: 979-988. [ Links ]
9. García A., Espinosa R., Delgado L., Casals E., González E., Puentes V., Barata C., Font X., Sánchez A. Acute toxicity of cerium oxide, titanium oxide and iron oxide nanoparticles using standardized tests. Desalination 2011; 269: 136-141. [ Links ]
10. Daohui L., Baoshan X. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environmental Pollution 2007; 150(2): 243-250. [ Links ]
11. Parola B., Serrano J.L. Materiales cerámicos y vidrios, Maestría en Ciencias de la ingeniería mención aeroespacial, Córdoba, España, 2011. [ Links ]
12. Arango-Parrado A., Rivera-Calvo D., Martínez-Salgado M.M., Carrascal-Camacho A.K., Pedroza-Rodríguez A.M. Elaboración de películas de TiO2 por sedimentación para el pos-tratamiento de un efluente anaeróbico generado en un relleno sanitario. Revista de Superficies y Vacío 2009; 22: 10-16. [ Links ]
13. Castañeda-Uribe O.A., Salcedo-Reyes J.C., Méndez-Pinzón H.A., Pedroza-Rodríguez A.M. Fabrication and optical characterization of a high-quality fcc-opal-based photonic crystal grown by the vertical convective self-assembly method. Universitas Scientiarum 2010; 15: 150-158. [ Links ]
14. APHA. In: A. E, Greenberg, L.S., Cleceri, A.D. Eaton, Editors, Standard Methods for the examination of water and wastewater (18th ed) American Public Health Association, Washington D.C 2005, p. 2-2, 5-12. [ Links ]
15. Fernández J.A., Henao L.M., Pedroza-Rodríguez A.M., Quevedo-Hidalgo B. Inmovilización de hongos ligninolíticos para la remoción del colorante negro reactivo 5. Revista Colombiana Biotenología 2009; 11: 59-72. [ Links ]
16. McInnis R. Daphnia magna. Toxicity Test Procedures. National Water Research Institute (NWRI). Environment Canadá. 1989. [ Links ]
17. ISO (International Organization for Standardization, Geneva, Switzerland), Water quality-determination of the inhibition of the mobility of Daphnia magna Starus (cladocera, Crustacea) acute toxicity test. ISO 6341, 1996. [ Links ]
18. Gustafsson J., Nordenswan E., Rosenholm J.B. Consolidation behavior in sedimentation of TiO2 suspensions in the presence of electrolytes. Journal Colloid Interface Science 2003; 258:235-243. [ Links ]
19. Allouni Z.E., Cimpan M. R., Hol P.J., Skodvin T., Gjerdet N.R. Agglomeration and sedimentation of TiO2 nanoparticles in cell culture medium. Colloid Surface B: Biointerfaces 2009; 68: 83-87. [ Links ]
20. Ao C.H., Leung M.K., Ringo H, Lama C.W., Dennis Y.C., Leung L., Vrijmoed L.P., Yamc W.C., Ng S.P., Photocatalytic decolorization of anthraquinonic dye by TiO2 thin film under UVA and visible-light irradiation. Chemical Engineering Journal 2007; 129: 153-159. [ Links ]
21. Krysa J., Keppert M., Jirkovsky J., Stengl V., Subrt J. The effect of thermal treatment on the properties of TiO2 photocatalyst. Material Chemistry Physics 2004; 86: 333-339. [ Links ]
22. Fernandez Machado N.R.C, Santana V.S. Influence of thermal treatment on the structure and photocatalytic activity of TiO2 P25. Catalsys Today 2005; 107-108: 595-601. [ Links ]
23. The-Vinh N., Hyun-Cheol L., O.-Bong Y. The effect of pre-thermal treatment of TiO2 nano-particles on the performances of dye-sensitized solar cells. Solar Energy Mater Solar Cell 2006; 90: 967-981. [ Links ]
24. Verma A., Agnihotry S.A. Thermal treatment effect on nanostructured TiO2 films deposited using diethanolamine stabilized precursor sol. Electrochimica Acta 2007; 52: 2701-2709. [ Links ]
25. Aguedach A, Brosillon S., Morvan J., Lhadi E.K. Influence of ionic strength in the adsorption and during photocatalysis of reactive black 5 azo dye on TiO2 coated on non woven paper with SiO2 as a binder. Journal Hazardous Materials 2008; 150: 250-256. [ Links ]
26. Belessia V., Romanosa G., Boukosa N., Lambropouloud D., Trapalis C. Removal of Reactive Red 195 from aqueous solutions by adsorption on the surface of TiO2 nanoparticles. Journal Hazardous Materials 2009; 170: 836-844. [ Links ]
27. Bergaminia R.B.M., Azevedo E.B., Raddi de Araújo L.R. Heterogeneous photocatalytic degradation of reactive dyes in aqueous TiO2 suspensions: Decolorization Kinetics. Chemistry Engineering Journal 2009; 149: 215-220. [ Links ]
28. Sh-Jie Y., Damodara R.A, Hou Sh.Ch. Degradation of Reactive Black 5 dye using anaerobic/aerobic membrane bioreactor (MBR) and photochemical membrane reactor. Journal Hazardous Materials 2010; 177: 1112-1118. [ Links ]
29. Wang K.S., Chen H.Y., Huang L.C., Chang Y.C.S.H. Degradation of Reactive Black 5 using combined electrochemical degradation-solar-light/immobilized TiO2 film process and toxicity evaluation. Chemosphere 2008; 72: 299-305. [ Links ]
30. Strigul N., Vaccari L., Galdun C., Wazne M., Liu X., Christodoulatos C., Jasinkiewicz K. Acute toxicity of boron, titanium dioxide, and aluminum nanoparticles to Daphnia magna and Vibrio fischeri. Desalination 2009; 248: 771-782. [ Links ]
31. Zhu X., Zhu L., Chen Y., Tian S. Acute toxicities of six manufactured nanomaterial suspensions to Daphnia magna. JournalNanoparticle Research 2009; 11: 67-75. [ Links ]
32. Lovern S.B., Klaper R. Daphnia magna mortality when exposed to titanium dioxide and fullerene (C60) nanoparticles. Environmental. Toxicology Chemistry 2006; 25: 1132-1137. [ Links ]














