Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Universitas Scientiarum
versión impresa ISSN 0122-7483
Univ. Sci. vol.19 no.2 Bogotá mayo/ago. 2014
Theoretical indications on the relationship between pyrogallol[4]arenes dynamics of assembling and geometry
Indicios teóricos entre la relación dinámica de ensamblaje y geometría de pirogalol[4]arenos
Indícios teóricos sobre a relação entre dinâmica de montagem e geometria de pirogalol[4]arenos
Robert A. Cazar1,2, F. Javier Torres2,3
Edited by Beynor Paez & Alberto Acosta
1ESPOCH, Facultad de Ciencias, Panamericana Sur Km 1.5, Riobamba, Ecuador
2Grupo Ecuatoriano para el Estudio Experimental y Teórico de Nanosistemas,-GETNano.
3Grupo de Química Computacional y Teórica (QCT-USFQ), Universidad San Francisco de Quito, Diego de Robles y Vía Interoceánica, Quito 17-1200-841, Ecuador.
Funding: Consorcio Ecuatoriano para el Desarrollo de Internet Avanzado, CEDIA.
Electronic supplementary material: NA
Received: 22-11-2013 Accepted: 25-03-2014 Published on line: 08-05-2014
Para citar este artículo / To cite this article
Cazar RA, Torres FJ (2014) Theoretical indications on the relationship between pyrogallol[4]arenes dynamics of assembling and geometry. Universitas Scientiarum 19(2): 133-137 doi: 10.11144/Javeriana.SC19-2.tirb
Resumen
Los pirogalol[4]arenos son macrociclos con gran potencial como bloques de construcción de nanocápsulas. Los dímeros precursores del 2,8,14,20-tetrametilpirogalol[4]areno y del 2,8,14,20-tetrafenilpirogalol[4]areno se estudiaron teóricamente para obtener entendimiento acerca de la dinámica de ensamble de estos compuestos. Las curvas de energía potencial a lo largo del ángulo de torsión del enlace R-pirogalol)CH-(R-pirogalol) de los dímeros han sido calculadas al nivel de teoría B3LYP/6-311G(d,p). Se encontró que las barreras de energía para rotación libre en torno al enlace seleccionado son 0.00133 Hartrees para el dímero con sustituyentes alquílicos y 0.77879 Hartrees para aquél con sustituyentes arílicos. Estos valores implican que la rotación libre en torno al enlace seleccionado es permitida para el primer dímero pero es prohibida para el segundo. Puesto que las orientaciones del sustituyente y el anillo de pirogalol en torno a este enlace posiblemente determinan la geometría de la estructura final, parece razonable proponer que el pirogalol[4]areno con sustituyentes alquílicos más probablemente adoptará una geometría de corona, mientras que el pirogalol[4]areno con sustituyentes arílicos mas probablemente adoptará una geometría de silla. Estas previsiones están en acuerdo con evidencia experimental la cual demuestra que la geometría de los pirogalol[4]arenos es dependiente del tipo de sustituyentes presentes en ellos.
Palabras clave: Pirogalol[4]arenos; química computacional; nanocápsulas.
Abstract
Pyrogallol[4]arenes are macrocycles with high potential as building blocks for nanocapsules. We theoretically studied the dimeric precursors of 2,8,14,20-tetramethylpyrogaUol[4]arene and 2,8,10,14-tetraphenylpyrogallol[4] arene to understand the dynamics of assembly of these compounds, and calculated the potential energy curves along the torsion angle of the (R-pyrogallol)CH-(R-pyrogallol) dimeric bond at the B3LYP/6-311G(d,p) level of theory. We found that the energy barriers for free rotation around the selected bond are 0.00133 Hartrees for the alkyl-substituted dimer and 0.77879 Hartrees for the aryl-substituted dimer. These values imply that the free rotation around the selected bond exists for the first dimer but not for the second one. Because the orientation of the substituent and the pyrogallol ring around this bond are likely to determine the geometry of the final structure, we propose that the alkyl-substituted compound will most likely adopt a crown-shaped geometry whereas the aryl-substituted compound will adopt a chair-shaped geometry. These predictions concur with experimental evidence, which shows that the geometry of pyrogallol[4]arenes depends on the substituents attached to them.
Keywords: Pyrogallol[4]arenes; computational chemistry; nanocapsules.
Resumo
O pyrogalol [4]arenes são macrocycles com grande potencial como blocos de construção da nanocapsules. Os dímeros precursores de 2,8,14,20-tetrametilpirogalol[4]arene e 2,8,14,20-tetrafenilpirogalol[4]arene foram estudados teoricamente para obter uma compreensão sobre a dinâmica do monte destes compostos. As curvas de energia potencial ao longo do ângulo de torção da link R-pkogdol)CH-(R-pirogalol) dos dímeros foram calculados ao nível da teoria B3LYP/ 6-311G(d,p). Verificou que as barreiras de energia da rotação livre em torno do link selecionado é 0,00133 Hartrees para ao dímero com substituintes alquil e 0,77879 Hartrees para que com substituintes aril. Esses valores implicam que a livre rotação em torno da link seleccionado é permitido para o primeiro dímero mas é proibida para o segundo. Uma vez que a orientação dos substituintes e o anel de pyrogalol em torno deste link possivelmente determinar a geometria da estrutura final, parece razoável sugerir que o pyrogalol[4]arene com substituintes alquil mais provavelmente vai ter uma geometria da copa, enquanto o pyrogalol[4]arene com substituintes resíduo mais provavelmente irá ter uma geometría de cadeira. Estas previsões estão de acordo com evidências experimentais que demonstram que a geometria do pyrogalol[4]arenes é dependente do tipo de substituintes presentes nos mesmos.
Palavras-chave: Pyrogallol[4]arenos; química computacional; nano-cápsulas.
Introducción
Pyrogallol[4]arenes (Figure 1) are macrocycles that provide dimeric and hexameric capsules and other assemblies with an internal volume large enough to accommodate and transport a variety of molecules and ions (Gerkensmeier et al. 1999, Atwood et al. 2001, Shivanyuk & Rebek 2001, Loustarinen et al. 2004, Maertz et al. 2010). Projected applications of these compounds include their usage as molecular filters, nano-carriers, nano-reactors and gas-storage units (Antesberger et al. 2005, Purse & Rebek 2005, Avram & Cohen 2006, Bassil et al. 2007, Jin et al. 2008, Dalgarno et al. 2009, Scott et al. 2009).
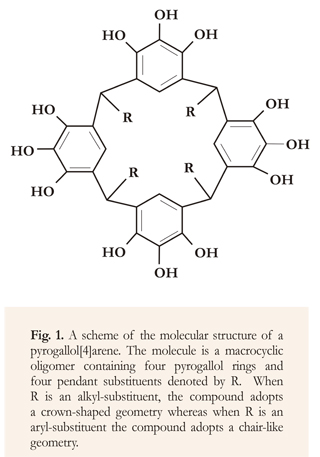
Pyrogallol[4]arenes are obtained by the acid-catalyzed condensation of a pyrogallol and an aldehyde (Hoegberg 1980). Depending on the aldehyde used in the reaction, two stereoisomers are most commonly produced. A crown-shaped conformer, denoted as rccc, is obtained when alkyl aldehydes are utilized; and a chair-shaped conformer, denoted rctt, is obtained when aryl aldehydes are utilized, denoted rctt is obtained (Gerkensmeier et al. 2001, Zambrano et al. 2010). In this work, we performed theoretical calculations (Jensen 2007) to obtain indications on the relationship between the dynamics of assembling of alkyl and aryl substituted pyrogallol[4]arenes and their final geometries (Weilnet & Schneider 1991, McKinlay et al. 2005). This information could be useful to project new, relevant applications of these compounds, such as their use as molecular hydrogen storage materials (Urbina et al. 2011, Gokel & Saeedeh 2013).
Materials and methods
We calculated the potential energy curves along the torsion angle of the (R-pyrogallol)CH— (R-pyrogallol) bond of the dimeric precursors of 2,8,14,20-tetramethylpyrogallol[4]arene and 2,8,14,20-tetramethylpyrogallol[4] arene using density functional theory (Koch & Holthausen 2001, Peverati & Baldridge 2008). A scheme of the dimers is shown in Figure 2; it highlights the bond that controls macrocycle assembly. We consider that the orientations adopted by the substituent and the pyrogallol ring with respect to such bond is likely to determine the geometry of the final product.
To generate the potential energy curves, we obtained the equilibrium geometries of the dimers at the B3LYP/6-311G(d,p) level of theory and used them as starting points of the curves. We then calculated the single-point energies of the structures; this was obtained by gradually increasing the torsion angle of the selected bond in increments of five degrees in the interval of zero to 180 degrees. The structure energy versus torsion angle increment data was plotted. All the calculations were performed using a Gaussian 09 software package (Frisch et al. 2009).
Results and discussion
The potential energy curve along the torsion angle of the selected bond for the precursor of the 2,8,14,20-tetramethylpyrogallol[4]arene is shown in Figure 3. This curve displays a minimum which corresponds to the starting structure, that is, the dimer equilibrium geometry and a maximum located at a torsion angle increment of 70 degrees. The energy difference between these two points provides the energy barrier for free rotation around the selected bond. This barrier is calculated to be 0.00133 Hartrees (8.3 kcal/mol). This value is low enough to suggest that, when R = methyl, the substituent and the pyrogallol ring can freely rotate around that bond and readily reorient from their initial positions with respect to the bond as the compound is assembled. That would lead to the formation of the crown-like conformer as the final product.
The potential energy curve along the torsion angle of the selected bond for the precursor of the 2,8,14,20-tetraphenylpyrogallol[4]arene is displayed in Figure 4. In this curve the minimum corresponds to the dimer equilibrium geometry and the maximum occurs at a torsion angle increment of 70 degrees. The energy barrier for free rotation around the selected bond is calculated to be 0.77879 Hartrees (488.7 kcal/mol). This high value implies that, when R = phenyl, the substituent and the pyrogallol ring may not rotate around that bond and remain in their original positions with respect to the bond as the compound is assembled. That would lead to the chair-like conformer as the final product.
These predictions concur with experimental evidence that has found that the most stable products for alkyl- and aryl-substituted pyrogallol[4]arenes adopt the rccc and rctt conformations, respectively (Barret et al. 2007, Thomas 2011).
Conclusion
We conducted a theoretical study of the dimers precursors of 2,8,14,20-tetra-methylpyrogallol[4] arene and 2,8,10,14-tetra-phenylpyrogallol[4]arene, and calculated the potential energy curves along the torsion angle of a selected bond of the dimers to get theoretical indications regarding the relationship
between the dynamics of assembly and the geometry of the final products. The results suggest that alkyl substituents lead to the rccc conformation while aryl substituents would lead to the rctt conformation. Such indications are in agreement with experimental evidence.
Acknowledgements
The authors wish to acknowledge the support of the Consorcio Ecuatoriano para el Desarrollo de Internet Avanzado, CEDIA, which provided the funds for this research (Grant CEPRA VI-2013-02).
Conflicts of interest
The authors declare that there are no conflicts of interest regarding the results published in this work.
References
Antesberger J, Cave GW Ferrarelli MC, Heaven MW Raston CL, Atwood JL (2005). Solvent-Free, Direct Synthesis of Supramolecular Nano-capsules. Chemical Communications 7:892-894. [ Links ]
Atwood J, Barbour L, Jerga, A (2001). Hydrogen-Bonded Molecular Capsules are Stable in Polar Media. Chemical Communications 22:2376-2377. [ Links ]
Avram L & Cohen Y (2006). Molecules at Close Range: Encapsulated Solvent Molecules in Pyrogallol[4] arene Hexameric Capsules. Organic Letters 8:219-222. [ Links ]
Barret E, Dale T, Rebek, J (2007). Synthesis and Assembly of Monofunctionalized Pyrogallolarene Capsules Monitored by Fluorescence Resonance Energy Transfer. Chemical Communications 41:4224-4226. [ Links ]
Bassil D, Dalgarno S, Cave G, Atwood J, Tucker S (2007) Spectroscopic investigations of adma encapsulated in pyrogallol[4]arene nanocapsules. Journal of Physical Chemistry B 111: 9088-9092. [ Links ]
Dalgarno S, Szabo T, Siavosh-Haghighi A, Deakyne C, Adams J et al (2009) Exploring the limits of encapsulating within hexameric pyrogallol[4]arene nanocapsules. Chemical Communications 11: 1339-1341. [ Links ]
Frisch MJ, Trucks G W Schlegel HB, Scuseria G E, Robb MA, et al (2009) Gaussian 09. [ Links ]
Gerkensmeier T, Iwanek W, Agena C, Frõhlich R, Kotila S et al (1999) Self-assembly of 2,8,14,20-tetraisobutyl-5,11,17,23-tetrahydroxyresorc [4]arene. European Journal of Organic Chemistry 9:2257-2262. [ Links ]
Gerkensmeier T, Agena C, Iwanek W Frõhlich R, Kotila Set al (2001) Synthesis and structural studies of 5,11,17,23-tetrahydroxyresorc[4]arenes. Zeitschriftfür Naturforschung B 56:1063-1073 [ Links ]
Gokel GW, Negin S (2013) Synthetic ion channels: From pores to biological applications. Accounts of Chemical Research 46: 2824-2833. [ Links ]
Hogberg A (1980) Two stereoisomeric macrocyclic resorcinol-acetaldehyde condensation products. Journal of Organic Chemistry 45:4498-4500. [ Links ]
Jensen F (2007) Introduction to Computational Chemistry. John Wiley & Sons New York, NY, USA [ Links ]
Jin P, Dalgarno SJ, Barnes C, Teat SJ, Atwood JL (2008) Ion transport to the interior of metal-organic pyrogallol [4] arene nanocapsules. Journal of the American Chemical Society 130: 17262-17263. [ Links ]
Koch W, Holthausen MA (2001) Chemist guide to density functional theory. Wiley-VCH Verlag Weinheim, Germany. [ Links ]
Luostarinen M, Ahman A, Nissinen M, Rissanen K (2004) Ethyl pyrogall[6]arene and pyrogall[4]arene: Synthesis, structural analysis and derivatization. Supramolecular Chemistry 16:505-512. [ Links ]
Maertz A, Thomas H, Power N, Deakyne C, Atwood J (2010) Dimeric nanocapsule induces conformational change. Chemical Communications 46:1235-1237. [ Links ]
McKinlay R, Cave G, Atwood J (2005) Supramolecular blueprint approach to metal-coordinated capsules. Proceedings of the National Academy of Sciences 102:5944-5948. [ Links ]
Peverati R, Baldridge K (2008) Implementation and performance of DFT-D with respect to basis set and functional for study of dispersion interactions in nanoscale aromatic hydrocarbons. Journal of Chemical Theory and Computation 4: 2030-2048. [ Links ]
Purse B, Rebek J (2005) Functional cavitands: Chemical reactivity in structured environments. Proceedings of the National Academy of Sciences 102:10777-10782. [ Links ]
Scott P, Dalgarno J, Warren J, Teat S, Atwood J (2009) Enhanced control over metal composition in mixed Ga/Zn y Ga/Cu coordinated pyrogallol[4]arenes nanocapsules. Chemical Communications 23:3348-3350. [ Links ]
Shivaniyuk A, Rebek J (2001) Hydrogen-Bonded Capsules in Polar, Protic Solvents. Chemical Communications 22: 2374-2375 [ Links ]
Thomas H (2011) Computational studies of three chemical systems. Doctorate thesis. University of Missouri-Columbia. [ Links ]
Urbina A, Saltos A, Torres FJ (2011) Estudio computacional B3LYP de la interacción del hidrógeno molecular con rccc R-Pyg[4]arenos (R = metil, fluor) funcionalizados con Li+. Avances en Ciencias e Ingeniería 3:A30-A34. [ Links ]
Weilnet F & Schneider H (1991) Mechanisms of macrocycle genesis. The condensation of resorcinol with aldehydes. Journal of Organic Chemistry 56:5527-5535. [ Links ]
Zambrano C, Manzano C, Saltos A, Dueno, E, Zeller, M (2010) Síntesis de 2,8,14,20-tetra-n-butilpirogalol[4] areno y estudio computacional conformacional. Avances en Ciencias e Ingeniería 2: A22-A29. [ Links ]













