Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Universitas Scientiarum
versión impresa ISSN 0122-7483
Univ. Sci. vol.20 no.2 Bogotá may./ago. 2015
https://doi.org/10.11144/Javeriana.SC20-2.pacv
Preservation of Azotobacter chroococcum vegetative cells in dry polymers
Preservación de células vegetativas de Azotobacter chroococcum en polímeros secos
Preservação de células vegetativas de Azotobacter chroococcum em polímeros secos
Daniel Rojas-Tapias1,2 , Oriana Ortega Sierra1, Diego Rivera Botia1, Ruth Bonilla1
Edited by Alberto Acosta
1Laboratorio de Microbiología de Suelos, Centro de Biotecnología y Bioindustria, Corporación Colombia de Investigación Agropecuaria, Corpoica, Mosquera 250014, Colombia.
2Graduate field at Department of Microbiology. Cornell University Ithaca, NY 14853.
Funding: Ministerio de Agricultura y Desarrollo Rural de Colombia.
Electronic supplementary material: N/A
Received: 28-07-2014 Accepted: 22-09-2014 Published on line: 19-10-2014
Para citar este artículo / To cite this article
Rojas-Tapias D, Ortega-Sierra O, Rivera Botía D, Bonilla R (2015) Preservation of Azotobacter chroococcum vegetative cells in dry polymers. Universitas Scientiarum 20(2): 201-207 doi: http://dx.doi.org/10.11144/Javeriana.SC20-2.pacv
Abstract
We studied the preservation of Azotobacter chroococcum C26 using three dry polymers: carrageenin, sodium alginate, and HPMC, using a method of accelerated degradation. Bacterial viability, as response variable, was measured at three temperatures in four different times, which was followed by calculation of bacterial degradation rates. Results showed that temperature, time of storage, and protective agent influenced both viability and degradation rates (P<0.05). We observed, using the Arrhenius thermodynamic model, that the use of polymers increased the activation energy of bacterial degradation compared to control. We obtained thermodynamic models for each polymer, based on the Arrhenius equation, which predicted the required time for thermal degradation of the cells at different temperatures. Analysis of the models showed that carrageenin was the best polymer to preserve A. chroococcum C26 since ~ 900 days are required at 4 °C to reduce its viability in two log units. We conclude, therefore, that long-term preservation of A. chroococcum C26 using dry polymers is suitable under adequate preservation and storage conditions.
Keywords: bacterial preservation; Arrhenius equation; Azotobacter chroococcum; polymers
Resumen
Se estudió la preservación de Azotobacter chroococcum C26 usando tres polímeros secos: carragenina, alginato de sodio y HPMC, usando un método de degradación acelerada. Viabilidad bacteriana, como variable de respuesta, fue medida a tres temperaturas en cuatro tiempos diferentes, lo cual fue seguido por el cálculo de tasas de degradación bacteriana. Los resultados mostraron que la temperatura, el tiempo de almacenamiento, y el agente protectivo influenciaron tanto la viabilidad como las tasas de degradación (P<0.05). Se observó, usando el modelo termodinàmico de Arrhenius, que el uso de polímeros incremento la energía de activación de degradación bacteriana comparado con el control. Adicionalmente, se obtuvieron modelos para cada polímero, basados en la ecuación de Arrhenius, para predecir el tiempo requerido para la degradación térmica de las células a diferentes temperaturas. El análisis de los modelos mostró que la carragenina fue el mejor polímero para preservar A. chroococcum C26 dado que un tiempo de aproximadamente 900 días a 4 °C son necesarios para reducir en dos unidades logarítmicas la viabilidad. Se concluye, por lo tanto, que la preservación a largo término usando polímeros es eficaz para la preservación de A. chroococcum C26 bajo condiciones adecuadas de preservación y mantenimiento.
Palabras clave: preservación; ecuación de Arrhenius; Azotobacter chroococcum; polímeros
Resumo
Estudamos a preservado do Azotobacter chroococcum C26 utilizando tres polímeros secos: carragenina, alginato de sòdio, e HPMC, utilizando um método de degradado acelerada. Viabilidade bacteriana, como variável de resposta, foi medida a tres temperaturas em quatro momentos diferentes, que foi seguido pelo cálculo das taxas de degradado bacteriana. Os resultados mostraram que a temperatura, tempo de armazenamento, e agente protetor influenciado as taxas de viabilidade e de degradado (P <0,05). Observou-se, utilizando o modelo de Arrhenius termodinàmico, que a utilizac.ao do polímeros de aumento da energia de activado do degradado bacteriana em comparado com o controlo. Adicionalmente, obtivemos modelos termodinámicos para cada polímero, com base na equação de Arrhenius, para prever o tempo necessàrio para a degradação térmica das células a diferentes temperaturas. Análise dos modelos mostrou que a carragenina é o melhor polímero para preservar A. chroococcum C26, porque ~ 900 dias são necessários a 4 °C para reduzir a viabilidade de duas unidades logarítmicas. Nós concluímos, portanto, a preservação a longo prazo de A. chroococcum C26 utilizando polímeros secos é adequado sob condic.öes de preservalo e armazenamento adequadas.
Palavras-chave: preservalo; equação de Arrhenius; Azotobacter chroococcum; polímeros
Introduction
Plant growth-promoting bacteria -PGPB- are microorganisms that can grow in, on, or around plant tissues and stimulate plant growth by numerous mechanisms (Vessey 2003). Azotobacter, a cyst-forming bacteria, is able to fix nitrogen under aerobic conditions. Interestingly, in some members of this bacterial genus, it has been shown that nitrogen fixation can be performed using up to three different nitrogenase enzymes that use different metal cofactors (Becking et al. 2006, Garrity et al. 2005). Because many members of Azotobacter exhibit plant-growth promoting features, this genus is included within the PGPB group (Kizilkaya 2008, Krumnow et al. 2009, Rojas-Tapias et al. 2012).
Preservation of bacteria is paramount for many fields of research due to the importance of maintaining the bacterial genetic consistency and viability (Malik & Claus 1987). Long-term preservation techniques are used when bacteria are maintained in biological collections, and viability and stability have to be maintained for years. So far, freeze-drying and ultra-freezing are the most used methods for long-term bacterial preservation. However, those methods require technology that may not be readily available, and generally require expensive equipment and advanced procedures (Krumnow et al. 2009). Mid-term preservation techniques are required when bacteria are active ingredient of bioproducts (e.g. biofertilizers). Here, viability, safety, and efficiency of the product are the most important features (Rivera et al. 2014). And although the same traditional techniques used for long-term preservation may be used for mid-term preservation, they are expensive and hence inapplicable. Therefore, new alternative methods that allow maintaining the bacterial stability throughout the time in almost any situation are required.
Krumnow et al. (2009) proposed the preservation of bacterial cells using natural polymers at room temperature. They found that Escherichia coli and Bacillus subtilis cells are well preserved using dry acacia gum or pullulan. This inexpensive and simple method for preserving microorganisms does not require special equipment and can be applied in the field or places where no refrigeration is available. Hence, this technique could be suitable for either long- or mid-term preservation of microorganisms. Here, we evaluated the usefulness of this method for preservation of A. chroococcum using a thermodynamic approach.
We previously studied the preservation of A. chroococcum C26 in dry polymers, and observed that alginate and carrageenin are suitable polymers to maintain the viability and activity of A. chroococcum C26 at room temperature (Rojas-Tapias et al. 2013). In this study, our objective was to perform a thermodynamic analysis to determine the efficiency of alginate, carrageenin, and a new polymer (HPMC) to maintain the viability of C26, select the best polymer in basis on the Arrhenius equation and its activation energy, and predict the viability of C26 cells in each polymer throughout the time using the Arrhenius plot.
Materials and methods
To study the suitability of the polymers to maintain the viability of A. chroococcum C26, we prepared bacterial suspensions in the following four sterile solutions: 1.5% carrageenin, 1.0% sodium alginate, 0.5% HPMC (hydroxypropyl methyl-cellulose), and PBS (phosphate buffered saline) (composition in g/l: NaCl 8.0, KCl 0.2, Na2HPO4 x 7H2O 1.15, KH2PO 0.2, pH 7.3). To prepare the polymer solutions, sterilized polymers were dispersed in sterile water. Before preparing the suspensions, the A. chroococcum cells were grown on Burk's solid medium (composition in g/l: Glucose 10, KH2PO4 0.44, K2HPO4 0.52, Na2SO4 0.05, CaCl2 0.2, MgSO4·7H2O 0.1, FeSO4·7H2O 0.0025, Na2MoO4·2H2O 0.0025, agar 15) for 48 h at 30 °C.
The concentrations of carrageenin and alginate were selected according to Rojas-Tapias et al. (2013), and the concentration of HPMC was based on previous experiments in our lab (data not shown). Sodium alginate and carrageenin were purchased from FMC BioPolymers (Ewing, USA), and HPMC from Sigma-Aldrich (St. Louis, USA). The concentrated solutions of the polymers were maintained at 4 °C prior to usage. For preparing each treatment, 200-ul bacteria-polymer mixtures were arranged into 1.5 ml vials and dried at 30 °C for 48 h until 10% humidity was reached. Immobilized cells were maintained at 4 °C, 22 °C, and 30°C during 30 days. To estimate bacterial survival, 200 [il deionized-sterilized water were added to each vial. Vials then were vortexed for 3 min and incubated for 30 min at room temperature before plate count. Bacterial survival was measured after 0, 8, 15 and 30 days after preservation by counting the number of viable cells on LG medium (Rojas-Tapias et al. 2013).
As experimental design a multilevel full-factorial design with 12 treatments was used. Polymers (three polymers and control) and temperature (three temperatures) were the factors for evaluation. As response variables, we measured cell viability in terms of CFU/ml in four different days. Each experiment was performed three times, each measurement was performed using three samples, and each cell count was performed four times using drop plates, for a total of 36 data per treatment per time. Viability data were studied using the ANOVA and HSD Tukey tests in the XLSTAT software. Bacterial degradation was analyzed through the Arrhenius equation as reported by Sorokulova et al. (2008).
Results and discussion
Temperature, time, and type of polymer affect the viability of A. chroococcum C26
Time and temperature had a significant effect on bacterial survival regardless of the protective agent used (p<0.05). Throughout the time, cell viability was progressively decreased and consequently the lowest bacterial counts were observed after 30 days of storage (Figure 1a-1c). Cell death plots followed first kinetic order, which was evident by straight regression lines in a semi-logarithmic plane. Because the greatest differences in bacterial survival were observed after 30 days, the following analyzes were performed using those data except for those analyzes based on the Arrhenius model. Differences in bacterial survival depended on the temperature of storage; cells stored at 4 °C maintained the greatest viability, followed by cells stored at 22 °C and 30 °C. Hence, higher temperatures and extended time resulted in a significant decrease in bacterial viability. Trivedi & Pandey (2008) showed that encapsulation of Bacillus subtilis and Pseudomonas corrugate, both PGPB, at 4 °C in alginate beads maintained bacterial populations in the order of 108 cfu/ml after three years of storage, retaining their biological properties. Similarly, Sorokulova et al. (2012) showed that Staphylococcus aureus and B. anthracis are well preserved on Whatman paper when acacia gum was also used, showing the same trend between viability and temperature.
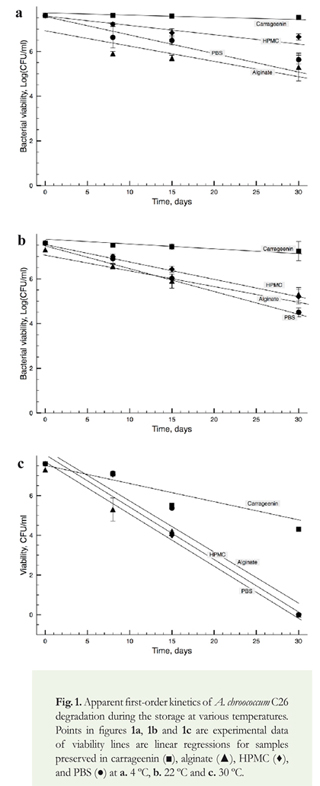
Protective agents are used to reduce the negative effect of long storage periods on bacterial survival (Diniz-Mendes et al. 1999). After 30 days, we observed significant differences associated to the protective agent used (p<0.05). Carrageenin proved to be the best polymer to maintain bacterial viability, and regardless of the temperature used, C26 could always be recovered from the stored vials. HPMC was effective to preserve bacteria at 4 °C and 22 °C, but viability was severely affected when cells were stored at 30 °C. Indeed, after 15 days of storage at 30 °C, cells preserved using HPMC showed the greatest loss of viability. Using alginate, we observed an important loss of viability at 22 °C and 30 °C, but minor effects at 4 °C. In contrast to our results for A. chroococcum, Bashan & Gonzales (1999) reported that dry alginate is suitable for preservation of Azospirillum brasilense and Pseudomonas fluorescens at room temperature. As expected, when BPS was used, we observed the greatest loss of viability irrespective of the temperature. Our observations suggest that the usage of polymers ameliorate the negative effects of long storage on viability of A. chroococcum C26.
Interpretation of the experiment using the Arrhenius equation
The model based on the Arrhenius equation (Equation 1) permits to evaluate the bacterial stability in different formulations via a method of accelerated degradation. This equation is used to express the dependence of the temperature of the first order activation kinetics:

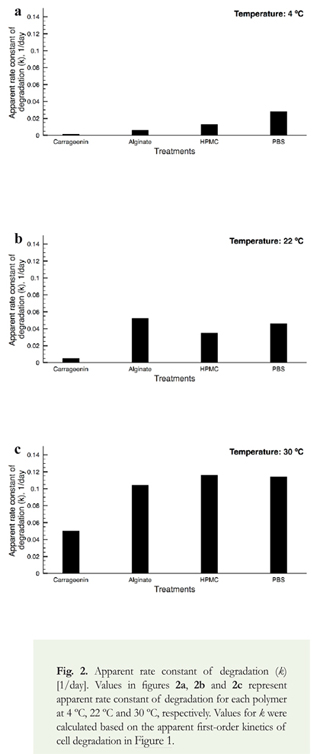
where k is the first-order rate constant of bacterial death (1/day), A is the pre-exponential Arrhenius factor (1/day), Ea is the apparent activation energy (cal/mol), R is the universal gas constant (1.985 cal/ mol K), and T is temperature (K). The method uses the apparent rates of thermal degradation of bacteria in each polymer at specific temperatures to determine the energy of cellular degradation. We observed that the apparent degradation rates (k) depended on the formulation used, and followed a first order kinetics in time across all temperatures (Figure 1a-1c). When we studied the apparent rates of bacterial death, we observed that the largest rates of bacterial degradation were exhibited at 30 °C, followed by 22 °C and 4 °C (Figure 2a-2c). Carrageenin was the polymer that led to the lowest rates of bacterial degradation, followed by alginate and HPMC. These results confirmed temperature plays a major role on bacterial degradation rates.
Identified constants were used to obtain an Arrhenius thermal degradation plot for C26 cells dried in carrageenin, alginate, HPMC, and PBS with relation to the storage temperature (Figure 3). The following expression (Equation 2) was used to determine the parameters:
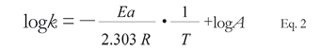
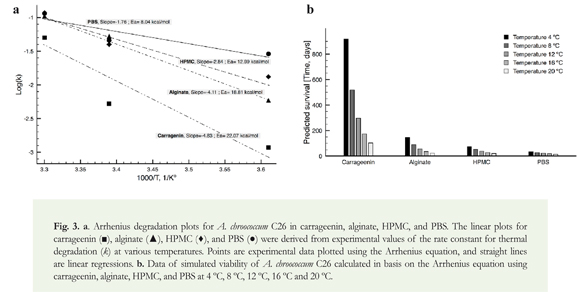
Data of bacterial viability of each polymer at all temperatures were used to estimate Ea for degradation of bacteria in different formulations (Figure 3). Importantly, Ea is the energy barrier that must be exceeded in order for a process to occur (Porterfield & Capone 1984), in our case cell degradation. To calculate the activation energy (Ea) of degradation of A. chroococcum C26 cells throughout preservation, we used the Arrhenius plot according to Sorokulova et al. (2008) (Figure 3a, Equation 2). We observed the highest Ea value was displayed by carrageenin, followed by alginate, HPMC, and PBS (Figure 3a). Based on the estimated values for Ea and A, we further conclude carrageenin is the best polymer to maintain the viability of A. chroococcum C26 throughout the time. Arrhenius' pre-exponential factor (A) accounts for entropy in the system, and as observed in equation 2, it also explains part of the estimated viability. The importance of this type of analysis, using the Arrhenius equation, lies in providing a way to analyze a system as a whole. Because different temperatures are considered to calculate the model parameters, the final result is independent of the temperature; therefore, it provides a manner to predict the future behavior of the system. Krumnow et al. (2009) observed lower results when preserved E. coli cells in pullulan and acacia gum (~ 6.1 and 11.2 kcal/mol), what suggests C26 is more resistant to the adverse conditions imposed by storage. We conclude that the chosen polymer has an important effect in increasing the amount of energy necessary to degrade bacterial cells, which may slow down the process of protein denaturation and degradation that lead to bacterial death.
Prediction of cell viability in time using the Arrhenius model
A further analysis using the Arrhenius equation and experimental values of apparent rate constants allowed predicting the time to reduce in two log units the viability of C26 at five different temperatures (Figure 3b) (Sorokulova et al. 2008). To calculate the time required to reduce the cell viability, we used the equation 4 derived from equation 3, with the k values obtained in equation 2 using the experimental Ea and A values.

k is the first-order rate constant of bacterial death (1/day), t is time (days), Nf number of cells after t days, and N0 initial number of cells. The results showed that the lower the temperature, the larger the viability throughout the time. The Arrhenius model predicted that three years at 4 °C using carrageenin as protective agent are necessary to reduce in two log units the initial concentration of cells, what suggests this method is suitable to preserve bacterial viability. Higher temperatures (> 4 °C) significantly reduced the necessary time to decrease in two log units the cell count (Figure 3b). For alginate, a reduction in two log units was predicted to be achieved in about six months at 4 °C, what indicates this polymer is either not as suitable as carrageenin to preserve the cellular viability or concentration of alginate tested was not the adequate to maintain viability throughout the time. HPMC proved less effective to maintain cell viability than carrageenin and alginate, and as little as two months were needed to reduce the viability in two log units at 4 °C. PBS showed the lowest protection to C26 cells (Figure 3b). Taken together, we conclude that although the use of dry polymers is a suitable technique to preserve cells of A. chroococcum, the selection of good storage conditions and protective agents is critical to successfully preserve them.
Conclusion
The present findings showed that preservation of A. chroococcum C26 using polymers is a suitable alternative to maintain its viability throughout the time. A general analysis evidenced that higher temperatures and longer times of storage have a significant role on bacterial viability. The type of polymer also had effect on bacterial survival. A further analysis using the Arrhenius equation showed differences with respect to Ea, in which carrageenin > alginate > HPMC were the polymers that better maintained viability of C26 cells compared to PBS. The present results suggested carrageenin was the best polymer to maintain the viability of C26 cells. Indeed, a mathematical analysis using the Arrhenius plot evidenced that more than 36 months are necessary to decrease the bacterial viability in two log units using carrageenin at 4 °C. Further experiments could be addressed to estimate the validity of the estimated degradation times using the Arrhenius equation, and test more physiological states in A. chroococcum as a way to increase its viability along the time for either basic or biotechnological applications.
Acknowledgments
We thank Ministerio de Agricultura and Desarrollo Rural de Colombia by its funding and support. In addition, we are grateful with people in the Bonilla lab for their useful suggestions and commentaries about the manuscript.
Conflict of interests
We declare no conflict of interests.
References
Bashan Y, Gonzalez LE (1999) Long-term survival of the plant- growth-promoting bacteria Azospirillum brasilense and Pseudomonas fluoresceins in dry alginate inoculant. Applied Microbiology and Biotechnology 51(2):262-267 doi: 10.1007/s002530051391. [ Links ]
Becking J (2006) The Family Azotobaderaceae. The Prokaryotes. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds). Springer New York, pp 759-783. [ Links ]
Diniz-Mendes L, Bernardes E, de Araujo PS, Panek AD, Paschoalin VMF (1999) Preservation of frozen yeast cells by trehalose. Biotechnology and Biooengneering 65(5): 572-578 doi: 10.1002/(SICI)1097-0290(19991205)65:5<572::AID-BIT10>3.0.CO;2-7. [ Links ]
Garrity GM, Bell JA, Lilburn T (2005) Pseudomonadales Orla-Jensen 1921, 270AL. Bergeys Manual® of Systematic Bacteriology. In: Brenner DJ, Krieg NR, Staley JT et al. (eds). Springer US, pp 323-442. [ Links ]
Kizilkaya R (2008) Yield response and nitrogen concentrations of spring wheat (Triticum aestivum) inoculated with Azotobacterchroococcum strains. Ecological Engineering 33:150-156 doi: 10.1016/j.ecoleng.2008.02.011. [ Links ]
Krumnow AA, Sorokulova IB, Olsen E, Globa L, Barbaree JM, Vodyanoy VJ (2009) Preservation of bacteria in natural polymers. Journal of Microbiological Methods 78:189-194 doi: 10.1016/j.mimet.2009.05.017. [ Links ]
Malik K, Claus D (1987) Bacterial culture collections: their importance to biotechnology and microbiology. Biotechnology and Genetic Engineering Reviews 5:137-197 doi: 10.1080/02648725.1987.10647837. [ Links ]
Porterfield RI, Capone JJ (1984) Application of kinetic models and Arrhenius methods to product stability evaluation. Medical Device and Diagnostic Industry 6:45-50 doi: 10.1007/s11746-001-0401-1. [ Links ]
Rivera D, Obando M, Rojas-Tapias D, Bonilla R, Barbosa H (2014) Evaluation of polymers for the liquid rhizobial formulation and their influence in the Rhizobium-Cowpea interaction. Universitas Scientarum 19(3):265-275. [ Links ]
Rojas-Tapias D, Moreno-Galván A, Pardo-Díaz S, Obando M, Rivera D, Bonilla R (2012) Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Applied Soil Ecology 61:264-272 doi: 10.1016/j. apsoil.2012.01.006. [ Links ]
Rojas-Tapias D, Ortiz-Vera M, Rivera D, Kloepper J, Bonilla R (2013) Evaluation of three methods for preservation of Azotobacter chroococcum and Azotobacter vinelandii. Universitas Scientarum 18(2):129-139 doi: http://dx.doi.org/10.11144/Javeriana.SC18-2.etmp. [ Links ]
Sorokulova IB, Krumnow AA, Pathirana S, Mandell AJ, Vodyanoy V (2008) Novel methods for storage stability and release of Bacillus spores. Biotechnology Progress 24: 1147-1153 doi: 10.1002/btpr.22. [ Links ]
Sorokulova I, Watt J, Olsen E, Globa L, Moore T, Barbaree J, Vodyanoy V (2012) Natural biopolymers for preservation of microorganisms during sampling and storage. Journal of Microbiological Methods 88:140-146 doi: 10.1016/j.mimet.2011.11.002. [ Links ]
Trivedi P, Pandey A (2008) Recovery of plant growth-promoting rhizobacteria from sodium alginate beads after 3 years following storage at 4 °C. Journal of Industrial Microbiology and Biotechnology 35:205-209 doi: 10.1007/s10295-007-0284-7. [ Links ]
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plantand Soil 255:571-586 doi: 10.1023/A:1026037216893. [ Links ]













