Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Universitas Scientiarum
Print version ISSN 0122-7483
Univ. Sci. vol.20 no.2 Bogotá May/Aug. 2015
https://doi.org/10.11144/Javeriana.SC20-2.efna
Ecological functions of neotropical amphibians and reptiles: a review
Funções ecológicas de anfibios e reptéis neotropicais: urna revisão
Funciones ecológicas de los anfibios y reptiles neotropicales: una revisión
Cortes-Gomez AM1, Ruiz-Agudelo CA2, Valencia-Aguilar A3, Ladle RJ4
Edited by Alberto Acosta & Juan Carlos Salcedo-Reyes
1Instituto de investigaciones en Recursos Biológicos Alexander von Humboldt, Laboratory of Conservation Genetics, Bogotá, Colombia. Herpetology Laboratory group, Biology Department, Universidad del Valle, Cali-Colombia.
2Socioeconomic Manager. Conservation International Colombia. Bogotá-Colombia.
3Instituto de Ciencias Biológicas e da Saúde, Universidade Federal de Alagoas, Maceió 57051-090, Brasil.
4School of Geography and the Environment, Oxford University, South Parks Road, Oxford, UK.
Funding: The Conservation Leadership Programme and Conservation International Foundation.
Electronic supplementary material: 2
Received: 17-07-2014 Accepted: 16-12-2014 Published on line: 26-02-2015
Para citar este artículo / To cite this article
Cortéz-Gómez AM, Ruiz-Agudelo CA, Valencia-Aguilar A, Ladle RJ (2015) Ecological functions of neotropical amphibians and reptiles: a review. Universitas Scientiarum 20(2): 229-245 doi: http://dx.doi.org/10.11144/Javeriana.SC20-2.efna
Abstract
Amphibians and reptiles (herps) are the most abundant and diverse vertebrate taxa in tropical ecosystems. Nevertheless, little is known about their role in maintaining and regulating ecosystem functions and, by extension, their potential value for supporting ecosystem services. Here, we review research on the ecological functions of Neotropical herps, in different sources (the bibliographic databases, book chapters, etc.). A total of 167 Neotropical herpetology studies published over the last four decades (1970 to 2014) were reviewed, providing information on more than 100 species that contribute to at least five categories of ecological functions: i) nutrient cycling; ii) bioturbation; iii) pollination; iv) seed dispersal, and; v) energy flow through ecosystems. We emphasize the need to expand the knowledge about ecological functions in Neotropical ecosystems and the mechanisms behind these, through the study of functional traits and analysis of ecological processes. Many of these functions provide key ecosystem services, such as biological pest control, seed dispersal and water quality. By knowing and understanding the functions that perform the herps in ecosystems, management plans for cultural landscapes, restoration or recovery projects of landscapes that involve aquatic and terrestrial systems, development of comprehensive plans and detailed conservation of species and ecosystems may be structured in a more appropriate way. Besides information gaps identified in this review, this contribution explores these issues in terms of better understanding of key questions in the study of ecosystem services and biodiversity and, also, of how these services are generated.
Keywords: ecological functions; Neotropical region; ecosystems; reptiles; amphibians
Resumen
Los anfibios y reptiles (herpetos) son dos de los grupos de vertebrados más abundantes y diversos en los ecosistemas Neotropicales. Sin embargo, poco se conoce sobre su papel en el mantenimiento y la regulación de las funciones del ecosistema y, por extensión, de su potencial aporte en el suministro de servicios ecosistémicos. En este estudio se realizó una revisión sobre las funciones ecológicas de los herpetos Neotropicales, usando diferentes recursos (búsqueda en bases de datos, capítulos de libros, etc.). Se revisó un total de 167 estudios de herpetología Neotropical, publicados en las últimas cuatro décadas (1970-2014). Estos estudios proporcionaron información sobre más de 100 especies que contribuyen al menos con cinco categorías de funciones ecológicas: i) ciclaje de nutrientes; ii) bioturbación; iii) polinización; iv) dispersión de semillas, y; v) flujo de energía a través de los ecosistemas. Muchas de estas funciones proveen servicios ecosistémicos claves para el bienestar humano, como el control biológico de especies plaga, dispersión de semillas y calidad del agua. Al conocer y entender las funciones que ejercen los herpetos en los ecosistemas se podrán estructurar de una forma más adecuada planes de manejo en paisajes transformados, proyectos de restauración o recuperación de paisajes que involucren sistemas acuáticos y terrestres, y planes completos y detallados de conservación de especies y ecosistemas. Además de los vacíos de información detectados en la presente revisión, esta contribución profundiza en la comprensión de algunas preguntas claves en el estudio de los servicios ecosistémicos y la biodiversidad y, además, de cómo se generan estos servicios.
Palabras clave: funciones ecológicas; región Neotropical; ecosistemas; reptiles; anfibios; revisión
Resumo
Os anfibios e répteis são dois dos grupos de vertebrados mais abundantes e diversos nos ecossistemas neotropicais. No entanto, pouco se sabe sobre o seu papel na manutenção e regulação das funções dos ecossistemas e sua potencial contribuição para o fornecimento de serviços ecossistémicos. Neste estudo, foi realizada uma avaliação sobre as funções ecológicas dos anfibios e répteis neotropicais, utilizando diferentes recursos (busca em bases de dados, capítulos de livros, etc.). Um total de 167 estudos publicados nas últimas quatro décadas (19702014) foram encontrados sobre as funções ecológicas dos anfibios e répteis neotropicais os quais forneceram informações sobre mais de 100 espécies que contribuem no: i) ciclo de nutrientes; ii) bioturbação; iii) polinização; iv) dispersáo de sementes, e; v) fluxo de energía através dos ecossistemas. Enfatizamos a necessidade de desenvolver estudos para ampliar o conhecimento das funções ecológicas dos ecossistemas neotropicais e os mecanismos por detrás destes, através do estudo das características funcionais e análise dos processos ecológicos. Muitas destas funções estao relacionadas com os principais serviços ecossistémicos para o bem estar humano como o controle biológico de espécies de pragas, dispersao de sementes e qualidade da água. Ao conhecer e compreender as funções que desempenham os repteis nos ecossistemas, podem ser estruturados planos de gestao nas paisagens culturais, projetos de restauração ou recuperação de paisagens que envolvem sistemas aquáticos e terrestres, e desenvolver planos abrangentes e detalhados de conservação de espécies e ecossistemas. Além das lacunas de informação identificadas nesta avaliação, esta contribuição explora essas questóes em termos de uma melhor compreensao das questóes-chave no estudo dos serviços dos ecossistemas e da biodiversidade além de como esses serviços são gerados.
Palavras-chave: funções ecológicas; região Neotropical; ecossistemas; répteis; anfibios; revisão
Introduction
Amphibians and reptiles (herps) are an abundant and diverse component of many terrestrial and freshwater ecosystems (Pough et al. 2004, Wells 2007, Collins & Crump 2009), contributing to a diverse range of ecological functions (Young et al. 2004, Pough et al. 2004, Tyler et al. 2007, Wells 2007, Collins & Crump 2009). However, they are much less studied than mammals (Kunz et al. 2011) and birds (Sekercioglu 2006, Whelan et al. 2008), and scientific knowledge of their role in ecosystem functioning is inconsistent and incomplete (Urbina-Cardona 2008, Valencia-Aguilar et al. 2013).
The generally low level of understanding regarding the roles that amphibians and reptiles play in ecosystems is well illustrated by recent global studies (Bickford et al. 2010, Hocking & Babbitt 2014). In response to evidence of rapid population declines of amphibians and reptiles worldwide (Rueda-Almonacid 1999, Gibbons et al. 2000, Lips et al. 2006, Reading et al. 2010), the International Union for the Conservation of Nature (IUCN) carried out a global evaluation and established criteria for determining the degree of threat to species of amphibians (Stuart et al. 2004), and a similar approach has been recently applied to reptiles (Bohm et al. 2012). Both studies identified considerable data shortfalls, and these were particularly apparent in the Neotropics - this region contain the largest number of amphibian species (49.2%) and has suffered the highest rates of population decline (63.1%) (Stuart et al. 2004).
The rapid and ongoing reduction of herp species richness worldwide has added urgency to efforts to understand the ecological roles that they play within ecosystems (Stuart et al. 2004, Lips et al. 2006, Connelly et al. 2011). The present work contributes to this goal by providing a heuristic framework for categorizing the functions of amphibians and reptiles in Neotropical ecosystems. Previously we explored the benefits to human society provided by elements of the neo-tropical herpetofauna (Valencia-Aguilar et al. 2013), here we explore their ecological roles; acknowledging these are not mutually exclusive. It is essential to understand the functional processes behind the ecosystem services, this new contributions seek to contribute to these gaps in knowledge. We describe the research trends of the past 20 years, identifying information gaps with respect to specific ecosystem functions and defining future investigation topics. As such, we aim to provide a robust database platform for future studies on how herps affect ecosystem functioning in the Neotropics, and how they are responding to the ongoing environmental changes within these ecosystems.
Materials and methods
This study was based on a review of the literature about the ecological functions of amphibians and reptiles in Neotropical ecosystems. Here, the term "function" denotes the relation between the parts (taxa) and the system, understood as the functions or roles that organisms perform within a system (Jax 2005).
Sources of information: Information was compiled from the following sources: a) the bibliographic databases ISI WEB OF KNOWLEDGE, JSTOR, EBSCO, Science Direct, SCOPUS, and Google Scholar; b) Book chapters containing information on Neotropical herps, and; c) Information provided by researchers. The search for information was restricted with keywords and boolean connectors (AND, OR). The following search words or terms were used: ecosystem function, ecological function, amphibian, reptile, tadpole, frog, toad, caecilian, salamander, lizard, snake, caiman, turtle, tortoise, role, bioturbation, decomposition, primary/secondary production, sediment, consumption, nutrient cycling, nutrient flow, excretion, biological control, mosquito control, diet, predation, food habit, seed dispersal, seed ingestion, dispersers, frugivore, saurocory, pollination, foraging, and flower.
Compilation of information: Studies published over the last four decades (1970 to 2014) and conducted in the 32 countries were compiled into a data matrix and analyzed by geographic region, year of publication, taxon (the study follows the current nomenclature for amphibians [Frost 2011] and reptiles [Uetz 2012]), type of experiment (field or laboratory), year of the study and ecological function.
Results and discussion
The total number of studies compiled resulted in 167, corresponding to: 67% on amphibians and 33% on reptiles. Most studies (76%) were based in South America, followed by Central America (14%) and the Caribbean (10%) (Figure 1). The vast majority (95%) of the sources were from electronic databases, 4.35% from libraries and less than 1% was provided by researchers.
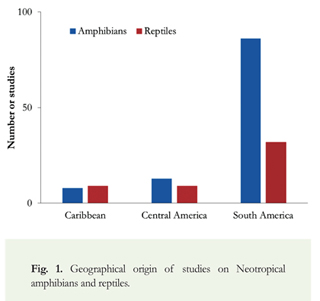
Several studies of ecological functions of amphibians and reptiles were grouped into the following five categories: nutrient cycling, energy flow through trophic chains (as predator and prey), bioturbation, seed dispersal, and pollination (Table 1), showing that the numbers of publications on these themes have increased considerably in recent years (Figure 2). Moreover, in the focus of the research, distinct historical trends were observed: in the 1970s, there was an emphasis on nutrient cycling and energy flow through trophic chains. Studies performed in the 1980s began to discuss the role of herps in seed dispersal and by the 1990s, bioturbation arose as a distinct research topic. Finally, in the 2000s there was an increase in the number of studies focused on energy flow and seed dispersal. In this last decade some researchers also began discussing the role of reptiles (turtles and lizards mainly) in the pollination of plants (mainly in insular ecosystems).
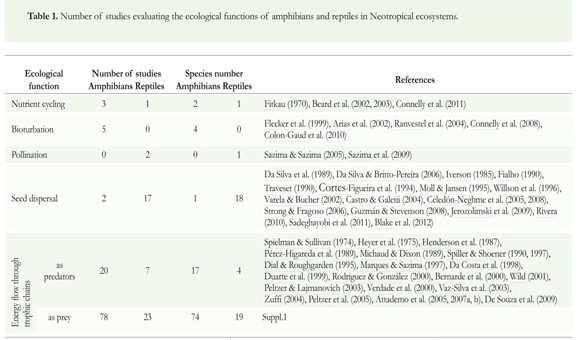
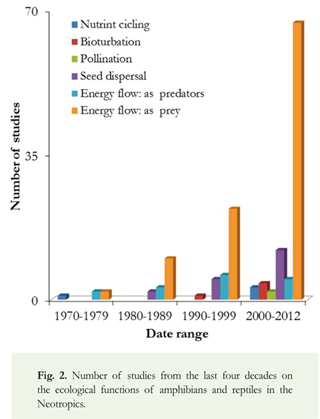
Eighty-two percent of the studies evaluated the role of amphibians and reptiles in energy flow through trophic chains, 12% documented the importance of reptiles (mostly lizards and tortoises) for seed dispersal and the viability of the seeds after being excreted. Only 1% of the studies investigated the role of herps in the pollination of plants, and all of these studies focused on reptiles. Three from five functions were reported through all of the studies for both amphibians and reptiles (Table 1).
Seventy-three percent of the papers reviewed were anecdotal field observations, half of which were carried out on reptiles. Only 27% of the studies were experimental (field and laboratory research), including 13% performed on amphibians and 14% on reptiles. Both experimental and observational studies were conducted at different spatial and temporal scales, with a minimum duration of a day or a month (for the observational and experimental studies, respectively) and a maximum of two years (in the case of one experimental study).
In the observational studies only qualitative variables were used to describe the data (mostly regarding predation). In contrast, both qualitative and quantitative data were collected in the experimental studies to describe feeding habits and behaviors, to characterize assemblages, to evaluate and measure the effects of the presence and absence of certain species of amphibians and reptiles on other animal populations (e.g., control over population densities of herbivore arthropods) and plant populations as in the case of seed dispersal). As well as to compare dynamics, to estimate population movements, and to study mutualism (e.g., pollination and the seed dispersal).
The roles of 142 species of amphibians and reptiles were documented for the five ecological functions identified (Table 1). Of these species, 93 were amphibians belonging to three orders and 17 families. Hylidae and Bufonidae exhibited the greatest number of functions documented, with four and three, respectively. Whereas for Centrolenidae, Eleutherodactylidae, Leiuperidae, Leptodactylidae, and Ranidae, only two functions were documented. For reptiles, 49 species were reported, belonging to three orders (two sub-orders) and 17 families. Alligatoridae, Iguanidae, Scincidae, Teiidae, Tropiduridae, and Testudinidae were the families with the greatest number of functions documented, with two each one.
Ecological Functions
Nutrient cycling: Animals are important in nutrient cycling through their effect on nutrient flow and consequently in the availability, inputs, recycling, and loss of these nutrients in the ecosystem (Vila 1998, Milton & Kaspari 2007). Animals can provide nutrients through excretion (e.g., nitrogen and phosphorus) at rates comparable with mineralization and biological fixation (Hickerson et al. 2012). For example, some fishes excrete nutrients in organic (urea) or inorganic forms (ammonia and phosphate) at rates potentially important for primary producers and heterotrophic microbes, supporting a substantial part of their demands for nutrients (Vanni 2002, Contosta et al. 2011). Given the high population densities (~20,000 individuals ha-1) of species such as Eleutherodactylus coqui (Stewart & Woolbright 1996) in tropical forests, herps clearly have the potential to significantly affect the concentrations and flow of nutrients (Sin et al. 2008). E. coqui and other similar abundant species, increase nutrient availability through their residues (feces, urine, and carcasses) and population movements increasing the rate of recycling in the ecosystems where they are present.
The coqui frog (E. coqui) has been observed to produce approximately 8.9 Kg of feces per hectare per year, which contain 34.2% C and 5.7% N. In addition, the urine contains 4.5% DOC, 3.3% Ca, 3.1% K, and more NH4+ than NO3-, elements and nutrients that are used by plants for their growth, development, and productivity (Beard et al. 2002, 2003). This increase in the rate of cycling is possible because, in spite of their size, E. coqui returns energy to the system in more assimilable forms (NH4+) as a result of the large quantity of the nutrients they ingest through their prey. Similarly, this frog can reduce by 28% the number of aerial invertebrates, reduce herbivory (by approximately 80% on a small scale), and increase the production of new foliage in plants and the rate of succession in forests after a perturbation, when the densities of plants in early succession and the density of the frogs are high. Conversely, the absence of E. coqui may reduce the quantity of K (between 5.7-6.6 Kg/ha) and P (between 3.1-3.7 Kg/ ha) available for the decomposition of the litter, with serious consequences for the rates of nutrient cycling, since these elements are essential for microbial activity and plant growth (Beard et al. 2002, 2003).
Tadpoles of Espadarana prosoblepon, Sachatamia albomaculata, Hyalinobatrachium colymbiphyllum, and Centrolene sp. have also been observed to play an important role in the cycling of nutrients in the aquatic systems they inhabit in Panama. These tadpoles feed by scraping the surface of decomposing leaves thereby stimulating fungal activity in the litter (Connelly et al. 2011). This increase in fungal biomass may be due to the high rates of excretion of nutrients by the tadpoles during the dry season, when their densities are high, tadpoles can excrete between 0.15 and 3.6 μg/hr of ammonia, which represents approximately 7% of the volume of absorption in these systems (Whiles et al. 2006).
The rate of nutrient cycling in ecosystems can also be affected by the relocation of nutrients caused by the behavior of the consuming organisms (Kitchell et al. 1979). For example, during the 1970s fish populations in some central Amazonian lakes were observed to be declining, a trend that was linked to the loss of caimans from these habitats (Fittkau 1970, 1973). Given that the waters in these lakes are quite poor in essential electrolytes, primary production is almost impossible, and the food chain is based on organic material that enters the water from the surrounding forests. Fishes originating from other tributaries migrate to these lakes to reproduce, serving as food for the caimans and other organisms, such as predatory fishes, turtles, and aquatic mammals. These and other allochthonous foods are rapidly transformed into nutrients and expelled by the caimans, and other predators, through their feces into the system, serving as a base for limited primary production that is then consumed by a new generation of fishes, thus maintaining the trophic network. This pattern probably explains why the disappearance of the caimans apparently affected the biological communities of the lake, especially of the fishes.
Further research is especially needed on the impact of the presence (or absence) of tadpoles, juveniles, and adults on the concentration and availability of nutrients, population density (of the species present at various trophic levels), plant biomass, and primary productivity at different temporal and spatial scales. Specifically, researchers need to quantify the degree to which activities such as feeding, excretion (feces and urine), the construction or use of burrows, and population movements may be affecting the energy and nutrient flow in aquatic and terrestrial systems.
Bioturbation: Bioturbation refers to the influence of organisms on the physical structure of the benthic habitat.,According to Moore (2006) , the bioturbators, are a type of ecosystem engineering in streams, and are defined as organisms that directly or indirectly control the availability of resources to other organisms through the "physical modification, maintenance, or creation of habitats" (Jones et al. 1994). For example, some freshwater vertebrates and invertebrates can significantly influence the deposition of sediments, sediment levels in the substrates, and the oxygen content in the substrate through their behavior and by the ingestion of fine particulate matter (Connelly et al. 2008, Creep et al. 2010). Tadpoles can be important bioturbators, directly or indirectly modifying the distribution of sediments and directly influencing the trophic dynamics and the energy flow in the system (Flecker et al. 1999, Ranvestel et al. 2004).
Tadpoles of Pseudisparadoxa and Lithobatespalmipes, which actively consume periphyton and sediments rich in organic matter, provide a clear example of amphibian induced bioturbation. These tadpoles modify the structure of the habitat during their foraging activities by reducing the accumulation of sediments. This, in turn, benefits other consumers that do not process the sediments as efficiently as benthic insectivorous fishes (Characidum), which must filter their food through sediment because they are not morphologically equipped to obtain nutrients from detritus (Flecker et al. 1999, Arias et al. 2002, Solomon et al. 2004).
Direct consumption of organic sediments by tadpoles is a mechanism for the resuspension of the sediments in rivers. Thus, tadpoles can represent a connection between deposited particles and those that are entrained (Whiles et al. 2006). In some aquatic systems in Panama, tadpoles of Atelopus Zeteki, Lithobates wars%ewitschii, and Hyla sp. consume periphyton, organic and inorganic sediments. This reduces the total quantity of these resources in the ecosystem and influences the biomass, diversity, and dynamics for periphyton and sediments. Furthermore, these grazers can facilitate primary production in tropical streams by removing sediments. Similarly, the physical perturbation associated with the feeding activities of tadpoles also influences the dynamics of the sediments (Ranvestel et al. 2004, Whiles et al. 2006). The absence of these tadpoles would cause an increase in benthic sedimentation and the biovolume of algae, potentially affecting basic resources and the functional structure of the trophic assemblage (Ranvestel et al. 2004, Connelly et al. 2008, Colon-Gaud et al. 2010a). Nevertheless, the impact of tadpoles on the structure and function of an aquatic system varies, and the removal of one or more species may not have significant effects due to ecological redundancy (Colon-Gaud et al. 2009, 2010b).
Pollination: Pollination can be directly affected by biological interactions between animals and plants, potentially benefiting both groups of organisms (Godínez-Álvarez 2004). Although the role of reptiles in pollination has rarely been considered, there is evidence that lizards can play an important role in plant reproduction, with this process apparently being more important on islands than on continents (Godínez-Álvarez 2004). In Brazil, during the dry season, the lizard Trachylepis atlantica visits the flowers of the tree Erythrina velutina, attracted by secretions of diluted nectar which the lizard uses as a source of water and energy. During the day, the lizard forages in the inflorescences, introducing its head into the flowers to lick up the nectar that accumulates at the base. As the lizard moves among the inflorescences, grains of pollen adhere to the scales of its head, shoulders, abdomen, and extremities. T. atlantica may thus be acting as a pollinating agent for the flowers of E. velutina, because foraging and bodily contact with the anthers and stigmas of the inflorescences would favor the cross-pollination of these trees (Sazima et al. 2005, 2009).
Seed dispersal: Most tropical plants produce fleshy fruits that are consumed principally by frugivores, which act as dispersers of numerous seeds. Differences in behavior among dispersers may influence the patterns of distribution of the seeds and thus the structure of the forest (Clark et al. 2001). Amphibians and reptiles are mainly considered to be carnivores. However, some herbivorous lizards, tortoises and turtles may play an essential role in the reproduction of some plants through their role in seed dispersion (Valido & Olesen 2007, Galindo-Uribe & Hoyos-Hoyos 2007). Few studies have evaluated the role of amphibians as potential disseminators of seeds. Nevertheless, there is evidence that this process can occur: Da Silva et al. (1989), Fialho (1990), and Da Silva & Britto-Pereira (2006) observed that Xenohyla truncata intentionally consumed fruits of Anthurium harrisii and Erythroxylum ovalifolium in a sand-dune ecosystem in Brazil. When A. harrisii fruit was given to X. truncata frogs, they consumed the fruit and excreted the seeds, which successfully germinated under laboratory conditions. Furthermore, it was noticed that fruits of these plants are commonly ingested by this frog in the dunes over the course of a few months. Based on these observations, the authors suggest that X. truncata consumes the fruit of both plants as part of its diet, and frequently defecates the seeds onto the axils of the bromeliad Neoregelia cruenta, increasing the possibility of germination in relation to those that fall onto the sandy ground.
Various lizard species have been recorded to consume fruits and seeds (Table 2), demonstrating their potential role in the reproduction of some plants (Godinez-Alvarez 2004). For example, the lizard Cyclura disperses the seeds of Eugenia uniflora, Genipa americana, Cereusperuvianus and Solanum viarum, distributing them in places favorable for germination and establishment (Castro & Galetti 2004). Likewise, Tropidurus torquatus actively consumes fruits of Erythroxylum ovalifolium during the dry season, defecating the seeds in open areas and sandy soils where they are usually preyed upon by insects and do not germinate (Fialho 1990). Even though T. torquatus is not an efficient disperser of E. ovalifolium, it is more effective dispersing the seeds of Melocactus violaceuss (seeds founded in the excreta of the lizards germinate more rapidly than those that are not consumed). Moreover, Melocactus sp. exhibits a number of specific adaptations (size and bright color of the fruit and its diurnal pattern of release), that make the fruits more likely to be dispersed by T. torquatus (Cortes-Figueira et al. 1994).
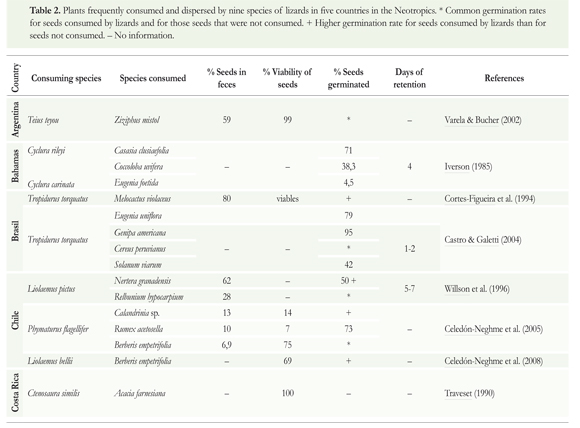
Many Neotropical lizards consume fruits that are very different in type, size and color, affecting seed germination (Godinez-Alvarez 2004). Consuming fruits can influence the dormancy and germination of the seed, the probability of survival, and the rate of growth of the seedling (Valido & Olesen 2007) (Table 2). For example, seeds of Coccoloba uvifera and Casasia clusiifolia germinated rapidly after passing through the tract of Cyclura sp., which expelled the seeds in its feces four days after consumption. Trials of germination and tests of viability conducted with seeds of Berberis empetrifolia, Rumex acetosella and Calandrinia sp. consumed by the lizard Phymaturus palluma indicated that pass through the digestive tract has various effects: although the viability of the seeds of B. empetrifolia consumed by the lizard was greater than the viability of those that were not consumed, there were no differences in the percentage of germination. In contrast, the viability of the seeds of R. acetosella and Calandrinia sp. consumed by the lizard was lower than those obtained from fruits. However, the percentage germination of consumed seeds of R. acetosella was greater than those not consumed (Celedôn-Neghme et al. 2005). Similarly, seeds of Berberis empetrifolia consumed by Liolaemus belii showed a higher rate of germination after passing through the digestive tract. This improvement is most likely due to the modification of the seed coat within the digestive tract, consumed seeds showed a greater deterioration than seeds extracted from fruits (Celedón-Neghme et al. 2008). Accordance to this information, P. palluma may be influencing the structure and diversity of the flora present in its habitat, and L. bellii may be acting as an effective disperser of B. empetrifolia because it improves the germination of seeds and deposits them in favorable sites for plant's development (Celedón-Neghme et al. 2005, 2008).
Some Neotropical tortoises (Chelonoidis carbonaria, C. chilensis, C. denticulata, Rhinoclemmys funerea and R. annulata) play an important role in the dispersion and germination of numerous plant species in forests in Argentina, Brazil, Costa Rica, Ecuador, and Peru. Seeds of plants from different species and a wide variety of families (Table 3) have been found in the feces of these tortoises. Most (91%) of these seeds showed no signs of external damage and were viable, almost all germinated after being consumed and excreted by the tortoises (Varela & Bucher 2002, Jerozolimski et al. 2009). A high percentage of seeds that were recovered from feces germinated more rapidly than those extracted directly from the fruits, possibly due to increases of the permeability of the seed endocarp (Varela & Bucher 2002, Jerozolimski et al. 2009).
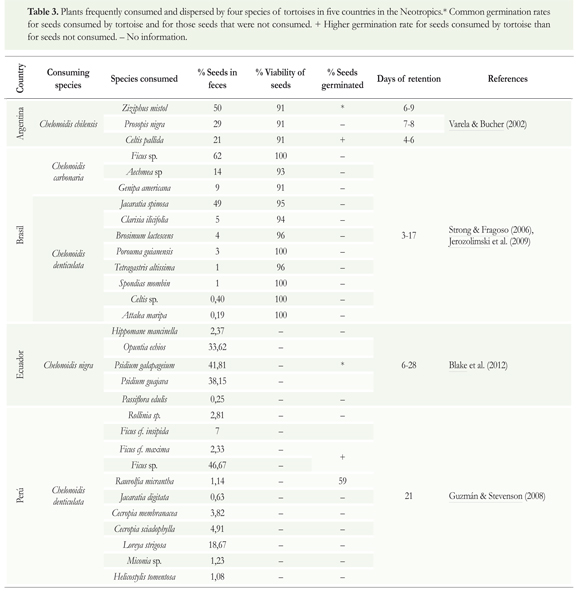
The high abundance and diversity of viable seeds found in tortoise feces is partly a consequence of the inability of tortoises to chew and their limited capacity to process what they ingest. Tortoises also exhibit a high mobility while they retain the seeds in their digestive tracts (Guzman & Stevenson 2008, Jerozolimski et al. 2009). These characteristics make tortoises important seed dispersers, contributing to the colonization of new habitats and promoting genetic flow between sub-populations of plants. Tortoise-assisted dispersal also increases seedling survival by reducing predation, herbivory and infestation by pathogens (Moll & Jansen 1995, Strong & Fragoso 2006).
Energy flow through trophic chains: predators and prey:
Herps as predators. Herps can regulate populations of certain organisms through biotic interactions such as predation and competition. These processes, in the case of arthropods eaters, can reduce the number of adult individuals or larvae (Schmitz & Sokol-Hessner 2002, Maerz et al. 2005, Schmitz 2008, Homyack et al. 2010, Mooney et al. 2010, Best & Welsh 2014). For example, in the Bahamas, leaves of the tree Coccoloba uiifera, or seagrape, are often affected by Homoptera (Cicadellidae and Aphididae), Hemiptera (Pentatomidae), Coleoptera (Scarabaeidae), and larvae of Lepidopterae (Tortricidae, Noctuidae) and Hymenoptera (Formicidae). These cause necrosis in parts of the plant tissue or create holes, resulting in loss of leaf area. Lizards, such as Anolis sagrei, are often the major predators of these herbivorous arthropods, significantly reducing both types of leaf damage through direct consumption of the arthropods (Spiller & Schoener 1990, 1997). Similarly, in Puerto Rico, there was an increase of 46% in the damage to rainforest canopy leaves, caused by arthropods (Blattaria and Orthoptera) in the absence of Anolis, whereas when the lizards were present, herbivory was reduced by up to 20% (Dial & Roughgarden 1995).
Given that competition for food among predators depends on prey availability (Blaustein & Chase 2007), evaluating predator-prey interactions may help to understand the role of a "natural enemy" in an ecosystem, either because it alters the food resources of other species or because it acts as a direct predator (Bellows 2001).
Tadpoles from many species are predators of insect larvae such as mosquitoes and dragonflies that use the same microhabitats. For example, tadpoles of Osteopilus septentrionalis predate the mosquito Culex quinquefasáatus in the Bahamas and Cuba, with individual tadpoles consuming between 13 and 21 larvae per day, a number that varies as a function of the density of the larvae (Spielman & Sullivan 1974, Rodríguez & González 2000). Culex sp. is never abundant when tadpoles were present and introduction of tadpoles to sites with Culex sp. larvae is often sufficient to make the mosquitoes disappear. The tadpoles clearly help to regulate the abundance of these mosquitoes during the rainy season in which the reproduction of O. septentrionalis coincides with the period of maximum abundance of C. quinquefasciatus (Spielman & Sullivan 1974, Rodríguez & González 2000).
In the Neotropical region, reptiles (snakes, lizards, turtles and alligators) feed on a variety of invertebrates and vertebrates (Myers et al. 1978; Heymann 1987; Michaud & Dixon 1989; Kluge 1981; Ortiz et al. 1997; Zamprogno & Zamprogno 1998; Grismer 2000; Prado 2003; Laverty & Dobson 2013). For example, the diet of the species of Crocodylia shows a marked difference during ontogeny. While neonates and juveniles are predominantly insectivore, adults can feed on mollusks, crustaceans, fish, iguanas, snakes, turtles, birds and mammals (Pérez-Higareda et al. 1989; Ortiz et al. 1997; Rivas et al. 1999; Morales-Betancourt 2013). Likewise, snakes, lizards and turtles feed on amphibians, other species of reptiles and mammals (Heymann 1987; Silva & Hillesheim 2004).
Herps as prey. The availability of nutrients and energy is an important driver of community dynamics (Bouchard & Bjorndal 2000). In this sense, food chains constitute an important part of the flow of energy in ecosystems through a network of nutritional relationships (Govenar 2012). Amphibians and reptiles may be responsible for a considerable part of the energy flow in aquatic and terrestrial ecosystems since they can represent an abundant source of protein, mainly for animals at higher trophic levels, and because they often achieve high population densities and are efficient at converting ingested energy into biomass (Boyd & Goodyear 1971, Wells 2007). Furthermore, because amphibians lack hard tissues such as feathers, beaks, hair, or chitin that are difficult to digest, they are a high-quality source of nutrition for other animals (Burton & Likens 1975, Bouchard & Bjorndal 2000, Wells 2007, Collins & Crump 2009).
The eggs, embryos, and tadpoles of frogs also offer a potential source of energy for other organisms due to their high protein content. In this sense, larval anurans are often key elements in the trophic networks of ponds and streams, where they can achieve high densities and biomass (Schiesari et al. 2009). Among the organisms that predate frogs are invertebrates, such as molluscs, crustaceans, arachnids, coleopterans, hymenopterans, orthopterans, hemipterans, and dipterans (Kluge 1981, Villa et al. 1982, Hayes 1983, Villa & Townsend 1983, Pramuk & Alamillo 2002, Menin & Giaretta 2003, Peltzer & Lajmanovich 2003, Jara & Perotti 2004, Vockenhuber et al. 2008, Ortega-Andrade 2008, Valencia et al. 2011). There are also many vertebrate predators, including caecilians, other anurans, snakes, and tortoises (Heyer et al. 1975, Heyer & Muedeking 1976, Vaira & Coria 1994, Mendes 1996, Verdade et al. 2000, Feltrim & Zaninicechin 2000, Solé & Kwet 2003, Cassimiro & Bertoluci 2003, Cuello et al. 2005, Mendes 2001, Verdade et al. 2000, Puente-Rolón 2001, Ortega-Andrade 2008) (Suppl. 1).
Although predation of adult amphibians by invertebrates is less common than egg and larvae predation, it may also have an important effect on population dynamics (Toledo 2005). Adult amphibians are especially vulnerable to predation when they gather at sites of reproduction (Wells 2007). Various invertebrate taxa have been observed to prey on juvenile and adult amphibians, including insects (Bastos et al. 1994, Buttenhoff 1995, Haddad & Bastos 1997, Pineda 2003, Brasileiro et al. 2003a, Toledo 2003, Figueiredo de Andrade et al. 2010, Costa-Pereira et al. 2010, Santos-Silva & Ferrari 2012), crabs (Caldart et al. 2011), and arachnids (Formanowicz et al. 1981, Hayes 1983, Bastos et al. 1994, Del Grande & Moura 1997, Bernarde et al. 1999, Gray et al. 1999, Summers 1999, Villanueva-Rivera et al. 2000, Aucone & Card 2002, Boistel 2002, Prado & Borgo 2003, Menin et al. 2005, Ortega-Andrade 2008, Manzanilla et al. 2008, Barbo et al. 2009, Santana et al. 2009, Costa-Pereira et al. 2010, Maffei et al. 2010, Caldart et al. 2011).
Amphibians and reptiles are a component of the diet of various species of fishes, birds, mammals, and even other amphibians and reptiles, playing an important role in population dynamics of predators (Duellman & Trueb 1994, Toledo 2005, Wells 2007, Caldart et al. 2011). More than 100 species of Neotropical amphibians (15 families) and reptiles (21 families) have been reported to be prey of numerous groups of vertebrates, such as fishes, amphibians, reptiles, birds and mammals (Suppl. 2).
Despite the large number of reports regarding amphibians and reptiles as prey and predators (Suppl. 2), consumption of dead animals (necrophagy) has frequently been underestimated or minimized (DeVault & Krochmal 2002). In Brazil, Sazima & Strussmann (1990) observed individuals of Helicops modestus, Hydrodynastes gigas and Liophis miliaris consuming carcasses of anurans Rhinella schneideri and Hypsiboas albomarginatus. In Costa Rica, Mora (1999) observed an individual of Leptodeira annulata consuming carcasses of Lithobates vaillanti in an advanced state of decomposition.
Some species of Neotropical insects, snakes, birds, and mammals have preferences for the consumption of various species of amphibians and reptiles. Although the majority of these reports are anecdotal, this information indicates these groups play a major role of in the diet of certain predators. For example, among the food items most frequently consumed by the snakes Uromacer catesbyi, U. frenatus and U. oxyrhynchus are the frog Osteopilus dominicensis, the lizards Anolis olssoni, A. semilineatus and Ameiva chrysolaema (Henderson et al. 1987). The falcon Leucopternis princeps frequently hunts individuals of the caecilian Caecilia orientalis (48.1% of its diet) and the snake Atractus occipitoalbus (34.6% of its diet) to feed its young (Greeney et al. 2008). Predation on the anurans Engystomops pustulosus and Hyalinobatrachium fleischmanni by the bat Trachops cirrhosus seems to have influenced the evolution of the various types of signals involved in the reproduction of these frogs species (Ryan et al. 1982, Delia et al. 2010).
Given that predator-prey relations between species can directly or indirectly affect a particular population (animal or plant), it would also be valuable to investigate associations that may occur between amphibians, reptiles, and communities of herbivorous arthropods. In particular, researchers should measure and quantify the effects of these interactions on the growth, predation, and productivity of plants. In this context, comparing the rate of folivory in the presence and absence of predatory species of certain herbivorous arthropods would help to demonstrate the importance of these predators in pest regulation and the possible positive effects for economically important plant species.
Conclusion
The loss of amphibians and reptiles highlights the need to improve the knowledge about this group, given that studies conducted over the last four decades demonstrate the importance of more than 100 herps species in several ecological functions. For example, the role of salamanders and turtles in matter and energy transfer between aquatic and terrestrial system have been widely studied in template regions, nevertheless in the Neotropic this information is limited. The existing herpetological literature, is incomplete and scattered, therefore is necessary to generate basic information about ecophysiology, reproductive biology, ethology, feeding ecology and population dynamics, especially with regard to structure (age, sex, size, and weight of individuals), population density, use of habitat (basic requirements for food and shelter), and population movements (dispersion of individuals of a species). These species traits can be studied via functional ecology, for the understanding of the relationships between these traits, their variability and effects on the ecosystems. Such characteristics shapes some of the ecosystem services provided by amphibians and reptiles, for example biological pest control, seed dispersal and water quality, through functions like energy flow through trophic chain (like predators), consuming of fruits, seeds, nutrient cycling and bioturbation, respectively.
We show how amphibians and reptiles contribute to different ecological functions, also highlighting the need to develop studies aimed to the understanding of the mechanisms that occur behind these general ecological functions, through the study of functional traits and ecological processes. We also emphasize the need for research involving more study time (at least one year), quantitative data collection and experimental models in field. The knowledge and understanding of the functions that the herps (organisms abundance and diversity) perform in the ecosystems, formulated in a more appropriate way, will aid to develop management plans for transformed landscapes, restoration or recovery projects of landscapes (involving aquatic and terrestrial systems), and the development of complete conservation strategies for species and ecosystems. Improving the understanding of how ecosystem services offered are generated for biodiversity, is undoubtedly the first step towards formal recognition of human benefits derived from the operation of those services. Keeping in mind this is one of the central topics from the perspective of ecosystem services.
Acknowledgements
This review was made possible by the support of the Conservation Leadership Programme (CLP) and Conservación Internacional Colombia (CI). We thank Dra. P. M. Peltzer, Dra. V Páez, Dra. K.H. Beard, Dr. B. Bock, Dr. M. Whiles and Dr. P.R. Stevenson, for their assistance in providing information for this review.
Conflicts of interest
There are no conflicts of interest with funding sources or institutions.
References
Arias MM, Peltzer PM, Lajmanovich RC (2002) Diet of the giant tadpole Pseudis paradoxa platensis (Anura, Pseudidae) from Argentina. Phyllomedusa 1:97-100. [ Links ]
Attademo AM, Peltzer PM, Lajmanovich RC (2005) Amphibians occurring in soybean and implications for biological control in Argentina. Agriculture, Ecosystems & Environment 106:389-394. [ Links ]
Attademo AM, Peltzer PM, Lajmanovich RC (2007a) Feeding habits of Physalaemus biligoonigerus (Anura, Leptodactylidae) from soybean field of Córdoba province, Argentina. Russian Journal of Herpetology 14:1-6. [ Links ]
Attademo AM, Cejas W Peltzer PM, Lajmanovich RC (2007b) Phenology in the diet of Chaunus arenarum (Anura: Bufonidae) in a soybean field of Córdoba province, Argentina. Revista Española de Herpetologia 21:41-48. [ Links ]
Aucone B, Card W (2002) Scinaxcruentomma (NCN) predation. Herpetological Review 33:48. [ Links ]
Barbo FE, Rodrigues MG, Couto FM, Sawaya RJ (2009) Predation on Leptodactylus marmoratus (Anura: Leptodactylidae) by the spider Ctenus medius (Araneae: Ctenidae) in the Atlantic Forest, southeast Brazil. Herpetology Notes 2:99-100. [ Links ]
Bastos RP, Oliveira OC, Pombal JP (1994) Hyla minuta. predation. Herpetological Review 25:118. [ Links ]
Beard KH, Vogt KV, Kulmatiski A (2002) Top-down effects of a terrestrial frog on forest nutrient dynamics. Oecologia 133:583-593. [ Links ]
Beard KH, Eschtruth AK, Vogt KA, Vogt DJ, Scatena FN (2003) The effects of the frog Eleutherodactylus coqui on invertebrates and ecosystem processes at two scales in the Luquillo experimental forest, Puerto Rico. Journal of Tropical Ecology 19:607-617. [ Links ]
Bellows TS (2001) Restoring population balance through natural enemy introductions. Biological Control 21:199205. [ Links ]
Bernarde PS, Souza MB, Kokubum MCN (1999) Predation on Hyla minuta Peters, 1872 (Anura, Hylidae) by Ancylometes spp. (Araneae, Pisauridae). Biociencias 7:199-203. [ Links ]
Bernarde PS, Machado RA (1999) Hyla faber (Smith frog). Larval cannibalism. Herpetological Review 30:162. [ Links ]
Bernarde PS, Moura-Leite JC, Machado RA, Kokobum MNC (2000). Diet of the colubnd snake, Thamnodynastes strigatus (Günther, 1858) from Paraná state, Brazil, with field notes on anuran predation. Revista Brasileira de Biologia 60:695-699. [ Links ]
Best M, Welsh H (2014) The trophic role of a forest salamander: impacts on invertebrates, leaf litter retention, and the humification process. Ecosphere 5(2): article 16. [ Links ]
Bickford D, Howard SD, Daniel JJN, Sheridan JA (2010) Impacts of climate change on the amphibians and reptiles of Southeast Asia. Biodiversity and Conservation 19:1043-1062. doi 10.1007/s10531-010-9782-4. [ Links ]
Blake S, Wikelski M, Cabrera F, Guezou A, Silva M, Sadeghayobi E, Yackulic CB, Jaramillo P (2012) Seed dispersal by Galápagos tortoises. Journal of Biogeography 39:1962-1972. [ Links ]
Blaustein L, Chase JM (2007) Interactions between mosquito larvae and species that share the same trophic level. Annual Review of Entomology 52:489-507. [ Links ]
Böhm M, Collen B, BailHe EMJ, Chanson J, Cox N. et al. (2012) The conservation status of the world's reptiles. Biological Conservation 157: 372-385. [ Links ]
Boistel R, Pauwels OSG (2002) Leptodactylus knudseni (Knudsen's Bullfrog). Predation. Herpetological Review 33:303. [ Links ]
Bouchard SS, Bjorndal KA (2000) Sea turtles as biological transporters of nutrients and energy from marine to terrestrial ecosystems. Ecology 81:2305-2313. [ Links ]
Boyd CE, Goodyear CP (1971) The protein content of some common reptiles and amphibians. Herpetologica 27:317-320. [ Links ]
Brasileiro CA, Sawaya RJ, Giraldelli G (2003a) Physalaemus cuvieri (Barker frog). Predation. Herpetological Review 34:137. [ Links ]
Burton TM, Likens GE (1975) Energy flow and nutrient cycling in salamander populations in the Hubbard Brook Experimental Forest, New Hampshire. Ecology 56:1068-1080. [ Links ]
Buttenhoff P (1995) Bolitoglossa rufescens (Northern Banana Salamander) predation. Herpetological Review 26:197. [ Links ]
Caldart VM, Iop S, Da Rocha MC, Cechin SZ (2011) Diurnal and nocturnal predators of Crossodactylus schmidti Gallardo, 1961 (Anura, Hylodidae) in southern Brazil. North-Western Journal of Zoology 7:342-345. [ Links ]
Cassimiro J, Bertoluci J (2003) Liophis maryellenae (Cobra-d' água). Diet. Herpetological Review 34:69. [ Links ]
Castro ER, Galetti M (2004) Frugivoria e dispersäo de sementes pelo lagarto teiú Tupinambis merianae (Reptilia: Teiidae). Paéis Avulsos de Zoología 44:91-97. [ Links ]
Celedón-Neghme C, Salgado CR, Victoriano PF (2005) Preferencias alimentarias y potencial dispersor del lagarto herviboro Phymaturus flagellifer (Tropiduridae) en los Andes. Guyana 69:266-276. [ Links ]
Celedón-Neghme C, San Marin LA, Victoriano PF, Cavieres LA (2008) Legitimate seed dispersal by lizards in an alpine habitat: The case of Berberis empetrifolia (Berberidaceae) dispersed by Liolaemus belii (Tropiduridae). Acta Oecologica 33:265-271. [ Links ]
Clark JC, Poulsen JR, Parker VT (2001) The role of arboreal seed dispersal groups on the seed rain of a lowland tropical forest. Biotropica 33:606-620. [ Links ]
Collins JP, Crump ML (2009) Extinction in our times: global amphibian decline. London, Oxford University Press. [ Links ]
Colón-Gaud C, Whiles MR, Klham SS, Lips KR Pringle CM, Connelly S (2009) Assessing ecological responses to catastrophic amphibian declines: Patterns of macroinvertebrate production and food web structure in upland Panamanian streams. Limnology & Oceanography 54:331-334. [ Links ]
Colón-Gaud C, Whiles MR, Lips KR, Pringle CM, Klham SS, Connelly S, Brenes R, Peterson SD (2010a) Stream invertebrate responses to a catastrophic decline in consumer diversity. Journal of the North American Benthological Society 29:1185-1198. [ Links ]
Colón-Gaud C, Whiles MR, Brenes R, Kilham SS, Lips KR, Pringle CM, Connelly S, Peterson SD (2010b) Potential functional redundancy and resource facilitation between tadpoles and insect grazers in tropical headwater streams. Freshwater Biology 55:2077-2088. [ Links ]
Connelly S, Pringle CM, Bixby RJ, Brenes R, Whiles MR, Lips KR, Kilham S, Huryn AD (2008) Changes in stream primary producer communities resulting from large-scale catastrophic amphibian declines: can small-scale experiments predict effects of tadpole loss? Ecosystems 11:1262-1276. [ Links ]
Connelly S, Pringle CM, Whiles MR, Lips KR Kilham S, Brenes R (2011) Do tadpoles affect leaf decomposition in neotropical streams? Freshwater Biology 56:1863-1875. [ Links ]
Contosta AR, Frey SD, Cooper AB (2011) Seasonal dynamics of soil respiration and N mineralization in chronically warmed and fertilized soils. Ecosphere 2:121. [ Links ]
Cortes-Figueira LE, Vasconcellos-Neto J, Garcia MA, Teixeira de Souza AL (1994) Saurocory in Melocactus violaceus (Cactaceae). Biotropica 26:295-301. [ Links ]
Costa-Pereira R, Martins FI, Sczesny-Moraes EA, Brascovit A (2010) Predation on young treefrogs (Osteocephalus taurinus) by arthropods (Insecta, Mantodea and Arachnida, Araneae) in Central Brazil. Biota Neotropica 10:469-472. [ Links ]
Creep RP, Taylor A, Pflaum JR (2010) Bioturbation by a dominant detritivore in a headwater stream: litter excavation and effects on community structure. Oikos 119:1870-1876. [ Links ]
Cuello ME, Jara F, Vidoz Q (2005) Atelognathus patagonicus (NCN) predation. Herpetological Review 36:298. [ Links ]
Da Silva HR, De Britto-Pereira MC, Caramaschi U (1989) Frugivory and seed dispersal by Hyla truncata, a neotropical treefrog. Copeia 1989:781-783. [ Links ]
Da Silva HR, Britto-Pereira MC (2006) How much fruit do fruit-eating frogs eat? An investigation on the diet of Xenohyla truncate (Lissamphibia: Anura: Hylidae). Journal of Zoology 270:692-698. [ Links ]
Delia J, Cisneros-Heredia DF, Whitney J, Murrieta-Galindo R (2010) Observations on the reproductive behavior of a Neotropical glassfrog Hyalinobatrachium fleischmanni (Anura: Centrolenidae) South American. Journal of Herpetology 5:1-12. [ Links ]
Del-Grande ML, Moura G (1997) Hyla sanborni (NCN). Predation. Herpetological Review 28:147. [ Links ]
De Souza CF, Palmuti JC, Bertoluci J (2009) Food habits of snakes from the RPPN Feliciano Miguel Abdala, an Atlantic Forest fragment of southeastern Brazil. Biota Neotropica 9-1: 263-269. [ Links ]
DeVault TL, Krochmal AR (2002) Scavenging by snakes: an examination of the literature. Herpetologica 58:429-436. [ Links ]
Dial R, Roughgarden J (1995) Experimental removal of insectivores from rain forest canopy: direct and indirect effects. Ecology 76:1821-1834. [ Links ]
Duarte CF, Vrcibradic D, Van SM (1999) Chironius multiventris foveatus (NCN). Prey. Herpetological Review 30:99-100. [ Links ]
Duellman WE, Trueb L (1994) Biology of amphibians. United States, The Johns Hopkins University press. [ Links ]
Feltrim AC, Zaninicechin ST (2000) Helicops infrataeniatus (NCN). Diet. Herpetological Review 31:46. [ Links ]
Fialho RF (1990) Seed dispersal by a lizard and a treefrog-effect of dispersal site on seed survivorship. Biotropica 22:423-424. [ Links ]
Figueiredo-de-Andrade CA, Santana DJ, De Carvalho-e-Silva SP (2010) Predation on Scinax x-signatus (Anura: Hylidae) by the giant water bug Lethocerus annulipes (Hemiptera: Belostomatidae) in a Brazilian Restinga habitat. Herpetology Notes 3:53-54. [ Links ]
Fittkau EJ (1970) Role of caimans in the nutrient regime of mouth-lakes of amazon affluents (An hypothesis). Biotropica 2:138-142. [ Links ]
Fittkau EJ (1973) Crocodiles and the nutrient metabolism of amazonian waters. Amazoniana 4:103-133. [ Links ]
Flecker AS, Feifarek BP, Taylor BW (1999) Ecosystem engineering by a tropical tadpole: density-dependent effects on habitat structure and larval growth rates. Copeia 1999:495-500. [ Links ]
Formanowicz DR, Stewart MM, Townsend K., Pough FH, Brussard PF (1981) Predation by giant crab spiders on the puerto rican frog Eleutherodactylus coqui. Herpetologica 37:125-129. [ Links ]
Frost DR (2011) Amphibian Species of the World: an Online Reference. Version 5.5 (31 January, 2011). Available at: http://research.amnh.org/vz/herpetology/amphibia/index.html. American Museum of Natural History, New York, USA. Accessed November 2012. [ Links ]
Galindo-Uribe D, Hoyos-Hoyos JM (2007) Relaciones planta-herpetofauna: nuevas perspectivas para la investigación en Colombia. Universietas Scientarium, Revista de la Facultad de Ciencias edición especial I 12:9-34. [ Links ]
Gibbons JW, Scott DE, Ryan TJ, Buhlmann KA, Tuberville TD, Metts BS, Greene JL, Mills T, Leiden Y, Poppy S, Winne CT (2000) The global decline of reptiles, deja-vu amphibians. Bioscience 50: 653-667. [ Links ]
Godínez-Álvarez H (2004) Pollination and seed dispersal by lizards: a review. Revista Chilena de Historia Natural 77:569-577. [ Links ]
Govenar B (2012) Energy transfer through food webs at hydrothermal vents: Linking the lithosphere to the biosphere. Oceanography 25:246-255. [ Links ]
Gray HM, Green DM, Peters MJ (1999) Physalaemus pustulosus (Túngara Frog). Predation. Herpetological Review 30:93. [ Links ]
Greeney HF, Gelis RA, Funk WC (2008) Predation on Caecilians (Caecilia orientali$) by Barred Hawks (Leucopternis princeps) depends on rainfall. Herpetological Review 39:162-164. [ Links ]
Grismer LL (2000) Two new species of skinks (Genus Sphenomorphus Fitzinger 1843) from the Seribuat Archipelago, West Malaysia. Herpetological Natural History 9: 151-162. [ Links ]
Guzmán A, Stevenson PR (2008) Seed dispersal, habitat selection and movement patterns in the Amazonian tortoise, Geochelone denticulata. Amphibia-Reptilia 29:463-472. [ Links ]
Haddad CF, Bastos RP (1997) Predation on the toad Bufo crucifer during reproduction (Anura: Bufonidae). Amphibia-Reptilia 18:295-298. [ Links ]
Hayes MP (1983) Predation on the Adults and Prehatching Stages of Glass Frogs (Centrolenidae). Biotropica 15:74-76. [ Links ]
Henderson RW, Schwartz A, Noeske-Hallin TA (1987) Food habits of three colubrid tree snakes (Genus Uromacer) on Hispaniola. Herpetologica 43:241-248. [ Links ]
Heyer R, Muedeking MH (1976) Notes on tadpoles as prey for naiads and turtles. Journal of the Washington Academy of Sciences 66:235-239. [ Links ]
Heyer WR, McDiarmid RW Weigmann DL (1975) Tadpoles, Predation and Pond Habitats in the Tropics. Biotropica 7:100-111. [ Links ]
Heymann EW (1987) A field observation of predation on a Moustached Tamarin (Saguinus mystx) by an anaconda. International Journal of Primatology 8(2): 193-195. [ Links ]
Hickerson C-AM, Anthony CD, Walton BM (2012) Interactions among forest floor guild members in structurally simple microhabitats. American Midland Naturalist 168: 30-42. [ Links ]
Hocking DJ, Babbitt KJ (2014) Effects of Red-Backed Salamanders on Ecosystem Functions. PLoS ONE 9(1): e86854. doi:10.1371/journal.pone.0086854. [ Links ]
Homyack JA, Sucre EB, Haas CA, Fox TR (2010) Does Plethodon cinereus affect leaf litter decomposition and invertebrate abundances in mixed oak forest? Journal of Herpetology 44: 447-456. [ Links ]
Iverson JB (1985) Lizards as seed dispersers? Journal of Herpetology 19:292-293. [ Links ]
Jara F, Perotti MG (2004) Pleurodema bufoninun (NCN) and Bufo spinulosuspapillosus (NCN). Predation. Herpetological Review 35:161. [ Links ]
Jax K (2005) Function and "functioning" in ecology: what does it mean? Oikos 11:641-648. [ Links ]
Jerozolimski A, Ribeiro MB, Martins M (2009) Are tortoises important seed dispersers in Amazonian forests?. Oecologia 161:517-528. [ Links ]
Jones CG, Lawton JH, Shackak M. (1994). Organisms as ecosystem engineers. Oikos 69: 373-386. [ Links ]
Kitchell JF, O'Neill RV, Webb D, Gallepp GW, Bartell SM, Koonce JF, Ausmus BS (1979) Consumer regulation of nutrient cycling. BioScience 29:28-34. [ Links ]
Kluge AG (1981) The life history, social organization, and parental behavior of Hyla rosenbergi Boulenger, a nest building gladiator frog. Miscellaneous Publications Museum of Zoology University of Michigan 160:1-170. [ Links ]
Kunz TH, de Torrez EB, Bauer D, Lobova T, Fleming TH (2011) Ecosystem services provided by bats. Annals of the New York Academy of Sciences 1223:1-38. [ Links ]
Laverty TM, Dobson AP (2013)Dietay overlap between black caimans and spectacle caimans in the Peruvian Amazon. Herpetologica 69(1):91-101. [ Links ]
Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, Voyle J, Carey C (2006) Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. PNAS 103:3165-3170. [ Links ]
Maerz JC, Karuzas JM, Madison DM, Blossey B (2005) Introduced invertebrates are important prey for a generalist predator. Diversity and Distributions 11: 83-90. [ Links ]
Maffei F, Ubaid FK, Jim J (2010) Predation of herps by spiders (Araneae) in the Brazilian Cerrado. Herpetology Notes 3:167-170. [ Links ]
Manzanilla OV, Manzanilla JP, Steines F (2008) Dos casos de anurofagia por Santinezia curvipes (Roewer, 1916) (Opiliones: Cranaidae). Boletín Sociedad Entomológica Aragonesa 42:317-319. [ Links ]
Marques OAV, Sazima I (1997) Diet and feeding behavior of the coral snake, Micrurus corallines, from the Atlantic forest of Brazil. Herpetological Natural History 5:88-93. [ Links ]
Mendes LC (1996) Phyllomedusa distinct (leaf-frog). Predation. Herpetological Review 27:141. [ Links ]
Mendes LC (2001) Phyllomedusa distinct (Leaf frog). Tadpole predation. Herpetological Review 32:103. [ Links ]
Menin M, Giaretta AA (2003) Predation on foam nests of leptodactyline frogs (Anura: Leptodactylidae) by larvae of Beckeriella niger (Diptera: Ephydridae). Journal of Zoology 261: 239-243. [ Links ]
Menin M, Rodrigues D, Azevedo CS (2005) Predation on amphibians by spiders (Arachnida, Araneae) in the Neotropical region. Phyllomedusa 4:39-47. [ Links ]
Michaud EJ, Dixon JR (1989) Prey items of 20 species of the neotropical colubrid snake genus Liophis. Herpetological Review 20:39-41. [ Links ]
Milton Y, Kaspari M (2007) Bottom-up and top-down regulation of decomposition in a tropical forest. Oecologia 153: 163-172. [ Links ]
Moll M, Jansen KP (1995) Evidence for a role in seed dispersal by two tropical herbivorous turtles. Biotropica 27: 121-127. [ Links ]
Mooney KA, Gruner DS, Barber NA, Van Bael SA, Philpott SM, "et al". (2010) Interactions among predators and the cascading effects of vertebrate insectivores on arthropod communities and plants. Proceedings of the Academy of Natural Sciences of Philadelphia 107: 7335-7340. [ Links ]
Moore JW (2006) Animal ecosystem engineers in streams. BioScience 56:237-246. [ Links ]
Mora JM (1999) Leptodeira annulata (culebra desteñida, banded cat-eye Snake). Diet. Herpetological Review 30:102. [ Links ]
Morales-Betancour MAC, Lasso A., De la Ossa J, Fajardo-Patiño A. (Ed) (2013) Biologia y conservación de los Crocodylia de Colombia. Serie editorial recursos hidrobiológicos y pesqueros continentales de Colombia. Instituto de Investigacion de recursos biologicos Alexander von Humboldt (IAvH). Bogotá, D.C, 336p. [ Links ]
Myers CW, Daly JW, Malkln B (1978) A dangerously toxic new frog (Phyllobates) used by Emberá Indians of western Colombia, with discussion of blowgun fabrication and dart poisoning. Bulletin of the American Museum of Natural Histoory 161:1-72. [ Links ]
Ortega-Andrade HM (2008) Agalychnis spurrelli Boulenger (Anura, Hylidae): variación, distribución y sinonimia. Papéis Avulsos de Zoologia 48:103-117. [ Links ]
Ortiz RM, Plotkln PT, Owens DW (1997) Predation olive ridley sea turtles (Lepidochelys olivacea) by the American crocodile (Crocodylus acutus) at playa Nanclte, Costa Rica. Chelonian Conservation and Biology 2:585-587. [ Links ]
Pérez-Higareda G, Rangel-Rangel A, Smith HM, Chiszar D (1898) Comments on the Food and Feeding Habits of Morelet's Crocodile. Copeia 1989:1039-1041. [ Links ]
Peltzer PM, Lajmanovich RC (2003) Hylapulchella (NCN). Predation. Herpetological Review 34:231. [ Links ]
Peltzer PM, Lajmanovich RC, Attademo AM, Cejas W (2005) Diversidad y conservación de anuros en ecosistemas agrícolas de Argentina: implicancias en el control biológico de plagas. Temas de la Biodiversidad del Litoral Fluvial Argentino II. INSUGEO, Miscelánea 14:263-280. [ Links ]
Pineda E (2003) Hyla miotympanum (Small-eared treefrog) predation. Herpetological Review 34:136-137. [ Links ]
Pough FH, Andrews RM, Cadle JE, Crump ML, Savitzky AH, Wells KD (2004) Herpetology. Upper Saddle River, Prentice-Hall. [ Links ]
Prado GM (2003) Leptodactylus chaquensis (NCN), Pseudis paradoxa (paradox frog), and Phrynohyas venulosa (veined treefrog). Predation. Snakes. Herpetological Review 34:231. [ Links ]
Prado GM, Borgo JH (2003) Sinax altera (NCN). Predation. Herpetological Review 34:238. [ Links ]
Pramuk JB, Alamillo H (2002) Hyla nana (Dwarf tree frog). Predation. Herpetological Review 33:46-47. [ Links ]
Puente-Rolón AR (2001) Arrhyton exiguum (Puerto Rican Garden Snake). Diet. Herpetological Review 32:261. [ Links ]
Ranvestel AW, Lips KR, Pringle CM, Whiles MR, Bixby RJ (2004) Neotropical tadpoles influence stream benthos: evidence for the ecological consequences of decline in amphibian populations. Freshwater Biology 49:274-285. [ Links ]
Reading CJ, Luiselli LM, Akani GC, Bonnet X, Amori G, Ballouard JM, Filippi E, Naulleau G, Pearson D, Ruggiero L (2010) Are snake populations in widespread decline? Biology Letters 6:1-4. [ Links ]
Rivas J, Thorbjarnarson JB, Muñoz CM, Owens RY (1999) Eunectes murinus (Green Anaconda). Caiman predation. Herpetological Review 30:101. [ Links ]
Rivera AL (2010) Comparación de la germinación de semillas de pimienta gorfa (Pimenta dioica), consumidas por la iguana verde (Iguana iguana) y la iguana espinosa (Ctenosaura acanthura). Trabajo de Grado. Facultad de la Biología de la Universidad Veracruzana. http://cdigital.uv.mx/bitstream/123456789/29359/1/LenzRivera.pdf. [ Links ]
Rodríguez RJ, González BR (2000) Evaluación de la capacidad depredadora de Osteopilus septentrionalis (Anura:Hylidae) sobre larvas de Culex quinquefasciatus (Diptera: Culicidae) en condiciones de laboratorio y de semicampo. Boletín de Malariokgíay Saneamiento Ambiental 11:12. [ Links ]
Rueda-Almonacid JV (1999) Anfibios y reptiles amenazadas de extinción en Colombia. Revista de la Academia Colombiana de Ciencias Exactas, Físicasy Naturales 23:475-498. [ Links ]
Ryan MJ, Tuttle MD, Rand AS (1982) Bat predation and sexual advertisement in a neotropical anuran. The American Naturalist 119:136-139. [ Links ]
Sadeghayobi E, Blake S, Wikelski M, James G, Mackie R, Cabrera F (2011) Digesta retention time in the Galápagos tortoise (Chelonoidis nigra). Comparative Biochemistry and Physiology, Part A 160:493-497. [ Links ]
Santana DJ, Da Silva ET, De Oliveira EF (2009) Predação de Dendropsophus elegans (Anura, Hylidae) por Phoneutria nigriventer (Araneae, Ctenidae) em Vicosa, Minas Gerais, Brasil. Boletim do Museu de Biologia Mello Leitào 26:59-65. [ Links ]
Santos-Silva CS, Ferrari SF (2012) Predation on Dendropsophus soaresi (Anura: Hylidae) by a diving beetle (Coleoptera: Dytiscidae) in Raso da Catarina, northeastern Brazil. Herpetology Notes 5:11-12. [ Links ]
Sin H, Beard KH, Pitt WC (2008) An invasive frog, Eleutherodactylus coqui, increases new leaf production and leaf litter decomposition rates through nutrient cycling in Hawaii. Biological Invasions 10: 335-345. [ Links ]
Sazima I, Strüssmann C (1990) Necrofagia em serpentes brasileiras: exemplos e previsöes. Revista Brasileira de Biologia 50:463-468. [ Links ]
Sazima I, Sazima C, Sazima M (2005) Little dragons prefer flowers to maidens: a lizard that laps nectar and pollinates trees. Biota Neotropica 5:185-192. [ Links ]
Sazima I, Sazima C, Sazima, M (2009) A catch-all leguminous tree: Erythrina velutina visited and pollinated by vertebrates at an oceanic island. Australian Journal of Botany 7:26-30. [ Links ]
Schiesari L, Werner EE, Kling GW (2009) Carnivory and resource-based niche differentiation in anuran larvae: implications for food web and experimental ecology. Freshwater Biology 54:572-586. [ Links ]
Schmitz OJ (2008) Effects of predat or hunting mode on grassland ecosystem function. Science 319: 952-954. [ Links ]
Schmitz OJ, Sokol-Hessner L (2002) Linearity in the aggregate effects of multiple predators in a food web. Ecology Letters 5: 168-172. [ Links ]
Sekercioglu CH (2006) Increasing awareness of avian ecological function. Trends in Ecology & Evolution 21: 464-471. [ Links ]
Silva JSB, Hillesheim R (2004) Tupinambis merianae (tegu). Diet. Herpetological Review 35:399. [ Links ]
Sin H, Beard KH, Pitt WC (2008) An invasive frog, Eleutherodactylus coqui, inceases new leaf production and leaf litter decomposition rates through nutrient cycling in Hawaii. Biology Invaions 10:335-345. [ Links ]
Spielman A, Sullivan J (1974) Predation on peridomestic mosquitoes by hylid tadpoles on grand Bahama island. The American Journal of Tropical Medicine and Hygiene 23:704-709. [ Links ]
Spiller DA, Schoener TW (1990) A terrestrial fiel experiment showing the impact of eliminating top predators on foliage damage. Letters to Nature 347:469-472. [ Links ]
Spiller DA, Schoener TW (1997) Folivory on Islands with and without Insectivorous lizards: an eight-year study. Oikos 78:15-22. [ Links ]
Solé M, Kwet A (2003) Liophis jaegeri (Jaeger's Ground Snake). Diet. Herpetological Review 34:69. [ Links ]
Solomon CT, Flecker AS, Taylor BW (2004) Testing the role of sediment-mediated interactions between tadpoles and armored catfish in a Neotropical stream. Copeia 2004:610-616. [ Links ]
Strong JN, Fragoso JM (2006) Seed dispersal by Geochelone carbonaria and Geochelone denticulata in northwestern Brazil. Biotropica 38:683-686. [ Links ]
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues AS, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306:1783-1786. [ Links ]
Stewart M, Woolbright L (1996) Amphibians. In: Reagan D, Waide R (eds) The Food Web of a Tropical Rain Forest. The University of Chicago Press, Chicago, USA, pp 363-398 [ Links ]
Summers K (1999) Dendrobates auratus (Green poison frog). Predation. Herpetological Review 30:91. [ Links ]
Toledo LF (2003) Predation on seven South American anuran species by water bugs (Belostomatidae). Phyllomedusa 2:105-108. [ Links ]
Toledo LF (2005) Predation of juvenile and adult anurans by invertebrates: current knowledge and perspectives. Herpetological Review 36:395-400. [ Links ]
Traveset A (1990) Ctenosaura similis Gray (Iguanidae) as a seed disperser in a central American deciduous forest. The American Midland Naturalist 123: 402-404. [ Links ]
Tyler MJ, Wassersug R, Smith B (2007) How frogs and humans interact: influences beyond habitat destruction, epidemics and global warming. Applied Herpetology 4:1-18. [ Links ]
Uetz P. (2012) The Reptile Database. http://www.reptile-database.org. Retrieved November 2012. [ Links ]
Urbina-Cardona JN (2008) Conservation of Neotropical herpetofauna: research trends and challenges. Tropical Conservation Science 1:359-375. [ Links ]
Vaira M, Coria G (1994) Leptodadylus ocellatus (Rana criolla). Predation. Herpetological Review 25:118. [ Links ]
Valencia A, Cortes AM, Ruiz CA (2013) Ecosystem services provided by amphibians and reptiles in Neotropical ecosystems. International Journal of Biodiversity Science, Ecosystem Services & Management 9(3): 2-16. http://dx.doi.org/10.1080/21513732.2013.821168. [ Links ]
Valencia A, Torres DM, Castro HF (2011) Depredación de huevos de Hyalinobatrachium aureoguttatum (Anura: Centrolenidae) por artrópodos. Boletín del Museo de Entomología de la Universidad del Valle 12:48-50. [ Links ]
Valido A, Olesen JM (2007) The importance of lizards as frugivores and seed dispersers. p 124-147. In: Dennis AJ, Schupp EW Green RJ, Westcott (Eds.): Seed dispersal: theory and its application in a changing world. Wallingford, CAB International. [ Links ]
Vanni MJ (2002) Nutrient cycling by animals in freshwater ecosystems. Annual Review of Ecology, Evolution, and Systematics 33:341-370. [ Links ]
Varela RO, Bucher EH (2002) Seed dispersal by Chelonoidis chilensis in the chaco dry woodland of Argentina. Journal of Herpetology 36:137-140. [ Links ]
Vaz-Silva W Rodrigues HL, Da Silva NJ (2003) Leptodactylus labyrinthicus (Labyrinth frog). Diet. Herpetological Review 34:359. [ Links ]
Verdade VK, Schiesari LC, Bertoluci JA (2000) Diet of juvenile aquatic caecilians, Typhlonectes compressicauda. Journal of Herpetology 34:291-293. [ Links ]
Vilá M (1998) Efectos de la diversidad de especies en el funcionamiento de los ecosistemas. Orsis 13:105-117. [ Links ]
Villa J, Townsend DS (1983) Viable Frog Eggs Eaten by Phorid Fly Larvae. Journal of Herpetology 17:278-281. [ Links ]
Villa J, McDiarmid RW, Gallardo JM (1982) Arthropod predators of Leptodactylid frog foam nests. Brenesia 19:577-589. [ Links ]
Villanueva-Rivera LJ, Joglar RL, Li-Objio FC (2000) Eleutherodactylus coqui (Coqui). Predation. Herpetological Review 31:100. [ Links ]
Vockenhuber EA, Hödl W Karpfen U (2008) Reproductive behaviour of the glass frog Hyalinobatrachium vakrioi (Anura: Centrolenidae) at the tropical stream Quebrada Negra (La Gamba, Costa Rica). Stapfia 88:335-348. [ Links ]
Wells KD (2007) The ecology and behavior of amphibians. USA, The University of Chicago Press. [ Links ]
Whelan CJ, Wenny DG, Marquis RJ (2008) Ecosystem services provided by birds. Annals of the New York Academy of Sciences 1134:25-60. [ Links ]
Whiles MR Lips KR, Pringle CM, Kilham SS, Bixby RJ, Brenes R, Connelly S, Colon-Gaud JC, Hunte-Brown M, Huryn AD, Montgomery C, Peterson S (2006) The effects of amphibian population declines on the structure and function of Neotropical stream ecosystems. Frontiers in Ecology and the Environment 4:27-34. [ Links ]
Wild ER (2001) Ceratophrys cranwelli (Cranwell's Horned Frog). Predation. Herpetological Review 32:102. [ Links ]
Willson MF, Sabag C, Figueroa JA, Armesto JJ, Caviedes M (1996) Seed dispersal by lizards in Chilean rainforest. Revista Chilena de Historia Natural 69: 339-342. [ Links ]
Young BE, Stuart SN, Chanson JS, Cox NA, Boucher TM (2004) Joyas que están desapareciendo: el estado de los anfibios en el nuevo mundo. Arlington-Virginia, NatureServe. [ Links ]
Zamprogno C, Zamprogno MG (1998) Siphonops hardyyi (Hardy's Caecilian). Predation. Herpetological Review 29:166. [ Links ]
Zuffi MAL (2004) Bothrops campbeli (Campbell's lancehead). Diet. Herpetological Review 35:57-58. [ Links ]













