Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Universitas Scientiarum
versión impresa ISSN 0122-7483
Univ. Sci. vol.20 no.2 Bogotá may./ago. 2015
https://doi.org/10.11144/Javeriana.SC20-2.xard
Xylanase activity and T2 (MRI) relationship during lulo la selva P32 ripening process
Relación entre la actividad de la xilanasa con medidas del T2 (IRM) durante el proceso de maduración del lulo La Selva P32
Relação entre e atividade com as medidas xilanase T2 (IRM) durante a maturação lulo A Selva P32
Yenny M. Dueñas1, Luis Agulles-Pedrós2, Carlos A. Rojas1, Adriana C. Agudelo1, Diana C. Alarcón1
Edited by Alberto Acosta & Juan Carlos Salcedo-Reyes
1Grupo de Biofísica y Bioquímica Estructural, Facultad de Ciencias, Pontificia Universidad Javeriana, Bogotá D.C., Colombia.
2Departamento de Física, Universidad Nacional de Colombia, Bogotá D.C.
Funding: Vicerrectoría de Investigación, Pontificia Universidad Javeriana.
Electronic supplementary material: N/A
Received: 30-05-2014 Accepted: 20-01-2015 Published on line: 21-03-2015
Para citar este artículo / To cite this article
Dueñas YM, Agulles-Pedrós L, Rojas CA, Agudelo AC, Alarcón DC (2015) Xylanase activity and T2 (MRI) relationship during lulo la selva P32 ripening process. Universitas Scientiarum 20(2): 247-259 doi: http://dx.doi.org/10.11144/Javeriana.SC20-2.xard
Abstract
The lulo La Selva P32 is a cross over different kinds of lulo. Though its sensory characteristics improvement, its life span is very short. Due to it is a new kind of lulo, there are not so many studies over the ripening process. In order to understand some softening processes, in this work it is evaluated the xylanase kinetics at the lulo peel during ripening, and the results are correlated with the MRI (Magnetic Resonance Imaging) relaxation time T2 . During ripening time, it is observed that the xylanase is one of the enzymes involved in the hydrolysis of the cell wall polymers. In addition, two T2 values regimes were distinguished by the xylanase kinetics. Although the correlation coefficient of 0.82 and p = 0.024 values, the results suggest that T2 weighted MRI can be useful as a non-invasive tool for ripening process monitoring.
Keywords: xylanase, lulillo, relaxation time T2 , MRI
Resumen
El Lulo La Selva P32 es un fruto producto del entrecruzamiento de varias especies de lulo. Aunque tiene mejores características organolépticas, su vida útil es corta. Dado que se trata de una variedad relativamente nueva de lulo, no hay muchos estudios sobre su proceso de maduración. En este trabajo, con el fin de entender algunos de los procesos de ablandamiento de esta variedad de lulo, por una parte, se evalúa la cinética de la xilanasa en la corteza durante la maduración y, por otra parte, estos resultados se correlacionan con el tiempo de relajación T2 , de la Imágenes de Resonancia Magnética Nuclear (IRM). Durante la maduración se observó que la xilanasa es una de las enzimas involucradas en la hidrólisis de los polímeros de la pared celular. Además, a partir de los resultados de la cinética de la xilanasa, se pudieron distinguir dos regímenes de valores T2 . Aunque el coeficiente de correlación fue de 0.82 y p = 0.024, los resultados sugieren que la determinación de T2 por medio de IRM se puede utilizar como una herramienta no invasiva en el proceso de monitoreo de maduración.
Palabras clave: xilanasa, lulillo, tiempo de relajación T2 , IRM
Resumo
O Lulo La Selva P32 é um produto fruto do cruzamento de várias espécies de lulo com um tempo de vida útil curto. Existem poucos estudos para entender os mecanismos que influenciam o amolecimento no lulillo. Este artigo se relaciona com a atividade da xilanase na casca do lulillo com a mobilidade das águas livres (tempo de relaxamento T2 ). O T2 se quantificou por imagens de ressonáncia magnética e a atividade da xilanase por métodos bioquímicos. Se observou uma tendencia no comportamiento dos valores do T2 na mobilidade das águas livres semelhantes á atividade da xilanase, associando-se a migração da água desde o exterior do fruto pela ação da enzima, involucrada na hidrólise de polímeros da parede celular. Apesar do coeficiente de correlação (0.82) e do p (0.024), os resultados sugerem que as imagens de ressonáncia poderiam ser uma alternativa nao destrutiva na indústria de exportação, visto que permite determinar o início da senescencia do fruto, baseado em condições fisiológicas e bioquímicas.
Palavras-chave: xilanase, lulillo, tempo de relaxamento T2 , IRM
Introduction
Colombia, due to its wide variety of climates, presents a lot of fruit crops, which are of interest for human consumption, both nationally and internationally, being the lulo one of them. The lulo belongs to the Solanaceae family, considered to be tropical fruit grown at temperatures around 20 °C, at an altitude higher than 1000 m.a.s.l. The most popular varieties of lulo in Colombia are Solatium septentrional and Solanum quitoense; the latter called lulo "La Castilla" (CORPOICA 2011).
In Colombia, lulo is grown in the departments of Antioquia, Cundinamarca, Cauca, Huila, Meta, Tolima, Valle, Caldas and Risaralda; being Huila the largest harvest area. However, in some regions, harvests have decreased due to phytosanitary problems, as well as the brief time of fruit ripening, which hinders commercialization and export.
Therefore, the State institution of Research and Accompaniment in the Agricultural Sector (CORPOICA), was given the work of studying a variety of lulo that could fulfill the nutritional and organoleptic characteristics, and also would show resilience to pest attack, thus raising the quality and production levels. As a result of this research, it was originated the Lulo La Selva P32 (Agro Invitro 2011), (to be henceforth called lulillo by the authors), which is a product of the inbreeding of two varieties of Lulo: Solatium Hirtium and Solanum Quitoense. The fruits used in this study come from the production plant located at 9 Km of the road Manizales -Neira, Guaicaica Rural District, San Antonio- Farm, Manizales-Caldas, Colombia.
The lulillo is a genetic enhancement that has a better internal quality and is characterized by an excellent yellow pulp, pleasant aroma, yellow bark, large industrial yield, little oxidation of pulp, good taste, between others. However, its size is smaller than the La Castilla, and its useful lifetime is too short. It should be noted that no scientific studies have been carried out, particularly in the mobility of the waters and their relation to the activity of enzymes related to softening of the peel; therefore, information is limited.
During the ripening of plant tissues occur biochemical phenomena that at the same time change the internal structure of the fruit, these changes can be seen when appreciating the variations of the relaxation times through magnetic resonance imaging images (MRI). The MRI technique has been used for the study of fruit for more than a decade (Muñoz-Barrio & Merodio 1997, Gôtz 2004, Aristizábal 2006, Musse et al. 2010, Shaarani et al. 2010, Mariette et al. 2012). Some fruits studied with this technique are: grape (Vitis pinífera) (Del Solar et al. 2002), apples (Pyrus malus L) (Létal et al. 2003, Marigheto & Hills 2005), citrus fruits (Galed et al. 2004) to tomatoes (Lycopersicon esculentum) (Musse et al. 2009, Zhang & McCarthy 2012, Mariette et al. 2012). In the literature, structural changes have been correlated primarily with changes in relaxation times, since, as mentioned, these depend on the magnetic environment due to surrounding molecules and movement. (Mazhara et al. 2015, Defraeye et al. 2013).
Magnetic resonance imaging (MRI) may detect nucleus of different elements, however, MRI images are generally more sensitive to the hydrogen nucleus (sometimes the term is used directly in protons) due to its abundance in biological tissues. On rare occasions, usually by hyperpolarization, can do MRI of another nucleus although it is not the case in this work. In any case, the signal is always sensitive to relaxation times. These can be described as follows: the detected signal has contribution from the signal emitted by all the hydrogens of a pixel: fats, sugars, proteins and water. However, the nucleus of the hydrogen which contribute most are those of the free water molecules, so assume those as the main responsible for the signal (Levitt 2008). These kind of hydrogen depends exponentially on two-stroke characteristic: one for the recovery of the longitudinal magnetization, i.e. parallel to the external magnetic field B0 and one transverse to said axis, i.e. the signal that is detected directly. The longitudinal relaxation or spin-lattice is due to the ability of the environment to absorb energy and is described by a characteristic time T1. Transverse relaxation or spin-spin is due to the difference between spins by small magnetic in-homogeneities within a same pixel and is related to a time T2 . According to the classical description, we can assume to spin like a small rotating magnet (magnetic moment), so it can move charges (electrons) and generate an electric current in an antenna; that generates the detected signal. The total signal is the sum of the current generated by each of these spins and depends on how coherent (in phase) they are turning. This mismatch is because, within a same pixel, the signal issued is averaged by the perpendicular projection of the magnetic moment being processed around of main magnetic field B0. The magnetic moment will not be under the influence of exactly the same local magnetic fields, so during the acquisition of the signal it is observed an exponential decay of the signal due to the difference on precession among magnetic moments during acquisition. Considering that the relaxation time T2 is due to the various in-homogeneities in the field, this time is associated with the movement capacity of the 1H within the pixel, during the application of the sequence. This mobility increases the probability that each nucleus is in a slightly different magnetic field, changing the frequency of precession and phase between them. Therefore, T2 is related to mobility and to changes in the structure of tissues to be studied that are affecting mobility (Ababneh et al. 2008, Levitt 2008, Musse et al. 2009, Musse et al. 2010).
Especially T2 , as a non-destructive technique, has been used to describe the structural changes in cells, such as tomatoes -another solanacea-, whose results in themselves are not satisfactory to describe the maturation process (Musse et al. 2009). Therefore, the action is compared with other variables such as the activity of enzymes, which indirectly contribute to this structural change at the cellular level.
Quality in fruits and vegetables with MRI studies, are related to the growth of fruit, evaluation of ripening, detection of internal defects, studies of physiological disorders and response to post-harvest treatments (Mariette et al. 2012).
In postharvest plant tissues, metabolic activity continues and therefore issues related to the physicochemical and sensory and nutritional characteristics, continue as ripening progresses. Example of these physiological changes corresponds to the phenomenon of softening, where polysaccharides such as pectin, cellulose and hemicellulose are hydrolyzed by enzymes of glucanase type (pectinases, cellulases, and xylanases, respectively); however, research shows that the involvement of these enzymes in the degradation of the cell wall is not always the same. This might be due to the moment that the enzymes act, the different enzyme speeds with which they pursue their activities, the type of fruit in which these events are running (Manrique & Lajolo 2004, Zainon et al. 2004) and differences in the design of the primary cell wall between the fruits, which also contributes to variations in its percentage of softening (Cosgrove 2001).
Concerning the xylanases (EC 3.2.1.8), these are enzymes that catalyze the hydrolysis of the (3-1, 4-glycosidic between D-xylose residues adjacent in the main series of xylan, to produce xilooligo-saccharides. The majority of the xylanases hydrolyze the main chain in regions where the substrate is not replaced (Ronen et al. 1991). The hydrolysis of xylans also requires the participation of the endo-(-1, 4-xylanases and (-xylosidases; of these enzymes, in plant tissue, it has been reported that they may be involved in the process of ripening, allowing not only the solubilization of hemi-cellulosic polymers of the cell wall, but its reorganization in other molecules of lower molecular weight, which could participate as substrates in other metabolic pathways (Buchanan et al. 2000).
The activity of the xylanaseas and (3-xylosidasas have been reported in ripe fruits such as avocado (Persea americana M) (Ronen et al. 1991), papaya (Carica papaya) (Manenoi & Paull 2007). In strawberries (Fragaria chiloensis and Fragaria x ananassa), although the amount of xylans has not been determined directly on the cell wall, it has been noted a high connection of xylose: glucose in the hemi-cellulosic fraction (Huber 1984, Koh & Melton 2002), suggesting the presence of polymers containing Xylose.
In order to understand some physiological and biochemical processes of ripening of the lulillo, it is important to know the behavior of the activity of various enzymes during postharvest; one of them, subject of this work, it is the xylanase. This has been studied in other plant tissues, reporting significant influence in the process of softening (Prabha & Bhagyalakshmi 1998, Srivastava & Dwivedi 2000, Koh & Melton 2002, Rosli et al. 2004, Dueñas et al. 2008). Thus, to carry out a comparative study of the activity of the enzyme as a function of T2 , the first was the periodical measurement of the xylanase activity using the bark of the fruits in ripening Stage 1, according to ICONTEC 5093 (ICONTEC 2002) standards, and through RMI was conducted and analysis of the mobility of free waters, related to the relaxation time T2 . In both cases, measurements were made at two day intervals until full ripening of the lulillo.
Materials and Methods
The enzyme and physicochemical assays (destructive measures), were conducted at the laboratory of food chemistry at Pontificia Javeriana University, and for magnetic resonance imaging (non-destructive measurements), they were done at the Hospital Universitario San Ignacio, in the Pontificia Javeriana University.
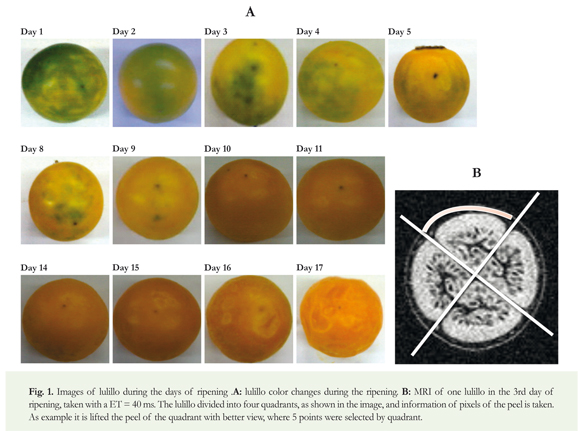
Selection of plant material: The fruits used in this study come from the production plant located at 9 Km of the road Manizales -Neira, Guaicaica Rural District, San Antonio- Farm, Manizales-Caldas, Colombia. About 90 lulos La Selva P32 with peel 90% green and 10% yellow were collected; stage of ripening 1 (ICONTEC 2002), healthy, free of extraneous odor and foreign matter in their apical orifice. Nine of them were for MRI studies, throughout the full ripening time, and the rest were for the biochemical studies.
The fruit postharvest stage: Once selected the fruits, they were distributed in batches of ten units, in baskets, throughout the period of research with environment temperature of 20 ± 2 °C, using the daylight and with a relative humidity of 75 ± 2%. Sampling was carried out periodically to evaluate the behavior of the xylanase activity until the lulillo completed the ripening process, that is, reached the stage of senescence. The whole process took 17 days.
Henceforth, each of the processes was performed in triplicate, using three fruits for each process:
Extration of xylanase: Daily, three peels of fruit were selected, to extract them with liquid nitrogen. The obtained powder was washed with acetone at 4 °C for removal of phenols. Subsequently, the residue was dissolved in phosphate buffer pH 7.0, 20 mM with 1.5 M of NaCl for 5 minutes, and then centrifuged at 4000 rpm / 4 °C. The recovered supernatant was used for the evaluation of the measurement of the xylanase activity and the quantification of this protein.
Activity of the xylanase: The supernatant obtained in the previous stage was used for the activity of xylanase, using buffer acetate 100mM pH 4.0, xylan as substrate 1.5% w/v and 30 μL of enzyme extract. Finally, incubating the reaction at 40 °C for 1 hour, reducing sugars were quantified using the technique of Nelson (Nelson 1944), modified by Somogyi (Somogyi 1952), where the generation of reducing sugars was determined spectrophotometrically by the action of xylanase on xylan. D-glucose was used a patron of quantification. One unit (U) of the enzyme catalyzes the release of umol of glucose in 1 minute in test conditions (Priya et al. 1995, Srivastava & Dwivedi 2000). The specific activity of the enzyme was expressed as U/mg protein (Dueñas et al. 2008).
Determination of proteins in enzyme extracts: Protein was determined by the method by the method of Bradford, modified by Zor and Sellinger (Zor & Sellinger 1996), using bovine albumin (BSA) as standard.
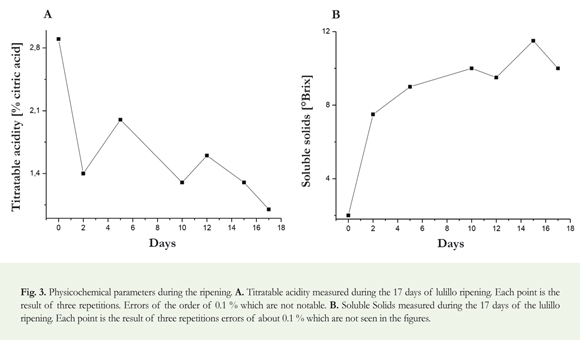
Total Soluble Solids: From each fruit pulp, total soluble solids were measured using a Brixco refractometer, reporting the Brix in percentage of 0-90%.
Total Titratable acidity: Using the pulp of each fruit, the acidity present in the sample was determined, titrating with NaOH 0,1 N and using phenolphthalein as an indicator. This value is expressed in percentage of citric acid.
Magnetic resonance imaging: The pictures were taken with a Philips MRI scan of 1.5 T. at the San Ignacio University Hospital, Bogotá, Colombia. On a previous study with lulillos, at different stages of ripening, the range of expected T2 was determined using different eco times (ET). The previous study showed the decay of the signal for different stages of ripening and ET. In this way, were defined two values of TE that revealed sufficient decay of the signal to obtain a good adjustment of the exponential decay without great loss of signal.
In parallel to the biochemical studies during the post-harvest of the fruit, 9 of 90 lulillos of the initial sample were selected. To the nine selected seven T_[2] weighted MRI were taken, in different days, during the seventeen days of storage. Each measurement day, two consecutive spin echo images (Levitt 2008) were taken with all the same parameters (FOV, TR, resolution...), except ET, which was 40 ms and 80 ms. Images were analyzed with the free software Image (http://rsbweb. nih.gov/ij/, date of query November of 2014). Using these images the signal to noise ratio (SNR) was measured in the peel and the relaxation time T2 for each fruit was determined. For this, the procedure was as follows: each lulillo peel was divided into 4 parts, to avoid the areas near to the segments (Figure 1B); in each zone, the signal of 5 pixel was taken and the average was divided by the noise. The noise was taken as the average signal per pixel of the upper, down, right and left region of the image given, in those areas where it was known that there were no protons. About 5*4 daily measures of SNR were taken for each of the nine lulillos. For the calculation of T2 it was proceeded to compare the SNR of the image with an ET of 40 ms, with that of 80 ms of ET, as follows:
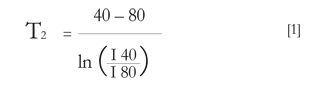
where I40 and I80 are the averaged SNR of the images with ET from 40 to 80 ms. T2 of each lulillo of the first day was taken as a reference value, on which the values for the remaining days were normalized. On this standardization, were averaged the nine lulillos, obtaining the daily measurement of T2 , whose error was taken as the standard deviation of the daily average.
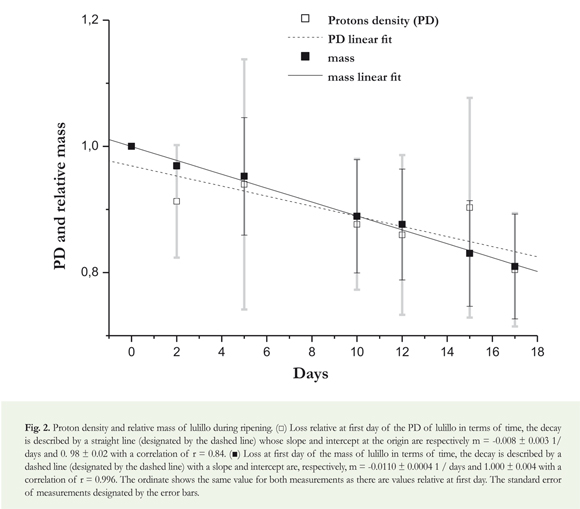
Relative loss of mass: The mass loss was measured with an analytical balance. Throughout the ripening period, periodic measurements of the mass of each lulillo were done. The mass loss of each lulillo was analyzed separately, normalizing to the first day and obtaining a mass relative to the first day. Finally, the relative loss of all lulillos was averaged per day, estimating the error as the standard deviation.
Density measurement for protons (PD): Proton density (PD) are assumed as the SNR. PD was measured using spin-echo sequence with a minor ET (40 ms). In a single image, it was possible to observe all lulillos. The lulillo's signal was measured assuming that the igure of each lulillo in the image was circular, averaging the total signal by pixel within this circle. This signal from each "circular" lulillo was divided by the pixel noise signal where there were lulillos—margins up, down, right and left, obtaining the SNR of that image, such as for the result of T2 . The SNR of each lulillo was standardized to the irst day, and the daily average of the nine standardized lulillos was used to establish the daily PD. Error is deined as the average standard deviation.
Statistical design: Pearson's correlation was used to compare the T2 and xylanase activity on the lulillo.
Results:
Relative mass:
A loss of approximately 1% of the mass per day for each lulillo, with a linear performance as shown in Figure 2, was observed. Figure 1A shows the ripening process of the lulillo on the 17th day, showing roughness and size change.
Density of protons
In Figure 2, more dispersed mesurements are observed than the mass, but with a similar behavior, that is a loss of around 1%.
Titratable acidity:
According to Figure 3A, the values of the total acidity, expressed as a percentage of citric acid will be reduced over time.
Soluble solids:
Figure 3B shows the performance of the SST expressed as Brix in the pulp of the lulillo, showing an increasing inclination with respect to the time of ripening of the lulillo.
Behavior of the xylanase during ripening of lullilo:
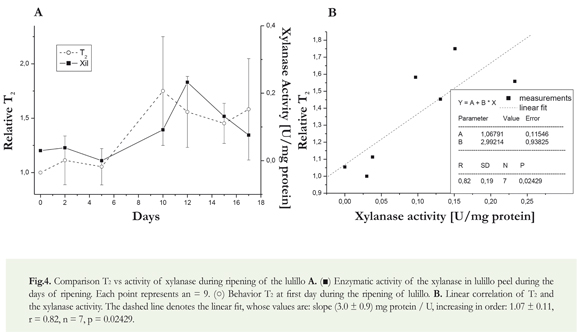
After picking the fruits at their ripeness stage 1, we proceeded to the quantification of xylanase activity. The behavior of the activity of the xylanase in the peel of the lulillo is evident in Figure 4A, during the 17 days of ripening of the fruit, showing a maximum activity of the enzyme towards the day 12th.
Transversal relaxation time: T2
In the T2 mesurements, shown in Figure 4B, a relative value increase is observed. This behavior could be divided into two periods with constant values; the first period lasted from day 0 to day 10th and the second, from day 10th to 17th. It is clearly observed a change in the 10th, simultaneous with a maximum the xylanase activity.
Discussion
Relationship among the various parameters evaluated during the ripening of the lulillo:
Commonly, it is expected that the modification of the cell wall of the fruit is associated with changes in firmness and texture, but the type and magnitude of these changes during ripening vary considerably between plant tissues (Brummell et al. 2004).
In Figure 1A is shown the ripening process of lulillo, where after the 9th day the fruit amounts to 100% of yellowing, associated with its sensory maturity and, comparing with the variety Solanum Quitoense in 5093 NTC, the 4 color corresponds to 100% of fruit peel and for the 5 color the fruit is over-ripe. For other lulo varieties, at temperatures of 20 °C, full yellow color is achieved by the day 15th in Solanum sp. (Burbano & Daza 2012), while for the Solanum Quitoense Lam is observed on days 24 (Rodríguez & Restrepo 2011) and 27 (Cortés 2006). These differences between fruits may possibly be due to their origin, to the moment when the fruits were harvested for starting the investigation, to possible genetic improvements made in the varieties of lulo, as well as to weather conditions both pre-harvest and post-harvest.
In terms of the mass loss and the proton density (Figure 2), despite any inaccuracies, a linear behavior is assumed in both the relative mass loss as in the proton density and the value of the slope is similar, within the slope adjustment errors. The high standard deviation of the PD signal loss is probably due to the inaccuracy in the measurement for assuming the lulillo as spherical. In addition, different internal structures have different intensity loss during the ripening process. Finally, another factor to consider is that a minor ET could minimize the influence of T2 and produce a more homogeneous behavior of the sample. However, this shows that the relative comparison of the signal (with the sequence and parameters used) can be used as an indicator of mass loss.
Systems of nuclear magnetic resonance images are mainly based on detection of proton density in tissues (influenced by the mobility of protons within the fruit core), revealing the onset of ripeness of the mesocarp (Angón et al. 2006). This explains the decrease in the values of proton density, since throughout the ripening of the fruits the breaking of hydrogen bridges increases, among other reasons, by the action of hydrolytic enzymes. Moreover, in the process of fruit ripening, mass loss is a result of transpiration and respiration processes; likewise, the water vapor pressure deficit between the fruit and the environment generates great water losses, which involves two things: proton losses and a consequently reduction of signal and a significant reduction in fresh weight (Lanchero et al. 2007, Cordeiro et al. 2013).
Sugars and organic acids in many plant tissues are important components of the flavor and, along with aroma, play an important role in maintaining the quality and nutritional value of the fruit (Jianhua et al. 2015). The behavior of the percentage of citric acid (Figure 3) was decreasing over time lulillo storage time, since the organic acids are, after carbohydrates, the most important energy deposit of fruits (Cordeiro et al. 2013). Furthermore maturity is reflected, among other aspects, by the behavior of soluble solids expressed as °Brix (Fischer & Martínez 1999). In this study, the behavior of the °Brix (Figure 3B) showed a tendency to increase with regard to the time, due to oxidative processes that are notorious in climacteric fruits, since respiratory rate increases and as a result it generates the hydrolysis of starch by the action of hydrolytic enzymes, which subsequently is converted into fructose and sucrose (Zainon et al. 2004). In this study, the xylanase activity is evident throughout the storage (Figure 4). The low activity in the early days is probably caused by the low amount of enzyme present in the fruit. It is evidenced an increase in the activity of the xylanase after the 5th day and until day 12th. In this time interval it is perhaps when, besides ethylene production, the fruit begins its physiological ripening phase, which among other aspects comprises diverse biochemical events associated with the phenomenon of softening of the fruit peel, hydrolysis of polysaccharides, synthesis of carotenes, possibly also due that in the fruit core are the optimal conditions for the interaction of the enzyme with the substrate, generating de-polymerization of the components that are part of the intermediate cell wall film. After reaching the maximum xylanase activity, it is decreased because the fruit is in the stage of senescence or apoptosis (Dueñas et al. 2008, Cordeiro et al. 2013).
Now, if the xylanase activity of lulillo is correlated with the color changes of fruit during its ripening process, which are shown in Figure 1A, it could be argued that these changes correspond to each of the physiological and metabolic processes developing inside the fruit. The low activity seen at the end of storage will then correspond to the stage of fruit aging. Therefore, the xylanase activity in the lulillo could generate the breaking of hemicellulose, and thus actively participate in softening, such as it happens during fruit ripening of papaya fruits (Carica papaya) (Manenoi & Paull 2007) and possibly yellow pitahaya (pitajaya amarilla) (Dueñas et al. 2008). Changes associated with the ripening in the lulillo not only correspond to the xylanase activity, but also to the possible activity of other enzymes endogenous to the post-harvest storage conditions (where, for example, variations in temperature during a single day interfere in the acceleration of this type of hydrolytic activity), to cases where the fruits differ markedly in their botanical origin; to the composition of polysaccharides and proteins; to the structure of the cell membrane; to the enzymatic metabolism; to the patterns of growth and ripening; as well as to the behavior during softening; whose answers are turned into changes in flesh firmness, in the rate of softening and in texture in general. These events also occur between different species, and they frequently occur in different cultivars, varieties and selections of the same species (Goulao & Oliveira 2008).
Comparison of T2 with xylanase activity:
Importantly, although different authors have made measurements similar to those proposed in this article, there are no known works that allow a comparison between the results of two different methodologies: biochemistry (enzymatic activity) and MRI (T2). In addition, for this last technique, no studies are reported in lulos of any species.
The Pearson correlation test was conducted to determine the relation between the xylanase enzyme activity and molecular mobility measured by T2 , which was obtained during the 17 days of ripening. The coefficient 0.82 is obtained, proving that there is no correlation between the enzyme activity and the relative T2 , with a P value of 0.024, (Figure 4B). If the graph of Figure 4A is observed, there is a similar pattern in the behavior of T2 and enzymatic activity; there is a similar change in the two parameters. The absolute values and the order of magnitude of change in the value of T2 , are consistent with those reported by Musse et al. (2009). The pattern was different when compared with our results, since it worked with various fruits, including another Solanaceae. These differences may be related to the different components and the behavior of the peel. Low values of T2 during the first days show the low mobility of waters present in the fruit, given the minimum conditions for enzymes to interact with their respective substrates in order to exercise the corresponding depolymerization of different polysaccharides that make up the fruit (Dueñas et al. 2008). Towards the 10th day, it was observed that the value of T2 increases approximately by 50%. Comparing this change with that of xylanase, which appears by day 12 and is shown as a maximum, it could be argued that the mismatch with the T2 is probably due to the action of other enzymes, which could have been activated by the maximum xylanase activity.
Metabolic events that are responsible for changes in the texture of the fruits, it is believed, involve a loss of turgidity and mass (Figure 2), to decomposition of starch (associated with increased in °Brix, Figure 3B), and to various dynamic mechanisms in the modifications of the structure of the cell wall. Although the relative contribution of each ripeness period of fruits like Apple (Pyrus malus L), strawberry (Fragaria chiloensis) and papaya (Caricapapaya) it is still not clear and probably depends on the species, on changes in the cellular wall composition especially the mechanics strength and on adhesion existing among cells, which are those that have been considered the most important factors (Hadfield & Bennett 1998, Jianmei et al. 2010, Figueroa et al. 2010, Mariette et al. 2012, Iniesta-González et al. 2013).
Biochemical studies indicate that structural changes and dispositions (rearrangements) in the structure of the cell wall during ripening, are produced together among pectins, hemicelluloses and celluloses (Huber 1984, Seymour et al. 1990, Jianmei et al. 2010, Figueroa et al. 2010) and as a result, in most cases, this is attributed to the activity of a certain group of enzymes and proteins of the same family that promote the growth and extension of tissue (Fischer & Bennett 1991, Iniesta-González et al. 2013). The disruption of the "red" of the cell wall probably involves the effect of the concerted and synergistic activity of many enzymes, where a family of enzymes that modify the cell wall could mediate the activity of another, resulting in changes in the cell wall (Rose et al. 1997).
The texture of fleshy fruit can be affected both by the cellular structure and the biochemical composition of the tissue. This organization of the tissue depends on the cell morphology, on the disposition of cells and the properties of cells and tissues. In this context, MRI can provide information about the cell, directly or indirectly and at macroscopic levels, with the advantage that this is a non-destructive analysis. This is particularly interesting for research on issues related to fruit ripening and/or development of physiological disorders (Musse et al. 2009).
Based on the results obtained for this fruit, and given that there are no studies of these cases, it is necessary to go in depth into the analysis of the purification of other enzymes that are related with the softening and darkening of the fruit. These studies should be complemented with MRI to provide structural information; diffusion, Ti, and spectroscopy images, in addition to T2 . Thus, the optimal harvest point could be set with basis on different parameters for this variety of lulillo, so that post-harvest farming losses are minimized. Furthermore a multi-parameter and interdisciplinary research helps to understand the mechanisms of ripening better.
Conclusion
For the variety of lulo La Selva P32, the results of the activity of the xylanase enzyme with T2 measurements, using MRI, were correlated. It is evident that when the enzyme exerts hydrological action in the peel of the fruit, increases the mobility of the free waters in response to the activity of the enzyme. These two techniques can complement and enable a better understanding of the fruit internal characteristics as goes the ripening process.
Acknowledgment
To Mr. Alirio Calderon owner of Finca San Antonio located in the village of Guacaica for his assistance in providing the lulillos used for this investigation. To Dr. Luis Felipe Uriza, Victor and Douglas for their advice, patience and use of MRI equipment, as well as to Pilar Infante Luna for her help with statistics.
This work was financed by the Vicerectory of the Pontificia Javeriana University (Bogotá D.C.) project ID 003175.
Conflict of interest
The authors declare that no conflicts of interest exist in relation to this work.
References
Ababneh ZQ, Ababneh R, Maier SE, Winalski CS, Oshio K, Ababneh AM, Mulkern RV (2008) On the correlation between T2 and tissue diffusion coefficients in exercised muscle: quantitative measurements at 3T with in the tibialis anterior. Magnetic Resonance Materials in Physics Biology and Medicine 21:273-278 doi: 10.1007/s10334-008-0120-8. [ Links ]
Agro Invitro (2011) http://www.agroinvitro.com/lulo/. Consultado en noviembre del 2014. [ Links ]
Angón-Galván P, Santos-Sánchez N, Hernández C (2006) Índices para la determinación de las condiciones óptimas de maduración de un fruto. Universidad Tecnológica de la Mixteca. Institut de Agroindustrias. Temas de Ciencia y Tecnología 10:30:3-8. [ Links ]
Aristizábal I (2006) Estudio, aplicación y propuesta de automatización del procesamiento de imágenes por resonancia magnética para la evaluación y detección de defectos internos de calidad en cítricos y melocotones. Doctorate thesis. Universidad Politécnica de Valencia. Valencia, España. [ Links ]
Burbano F, Daza J (2012) Image analysis application to determine variations in shape, size and color of biological structures: Determination of changes in size and color of the fruit of Solanum sp. in the process of maturation. 5th International Conference on BioMedical Engineering and Informatics 286-289 doi: 10.1109/BMEI.2012.6512945. [ Links ]
Brummell D, Cin V, Crisosto C, Labavitch J (2004) Cell wall metabolism during maturation, ripening and senescence of peach fruit. Journal of Experimental Botany 55:2029-2930 doi: 10.1093/jxb/erh227. [ Links ]
Buchanan B, Gruissem W Jones R (2000) The cell wall. In: Biochemistry and molecular biology of plants. American Society of Plant Physiologists 52-158. [ Links ]
Cordeiro N, Sousa L, Freitas N, Gouveia M (2013). Changes in the mesocarp of Annona cherimola Mill. "Madeira" during postharvest ripening. PostharvestBiology and Technology 85:179-184 http://dx.doi.org/10.1016/j.postharvbio.2013.05.014. [ Links ]
CORPOICA (2011) http://www.corpoica.org.co/SitioWeb/Archivos/Publicaciones/Lulo.pdf. Retrieved November 2014. [ Links ]
Cortés R (2006) Efecto de la refrigeración y el choque térmico sobre algunos indicadores de actividad antioxidante en lulo (Solanum quitoense Lam). Master thesis. Faculty of Sciences. Universidad Nacional de Colombia, Bogotá [ Links ].
Cosgrove D (2001) Wall structure and wall loosening: a look backward and forwards. Plant Physiology 125:131-134. [ Links ]
Defraeye T, Lehmann V, Gross D, Holat C, Herremans E, Verboven P, Verlinden B, Nicolai B (2013) Application of MRI for tissue characterisation of 'Braeburn' apple. Postharvest Biology and Technology 75:96-105 http://dx.doi.org/10.1016/j.postharvbio.2012.08.009. [ Links ]
Del Solar D C, Irarrázaval P, SozaJA, Depallens D, Esquivel J (2002) Resonancia Magnética (Scanner- MRI) en CV Thompson (Vitis vinifera L.) seedless como posible técnica para evaluar condición en postcosecha. Pharos 9(2):29-64 [ Links ]
Dueñas Y, Narváez C, Restrepo L (2008) Búsqueda de las mejores condiciones para la extracción y medida de actividad de celulasa y xilanasa extraídas de la corteza de pitaya amarilla (Acanthocereus pitajaya). Acta Biológica Colombiana 13 (1):217-228 [ Links ]
Figueroa CR, Rosli HG, Civello PM, Martínez GA (2010) Changes in cell wall polysaccharides and cell wall degrading enzymes during ripening of Fragaria chiloensis and Fragaria xananassa fruits. Scientia Horticulturae 124:454-462 doi: 10.1016/j.scienta.2010.02.003. [ Links ]
Fischer RL, Bennett AB (1991) Role of cell wall hydrolases in fruit ripening. Annual Review of Plant Physiology and Plant Molecular Biology 42:675-703. [ Links ]
Fischer G, Martínez O (1999) Calidad y madurez de la uchuva (Physalis peruviana L.) en relación con la coloración del fruto. Agronomía Colombiana 16(1-3) :35-39. [ Links ]
Galed G, Fernández M, Valle A, Martínez A (2004) Application of MRI to monitor the process of ripening and decay in citrus treated with chitosan solutions. Magnetic Resonance Imaging 22:127-137 doi: 10.1016/j.mri.2003.05.006. [ Links ]
Götz J (2004) Applications of NMR to Food and Model Systems in Process Engineering. Doctoral thesis. Technische Universität München, Alemania. [ Links ]
Goulao L, Oliveira C (2008) Cell wall modifications during fruit ripening: when a fruit is not the fruit. Trends in Food Science and Technology 19:4-25 doi: 10.1016/j. tifs.2007.07.002. [ Links ]
Hadfield K, Bennett A (1998) Polygalacturonases: many genes in search of a function. Plant Physiology 117:337-343. [ Links ]
Huber D (1984) Strawberry fruit softening: the potential roles of polyuronides and hemicelluloses. Journal of Food Science 49:1310-1315. [ Links ]
IMAGE J http://rsbweb.nih.gov/ij/. Retrieved November 2014. [ Links ]
Iniesta-González JJ, Lino-López GJ, Paullc RE, Barba de la Rosad AP, Mancilla-Margallie NA, Sañudo-Barajas JA, Ibarra-Junquera V, Chenc NJ, Hernández-Velasco MA, Osuna-Castro JA (2013) Papaya endoxylanase biochemical characterization and isoforms expressed during fruit ripening. Postharvest Biology and Technology 81:13-22 http://dx.doi.org/10.1016/j.postharvbio.2013.02.001. [ Links ]
Instituto Colombiano de Normas Técnicas y Certificación. Frutas Frescas. Lulo de Castilla. Especificaciones. Bogotá: ICONTEC, 2002 (NTC 5093) [ Links ]
Jianhua Z, Haoxia L, Wanpeng X, Wei A, Linlin N, Youlong C, Huafang W (2015) Changes in sugars and organic acids in wolfberry (Lyciumbarbarum L.) fruit during development and maturation. Food Chemistry 173: 718-724 http://dx.doi.org/10.1016/j.foodchem.2014.10.082. [ Links ]
Jianmei W Fengwang M, Shouguo S, Xiudong Q, Xiangqiu Z, Junwei Y (2010) Changes and postharvest regulation of activity and gene expression of enzymes related to cell wall degradation in ripening apple fruit. Postharvest Biology and Technology 56:147-154 doi: 10.1016/j. postharvbio.2009.12.003. [ Links ]
Koh TH, Melton LD (2002) Ripening related changes in cell wall polysaccharides of strawberry cortical and pith tissues. Postharvest Biology and Technology 26:23-33. [ Links ]
Lanchero O, Velandia G, Fischer G, Varela NC, García H (2007) Comportamiento de la uchuva (Physalis peruviana L.) en poscosecha bajo condiciones de atmósfera modificada activa. Revista Corpoica - Ciencia y Tecnología Agropecuaria 8:61-68. [ Links ]
Létal J, Jirák D, Suderlová L, Hájek M (2003) MRI 'texture' analysis of MR images of apples during ripening and storage. Lebensm. -Wiss.u.-Technology 36:719-727 doi: 10.1016/S0023-6438(03)00099-9. [ Links ]
Levitt M (2008) Spin Dynamics: Basic Principles of NMR Spectroscopy. John Wiley & Sons. [ Links ]
Manenoi A, Paull R (2007) Papaya fruit softening, endoxylanase gene expression, protein and activity. Physiologia Plantarum 131:470-480 doi: 10.1111/j.1399-3054.2007.00967.x. [ Links ]
Manrique J, Lajolo F (2004) Cell-wall polysaccharide modifications during postharvest ripening of papaya fruit (Carica papaya). Postharvest Biology and Technology 33:11-26 doi: 10.1016/j.postharvbio.2004.01.007. [ Links ]
Mariette F, Collewet G, Davenel A, Lucas T, Musse M (2012) Quantitative MRI in Food Science & Food Engineering. Encyclopedia of Magnetic Resonance by John Wiky & Sons Ltd. doi: 10.1002/9780470034590.emrstm1272. [ Links ]
Marigheto N, Hills B (2005) MRI as a Potential On-line Sensor of Apple Quality. In: Cemagref (Ed.) FRUTIC 05 Information and Technology for Sustainable Fruit and Vegetable Production. Montpellier, France 455-463. [ Links ]
Mazhara M, Cowinc G, Breretonc I, Hofmand P, Collinsa R, Guptaa M (2015) Non-destructive 1H-MRI assessment of flesh bruising in avocado (Persea americana M.) cv Hass. Postharvest Biology and Technology 100:33-40 http://dx.doi.org/10.1016/j.postharvbio.2014.09.006. [ Links ]
Muñoz-Barrio MT, Merodio C (1997) Magnetic Resonance Imaging/ A non-destructive approach to ripening state in fruits. Acta Horticulturae 463:385-390. [ Links ]
Musse M, Quellec S, Cambert M, Devaux M-F, Lahaye M, Mariette F (2009) Monitoring the postharvest ripening of tomato fruit using quantitative MRI and NMR relaxometry. Postharvest Biology and Technology 53:22-35 doi: 10.1016/j.postharvbio.2009.02.004. [ Links ]
Musse M, De Guio F, Quellec S, Cambert M, Challois S, Davenel A (2010) Quantification of microporosity in fruit by MRI at various magnetic fields: Comparison with X-ray microtomography. Journal of Magnetic Resonance Imaging 28, 10. 1525-1534 doi: 10.1016/j.mri.2010.06.028. [ Links ]
Nelson N (1944) A photometric adaptation of the Somogyi method for determination of glucose. Journal of Biological Chemistry 153:378-380 [ Links ]
Prabha T, Bhagyalakshmi N (1998) Carbohydrate metabolism in ripening banana fruit. Phytochemistry 48(6):915-920. [ Links ]
Priya K, Prabha T, Tharanathan R (1995) Postharvest biochemical changes associated with the softening phenomenon in Capsicum annuum fruits. Phytochemistry 42:961-966. [ Links ]
Rodríguez J, Restrepo LP (2011) Activity of pectic enzymes involved in the ripening process of lulo (Solanum quitoense Lam.). Agronomía Colombiana 29:63-71. [ Links ]
Ronen R, Zauberman G, Akerman M, Weksler A, Rot I, Fuchs Y (1991) Xylanase and xylosidase activities in avocado fruit. Plant Physiology 95:961-964. [ Links ]
Rose JK, Lee HH, Bennett AB (1997) Expression of a divergent expansin gene is fruit-specific and ripening-regulated. Plant Biology 94:5955-5960. [ Links ]
Rosli G, Civello M, Martínez A (2004) Changes in cell wall composition of three Fragaria x ananassa cultivars with different softening rate during ripening. Plant Physiology and Biochemistry 42:823-831 doi: 10.1016/j.plaphy.2004.10.002. [ Links ]
Shaarani, S Md, Cardenas-Blanco A, Amin MHG, Soon NG, Hall LD (2010) Monitoring development and ripeness of oil palm fruit (Elaeisguneensis) by MRI and bulk NMR. International Journal of Agriculture and Biology 12: 101-105. [ Links ]
Seymour G, Colquhoun I, Dupont M, Parsley K, Selvendran R (1990) Composition and structural features of cell wall polysaccharides from tomato fruits. Phytochemistry 29:725-731 doi: 10.1016/0031-9422(90)80008-5. [ Links ]
Somogyi M (1952) Notes on sugar determination. Journal of Biological Chemistry 195:19-23. [ Links ]
Srivastava M; Dwivedi U (2000) Delayed ripening of banana fruit by salicylic acid. Plant Scientarum 158:87-96. [ Links ]
Zainon M, Lieng-Hong C, Hamid H (2004) A comparative study on wall degrading enzymes, pectin modifications and softening during ripening of selected tropical fruits. PlantScience 167:317-327 doi: 10.1016/j.plantsci.2004.03.030. [ Links ]
Zhang L, McCarthy M (2012) Measurement and evaluation of tomato maturity using magnetic resonance imaging. Postharvest Biology and Technology 67:37-43 doi: 10.1016/j.postharvbio.2011.12.004. [ Links ]
Zor T, Sellinger Z (1996) Linearization of the Bradford protein assay increases its sensivity: theorical and experimental studies. Analytical Biochemistry 236:302-308. [ Links ]













