Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Universitas Scientiarum
versión impresa ISSN 0122-7483
Univ. Sci. vol.20 no.3 Bogotá sep./dic. 2015
https://doi.org/10.11144/Javeriana.SC20-3.eobp
Evaluation of biological production of lactic acid in a synthetic medium and in Aloe vera (L.) Burm. f. processing by-products
Evaluation of biological production of lactic acid in a synthetic medium and in Aloe vera (L.) Burm. f. processing by-products
Avaliação da produção biológica de ácido lático em um meio sintético e no processamento de subprodutos de Aloe vera (L.) Burm. f.
Javier Antonio Gómez-Gómez1, Catalina Giraldo-Estrada2, David Habeych3, Sandra Baena1
Edited by Alberto Acosta & Juan Carlos Salcedo-Reyes
1Unidad de Saneamiento y Biotecnología Ambiental - Departamento de Biología — Facultad de Ciencias - Pontificia Universidad Javeriana, POB 56710, Bogotá D.C, Colombia
2Departamento de Ingeniería de Procesos — Escuela de Ingeniería -Universidad EAFIT, Medellín, Colombia
3Verlengde Meeuwerderweg 97 9723ZM, Groningen, Netherlands
Funding: Colciencias, Pontificia Universidad Javeriana, EAFIT and Agrobamboo de Colombia (Research project 120348925362; ID 3676), and had permit No. 33 2009 of the Ministry of Environment and Sustainable Development of Colombia to access genetic resources.
Electronic supplementary material: N/A
In memory of Michael Tistl.
Received: 07-02-2014 Accepted: 21-09-2015 Published on line: 17-11-2015
Para citar este artículo / To cite this article
Gómez-Gómez J A, Giraldo-Estrada C, Habeych D, Baena S (2015) Evaluation of biological production of lactic acid in a synthetic medium and in Aloe vera (L.) Burm. f. processing by-products. Universitas Scientiarum 20(3): 369-385 doi: http://dx.doi.org/10.11144/Javeriana.SC20-3.eobp
Abstract
This study evaluated lactic acid production through batch fermentation in a bioreactor with Thermoanaerobacter sp. strain USBA-018 and a chemically defined culture medium and with hydrolyzed pressed extract of Aloe vera peel (AHE). The strain USBA-018 fermented various sugars, but its primary end-product was L-lactic acid. Factors which influenced L- lactic acid production were pH, addition of yeast extract (YE) and manganese chloride. Under the most favorable growing conditions for the production of lactic acid, yield (Yp/s) increased from 0.66 to 0.96 g/g with a productivity (Qp) of 0.62 g.l-1.h and a maximum lactic acid concentration of 178 mM at 26 hours of fermentation. When AHE was used, 93.3 mM, or 0.175 g.l-1.h, was obtained. These results show the potential for transformation of sugars that strain USBA-018 offers, but additional studies are needed to find out if different strategies using AHE as carbon source can produce large enough quantities of lactic acid to allow AHE to become a low-cost alternative substrate.
Keywords: Aloe vera, lactic acid, Thermoanaerobacter sp. USBA-018, thermophilic fermentation
Resumen
Este estudio evaluó la producción de ácido láctico de la cepa de Thermoanaerobacter sp. USBA-018 en un biorreactor de fermentación por lotes, utilizando como medios de cultivo una formulación químicamente definida y un extracto prensado e hidrolizado de cáscara de Aloe vera (AHE). La cepa USBA-018 fermentó varios azúcares, pero su principal producto final fue L-ácido láctico. Los factores que influyeron en la producción de L-ácido láctico fueron pH, adición de extracto de levadura (YE) y de cloruro de manganeso. Bajo las condiciones más favorables de crecimiento para la producción de ácido láctico el rendimiento (Yp/s) aumentó de 0.66 a 0.96 g/g, con una productividad (Qp) de 0.62 g.l-1.h y una máxima concentración de ácido láctico de 178 mM a las 26 horas de fermentación. Cuando se usó AHE, se obtuvieron 93.3 mM, o 0.175 g.l-1.h. Estos resultados muestran el potencial de transformación de azúcares que ofrece la cepa USBA-018, pero se requieren estudios adicionales para determinar si diferentes estrategias de uso de AHE como fuente de carbono producen cantidades suficientemente grandes de ácido láctico como para permitir que el AHE se convierta en un sustrato alternativo de bajo costo.
Palabras clave: Aloe vera, ácido láctico, Thermoanaerobacter sp. USBA-018, fermentación termofílica
Resumen
Este estudo avaliou a produção de ácido lático por meio de fermentação descontinua em um biorreator com Thermoanaerobacter sp. cepa USBA-018 e um meio de cultura químicamente definido e com extrato hidrolisado de casca de Aloe vera (AHE). A cepa USBA-018 fermentou vários acucares, mas seu produto final primàrio foi L-ácido lático. Os fatores que influenciaram a produção de L-ácido lático foram o pH, adição de extrato de levedura (YE) e cloreto de manganès. Sob as condicoes de crescimento mais favoráveis para a produção de ácido lático, o rendimento (Yp/S) aumentou de 0,66 a 0,96 g/g com uma produtividade (Q) de 0,62 g.L4.h e um máximo de concentração de ácido lático de 178 mM em 26 horas de fermentação. Quando o AHE foi utilizado, se obteve 93,3 mM ou 0,175 g.L-1.h. Estes resultados mostram o potencial para transformação de acucares que a cepa USBA-018 oferece, entretanto estudos adicionais sáo necessários para descobrir se diferentes estrategias utilizando o AHE como fonte de carbonos podem produzir quantidades de ácido lático grandes o suficiente para permitir que AHE se converta em um substrato alternativo de baixo custo.
Palabras clave: Aloe vera, ácido lático, Thermoanaerobacter sp. USBA-018, fermentação termofilica.
Introduction
Current trends toward environmental sustainability and the use of renewable resources such as biomass fermentation processes have led to growing interest in the biological production of organic acids including lactic acid. Demand for lactic acid is high in the food, pharmaceutical, textile, and chemical feedstock industries where it is used for production of lactate esters, propylene glycol, 2,3-pentanedione, and propanoic acid. It is used in the cosmetic industry as a humectant in the preparation of creams and soaps (Wasewar, 2005, Wee et al, 2006, Lee et al, 2011, Ma et al., 2014), and consumption of lactic acid has increased considerably because of its use as a basic input in the production of polylactates and polylactic acid (PLA) for the manufacture of biodegradable plastics (Gao et al., 2011, Xu & Xu, 2014). Estimated global demand for lactic acid is 200,000 to 350,000 metric tons per year with annual growth rates between 12% and 15% (Corma et a/., 2007, Wee & Ryu, 2009, Silveira et al, 2012). According to Abdel-Rahman et al. (2013) it may reach 370,000 metric tons by 2017.
Biological production of lactic acid has significant advantages over chemical production of this acid. Advantages include the high specificity of the product (production of optically pure isomeric form), the capacity to transform raw materials such as whey, molasses, sugar cane bagasse, and starch which are rich in fermentable sugars, reduction of the use of substrates derived from petrochemicals, and low energy consumption (Qin et al., 2009, Tongpim et al., 2013). Nevertheless, commercial use of this raw material would require that it have low costs, achieve high production rates, be consistently available, produce few byproducts, and ferment easily with little pre-treatment (Wee et al., 2006). Ease of fermentation and the need to add complex nitrogen sources to the fermentation medium (Akerberg & Zacchi, 2000, Romani et al., 2008) may be limitations on biological production. Nitrogen sources are required for the growth of organisms producing lactic acid. These factors can also increase the costs of biotechnological production, so the choice of raw materials is of great importance for any fermentation process (Eiteman & Ramalingam, 2015).
The residue left over after Aloe vera leaves have been processed constitute an unexplored fermentable raw material that could potentially be used as a raw material that can be processed into lactic acid. Currently, demand for A. vera derivatives is high in Colombia where they are used as inputs in cosmetics, medicines and over the counter processed foods. Since domestically produced A. vera does not currently cover demand, land devoted to this crop is likely to increase, and along with it the amount of the by-products of A. vera processing available for other lactic acid production is also likely to grow (Gomez, 2013).
By-products from the processing of A. vera leaves are suitable for fermentation because they are high in fermentable sugars such as mannose and glucose which facilitates pre-treatment through hydrolysis. These by-products also contain traces of other sugars such as arabinose, galactose and xylose (Moghaddasi & Kumar, 2011). Importantly, they are also low in lignin and hemicellulose (Reynolds, 2004) whose fermentation may be a limiting step. Another feature that makes them potentially useful is that they contain sufficient amounts of nitrogen compounds to meet the metabolic needs of lactic acid producing organisms.
Traditionally, biological production of lactic acid has used mesophilic lactic acid bacteria (LAB) or fungi. The bacteria are mainly from the genera Bacillus, Lactobacillus, Lactococcus, Streptococcus, Enterococcus and Leuconostoc, and the fungi are from the genus Rhizopus (Jin et al., 2003, Maas et al., 2006). Optimum fermentation temperatures have been between 30 and 42°C for industrial applications (Qin et al., 2009). The use of LAB to produce lactic acid has several limitations. They produce either L-lactic acid through the L-lactate dehydrogenase pathway (L-LDH), or D-lactic acid through the D-lactate dehydrogenase (D-LDH) pathway. They require nutrition that is rich in amino acids, peptides, nucleotides and vitamins and, because their biosynthetic pathways are limited, they have high risks of infection by bacteriophages which produce cell lysis (Abdel-Rahman et al., 2013). In addition, they have limited ability to degrade complex carbohydrates such as starches since they have higher preferences for simple monosaccharide and disaccharide sugars. Within the field of metabolic engineering several alternatives have been proposed to address these limitations. They include improvement of optical purity via deletion of the D- or L-LDH genes and development of bacterial strains that can produce lactic acid in chemically defined media (de Vos & Hugenholtz, 2004).
The significant advantages that the use of thermophilic organism fermentation has over the use of mesophilic fermentation by LAB include reduced risk of contamination during the process, elimination of the need for sterile conditions for fermentation, and decreased viscosity of the substrate to be fermented (Payot et al., 1999, Qin et al., 2009). In addition, thermophilic organisms frequently degrade polymeric substrates such as starch, transforming them into fermentable sugars that can then be fermented again into metabolites of industrial interest (Carlier et al., 2006). Also, thermophilic temperatures may be compatible with the use of hydrolytic enzymes that function at the high temperatures required in certain processes for saccharification of lignocellulosic substrates. For all of these reasons, the search for thermophilic species capable of transforming sugars into organic acids or alcohols with industrial applications has intensified in recent years.
This study evaluates the capacity of Thermoanaerobacter sp. USBA-018 to produce lactic acid from simple and complex carbohydrates. Initially, factors favoring the synthesis of this acid in a chemically defined medium using glucose as the carbon source were determined. Based on those results, the production of lactic acid by this bacterium using a substrate of the by-products left over after the processing of Aloe vera leaves was evaluated. This evaluation demonstrates the potential of this organism for biological production of lactic acid.
Materials and methods
Microbial growth
Strain USBA-018 (CMPUJ U018) was cultured in Hungate tubes with 5 ml of a chemically defined basal salts medium (BM) that was supplemented with 20 mM D-glucose, under an atmosphere of N2:CO2 (80:20), and incubated at 65°C. The basal salts medium (g.l-1) contained 1.0 g of INM; 0.3 g of K2HPO4; 0.3 g of KH2PO4; 10.0 g of NaCl; 0.1 g of CaCl2H2O; 0.1 g of KCl; 0.5 g of cysteine-HCl; 1.0 g of yeast extract (YE); 1.0 ml of Balch micronutrients (Balch et al., 1979); and 1.0 ml of resazurin (Sigma) 0.1% (w/v). The pH of the BM was adjusted to 7.0 ± 0.1 using 0.1N NaOH. Prior to use, each Hungate tube was injected with 0.05 ml of 2% (w/v) Na2S'9H2C and 0.05 ml of 10% (w/v) NaHCO3.
The purity of the cultures used in the experiments was verified by confirming the presence of a single morphology through microscopic observation (Eclipse 50i; Nikon) of the growth of the microorganism in BM supplemented with 2 g.l-1 YE and 20 mM D-glucose. The range of pH and the temperature in which the strain USBA-018 grew, were evaluated in BM supplemented with 2 g.l-1 YE and 20 mM D-glucose. pH was evaluated between 3.2 and 8.8 using anaerobic stock solutions of NaHCO3 (10% w/v) and Na2CC3 (10% w/v). The range of temperatures in which this strain was evaluated was from 50 to 75°C.
Evaluation of carbon sources and final metabolites from fermentation
The following substrates were evaluated at concentrations of 20 mM: succinic acid, glucose, cellobiose, trehalose, mannose, sucrose, raffinose, galactose, glycerol, maltose, xylose, lactose, fructose and mannitol. Degradation of complex substrates was also evaluated. They included starch, pectin, and xylan which were evaluated at 1% (w/v) of the final concentration. In all evaluations, the BM was supplemented with 1g.l-1 YE and strain USBA-018 was cultivated twice in succession under the experimental conditions described above. Each time, three Hungate tubes were used for batch fermentation without pH control and without agitation.
Evaluation of the effects of the conditions of the culture on the formation of lactic acid from glucose in BM
Various culture conditions that could affect the formation of lactic acid in the BM described above were evaluated. In every evaluation, strain USBA-018 was cultured twice in succession under the same experimental conditions. For each culture, three Hungate tubes were used for batch fermentation without pH control and without agitation. Hungate tubes containing BM and glucose were injected with 10% (v/v) of bacterial seed culture in the exponential growth phase as determined by optical density (OD) (ABS580nm: 0.4 - 0.5 equivalent to 820 - 1025 ug dry biomass.ml-1) measured with a Hach DR 5000 spectrophotometer with incubation at 65 °C.
Using glucose as the carbon source, the following culture conditions were evaluated: (1) Variations of the initial pH of the culture; (2) Variations of initial glucose concentrations. This was done to determine the maximum concentration tolerated by the strain USBA-018 and the concentration that resulted in the production of the highest lactic acid concentration; (3) Different concentrations of organic nitrogen sources (yeast extract, peptone and trypticase); (4) An inorganic nitrogen source (ammonium chloride) with added vitamins, and (5) Addition of manganese chloride.
Effects of additions of vitamins, yeast extract and ammonium chloride on the formation of lactic acid
The effects of the addition of a mixture of five vitamins, yeast extract and ammonium chloride were evaluated in a 23 factorial design (three factors at two levels). The vitamins used were Vitamin B1 (thiamine), Vitamin B2 (riboflavin), Vitamin B5 (pantothenic acid), Vitamin B6 (pyridoxine) and Vitamin H (biotin). Vitamins were added to the BM from sterile filtered solutions that had been prepared under anaerobic conditions.
Effects of variations of pH on fermentation and the effects of adding manganese chloride on the formation of lactic acid
Several series of controlled pH batch fermentations were done. Fermentations were done in Hungate tubes using BM with glucose (40 mM) and the three organic nitrogen sources previously evaluated at concentrations of 1.0 g.l-1. The aim was to identify the effect of pH control on the formation of lactic acid. On the basis of these results, the addition of 0.1 g.l-1 of manganese chloride using only 1.0 g.l-1 of YE as the organic nitrogen source was subsequently evaluated. Control of pH was achieved by adding 0.1 ml of NaHCO3 (10% w/v) every 12 hours to maintain the pH of fermentation between 5.5 and 7.0.
Effect of manganese chloride concentration on the formation of lactic acid
Based on these results, the formation of lactic acid in BM with different concentrations of manganese chloride (0.05, 0.1, 0.15, 0.2, 0.25 g.l-1) was evaluated.
Batch fermentations were done with pH control in Hungate tubes with glucose (20 mM) as the carbon source and yeast extract (1 g.l-1) as the nitrogen source.
Controls
Abiotic and biotic controls were used. The abiotic controls consisted of preparing Hungate tubes under the evaluated conditions without inoculation, and biotic controls consisted of preparing Hungate tubes under the evaluated conditions and inoculating them but without adding a carbon source.
Controlled pH batch fermentation with bioreactor agitation
Subsequently, batch fermentations were performed in a 3 L heating mantle bioreactor (New Brunswick BioFlo®/CelliGen® 115) and an effective working volume (EWV) of 1 L. Anaerobic conditions were maintained and fermentation occurred in an atmosphere of N2/CC2. The manufacturer's recommendations were followed, and resazurin was used as an indicator of redox potential. The pH was automatically adjusted to 7.0 ± 0.2 with a 5N solution of NaOH. The fermenter was inoculated with 10% of bacterial inoculum in exponential phase of growth under the same experimental conditions described above. Agitation (250 rpm) and pH (7.0) were controlled automatically by the computer. Samples (~5mL) of metabolites produced at different fermentation times produced were taken for analysis.
Effects of variations in the ratio between the carbon source and the organic nitrogen source on lactic acid production in batch fermentations
Different ratios between the amount of glucose, the carbon source, and the amount of yeast extract, the organic nitrogen source (CS:NS), were tested in duplicate batch fermentations in the bioreactor. The BM used had initial concentrations of 100 mM glucose. YE was added in differing amounts depending on the ratio to be evaluated (18:1, 9:1, 6:1, 3:1). Samples were taken at various fermentation times.
Based on the results obtained, three CS:NS ratios (3:1, 1:1 and 1:2) were selected for evaluation of lactic acid production when 0.1 g.l-1 of manganese chloride was added to duplicate batch fermentations in a bioreactor.
Evaluation of hydrolyzed Aloe vera peel extracts (AHE) as a culture medium for producing lactic acid and evaluation of the addition of manganese chloride
Once the factors influencing the formation of lactic acid in the BM were identified, pressed and hydrolyzed A. vera peel extract (AHE) was evaluated as a culture medium for batch fermentations in a bioreactor with an EWV of 1 L without any type of supplementation. Growth of Thermoanaerobacter sp. USBA-018 was observed and the end products of fermentation of sugars from the hydrolyzate were identified.
The AHE was produced from A. vera peel by-products obtained from Agro Bamboo de Colombia, which uses A. vera for the production of cosmetics. The by-products were subjected to enzymatic hydrolysis and then dried, ground and sieved according to the protocols described by Giraldo-Estrada & Ramirez (2010). The resulting solid was hydrolyzed with an enzyme extract produced from semi-solid state fermentation of Aspergillus niger in a culture medium with A. vera peels as a substrate. This was run for 72 hours at 30°C with constant agitation at 150 rpm (Giraldo-Estrada & Ramirez, 2010).
The enzymatic hydrolysis reaction was run at 50 °C ± 2 °C for 8 hours. The concentration of sugars present in the AHE was standardized at 18 g.l-1 of reducing sugar equivalents of glucose using the 3,5-dinitrosalicylic (DNS) acid colorimetric technique (Miller, 1959). The AHE's composition, which had been analyzed previously (Giraldo-Estrada & Ramirez, 2010), had a total carbohydrate content of 22 g.l-1 as quantified by the method of Dubois (DuBois et al., 1956), 0.2% protein content as quantified by the Kjeldahl method (NTC4657, 1999) and a mineral content of 20 ppm of Mn, 829 ppm of Ca, 47 ppm of P, 203 ppm of Mg and 72 ppm of Na as determined by atomic absorption spectrophotometry (NTC5151, 2003).
On the basis of these results, the AHE was supplemented with 6 g.l-1 of YE to maintain a CS:NS ratio of 3:1. Afterwards, 0.1 g.l-1 of manganese chloride was added to reevaluate the formation of lactic acid. Duplicate batch fermentation using 1 L EWV with pH controlled at 7.1 and with constant agitation at 250 rpm was then conducted. Samples of approximately 5 ml each were taken at various times during the process.
Analytical methods
Growth of Thermoanaerobacter sp. USBA-018 was monitored under the various culture conditions by optical density (OD) at 580 nm, and growth was verified by phase contrast microscopy. Measurements of absorbance obtained were converted into concentrations of dry biomass.ml1 (μg.ml-1) by means of a biomass dry weight standard curve (data not shown). Samples were collected at 0, 24 and 48 hours after the start of fermentation in the bioreactor. The end products of fermentation and glucose consumption were quantified by high performance liquid chromatography (HPLC) in reverse phase in a Shimadzu Prominence chromatograph LC20AT (Japan) using a 300 x 8 mm Shodex SH1011 column (Japan). The mobile phase was 0.01 N H2SO4 at a constant flow of 1.0 ml.min-1 and a column temperature of 50°C. A Shimadzu SPDM20Aa 210 nm diode array detector was used for organic acids, and a Shimadzu RID10A refractive index detector was used for sugars and alcohols. The optical isomer produced by Thermoanaerobacter sp. USBA-018 was analyzed by the UV EnzytecTM colorimetric method for determination of D/L of lactic acid (RBiopharm AG, Germany) according to the manufacturer's protocol.
Statistical analysis
Statistix 9.0 for Microsoft Windows was used to Statistix 9.0 for Microsoft Windows analyze the lactic acid levels produced in the various fermentations. The Shapiro Wilk test was used to test normality, and the homogeneity of variances was tested with Levene's Test. ANOVA (Analysis of Variance) and LSD (Least Significance Differences) were used to analyze normally distributed data. For non-normally distributed data, statistic Kruskal Wallis was applied. In all cases the confidence level was 95% and p = 0.05.
Results
In addition to fermenting glucose, Thermoanaerobacter sp. USBA-018 catabolizes most of the carbon sources tested including monosaccharides, disaccharides, pentoses and hexoses (Table 1). This bacterium primarily exhibited fermentative metabolisms which produced L-lactic acid from glucose, mannose, sucrose and xylose, and lesser amounts from cellobiose, trehalose, galactose and fructose. Lactic acid production was measured by the UV method of EnzytecTM. The D-lactic acid isomer was not observed, which is an indication of the optical purity of metabolite produced. This is an advantage over other types of fermentation with LAB and over chemical synthesis of lactic acid. Fermentation from maltose produced mainly ethanol (10.1 mM) and only traces of lactic acid (~1.5 mM) while fermentation from mannitol produced equimolar amounts of ethanol and lactic acid (11.7 mM and 10.5 mM respectively). Unlike the lactic acid bacteria of the genus Lactobacillus which are widely used in the production of lactic acid, Thermoanaerobacter sp. USBA-018 strain degraded complex substrates, such as starch and xylan, primarily into lactic acid. Fermentation from pectin produced only traces of galacturonic acid, formic acid, lactic acid, acetic acid and ethanol. Strain USBA-018 did not grow on arabinose, glycerol, lactose, succinic acid or ethanol when any of them were added to the culture medium as the sole carbon source. Only low levels of fermentation occurred when raffinose was added. It produced traces of lactic and acetic acids.
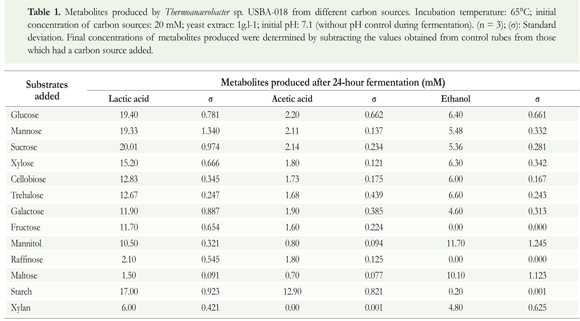
Evaluation of lactic acid formation in the chemically defined culture medium
Effect of initial pH of the culture on the production of lactic acid
Thermoanaerobacter sp. USBA-018 grew in a pH range between 6.0 and 8.0. Beyond this range, observation under a microscope showed changes in cell morphology indicating cellular stress. Optimal pH for growth was 7.1 -7.2.
Varying the initial pH of the culture during glucose fermentation, produced results in which the largest proportion of synthesized metabolites continued to be lactic acid with acetic acid, succinic acid and ethanol in smaller proportions. (Figure 1a). In other words, the profile of the synthesized metabolites was similar in all cases despite variations in initial pH. Nevertheless, the initial pH of the culture had a significant effect on the production of lactic acid (p = 0.0188), on the productivity of the process (Qp) (p = 0.0203) and on the yield Yp/s (p = 0.0377). The concentration of lactic acid decreased during fermentations performed at pH values outside the optimal levels for growth of the bacterium (7.1 - 7.2).
Effect of initial glucose concentration on lactic acid production
Thermoanaerobacter sp. USBA-018 grew in all of the glucose concentrations tested (10 mM - 200 mM). The largest portion of the metabolites synthesized after 48 hours of culturing was again lactic acid, followed by acetic acid. Only trace amounts of succinic acid and ethanol occurred.
The results obtained in the tests showed no statistically significant differences (p = 0.0656) among the final concentrations of lactic acid. Lactic acid values ranged from a low of 13.9 mM (from 200 mM glucose) to a high of 18.2 (20 mM glucose). Similarly, productivity among the treatments showed no statistically significant differences (p = 0.073), but the yield was highest when the glucose concentration was 10 mM (0.92 g/g) as shown in Figure 1b.
Effects of different organic nitrogen sources and concentrations on the formation of lactic acid
Three sources of organic nitrogen, yeast extract, peptone (PEP) and trypticase, were all evaluated with 40 mM glucose at pH of 7.1 -7.2. Thermoanaerobacter sp. USBA-018 grew with its characteristic morphology and produced lactic acid as the primary metabolite with lesser amounts of acetic acid, succinic acid and ethanol. Evaluations of different concentrations of YE (Figure 1c) showed that, as the concentration of YE increased, the consumption of glucose increased, and along with it, the production of lactic acid (p = 0.012). It reached a maximum value of 16.18 mM using 5 g.l-1 yeast extract. Measures of yield (Yp/s) and productivity (Q) had the same behavior with maximum values obtained at a concentration of YE of 5 g.l-1 (0.64 g/g and 0.06 g.l-1.h). Nevertheless, at YE concentrations over 5 g.l-1, lactic acid production decreased from 14.94 mM at 10 g.l-1 to 14.26 mM at 20 g.l-1 (Figure 1c).
The behavior of PEP was similar to that observed with YE. There was a significant direct relationship (p = 0.0125) between the concentration of PEP and lactic acid production. Increases in PEP concentration up to a maximum point resulted in increased lactic acid production, but after the maximum had been reached, additional increases in PEP concentrations resulted in falling lactic acid production. The maximum amount of lactic acid was 17.02 mM which was produced when the BM was supplemented with 10 g.l-1 of peptone. Yields remained constant between 0.22 and 0.36 g/g (lower amounts than those observed with YE). Maximum productivity was 0.064 g.l-1.h with PEP at 10 g.l-1 (Figure 1c).
Different concentrations of trypticase mixed with the BM behaved similarly. A maximum concentration of 30.6 mM lactic acid with productivity of 0.115 g.l-1.h and yield of 0.62 g/g was obtained when the BM was supplemented with 30 g.l-1 of trypticase (Figure 1c).
In contrast to the other nitrogen sources tested, the lowest concentration of trypticase tested (0.1 g.l-1) did not produce lactic acid. Furthermore, at concentrations of 1 g.l-1 and 5 g.l-1 of trypticase lactic acid production was lower than at the same concentrations of YE and PEP. This indicates that higher concentrations of trypticase are required to obtain the same effect achieved with lower concentrations of YE and PEP.
Effect of adding vitamins and an inorganic nitrogen source on production of lactic acid
Vitamins were also evaluated as additives for increasing production of lactic acid because of their roles as essential micronutrients for metabolism. Vitamins act as precursors of enzyme cofactors according to various authors (LeBlanc et al., 2011). Figure 2a shows the lactic acid produced in the eight vitamin treatments tested. The maximum amount of lactic acid produced was 19.53 mM which was achieved in culture control experiment 1 with 1 g.l-1 of YE and 1 g.l-1 of NH4CI. Then followed culture control experiment 2, which added a mixture of vitamins, with a production of 19.28 mM of lactic acid.
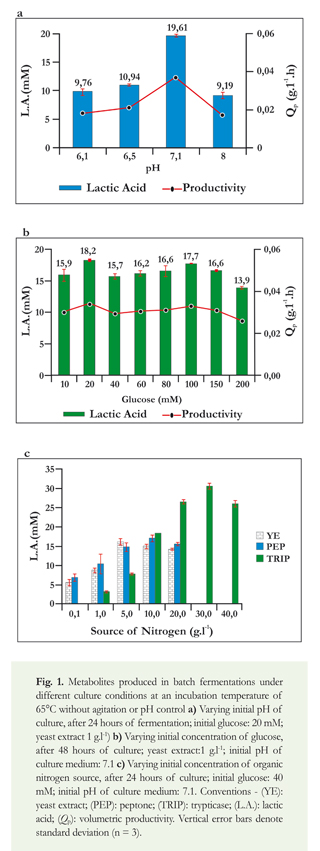
To determine if the addition of ammonium chloride could supply the nitrogen needed in addition to that supplied by the YE, a higher concentration (10 g.l-1) of ammonium chloride was compared to two concentrations of YE (0.05 g.l-1 and 1.0 g.l-1). Tests were run with and without vitamins (Treatments 3 to 8 in Figure 2a). It was observed that lactic acid production was significantly lower with YE (1 g.l-1) and NH4Q (10 g.l-1) with and without vitamins than it was under control conditions (8.56 mM and 4.88 mM, respectively). Furthermore, when the concentration of YE in the BM was reduced to its minimum value of 0.05 g.l-1 and concentration of the inorganic source was increased with and without vitamins, lactate production was minimal (between 1.76 and 3.2 mM). These results show that the addition of vitamins to the strain USBA-018 had no statistically significant effect (p = 0.564) in the presence of ammonium chloride or YE.
Effect of pH control and addition of manganese chloride on lactic acid production
Considering the results of the effect of acidic pH and possible inhibition of growth of this strain, batch fermentations in Hungate tubes were done using pH controls. BM supplemented with glucose was used as the carbon source, and the same three organic nitrogen sources previously evaluated were each tested at concentrations of 1 g.l-1. Subsequently, the addition of 0.1 g.l-1 manganese chloride and 1 g.l-1 YE to the BM was evaluated.
Previously, it had been observed that, when 1 g.l-1 of YE without pH control and 40 mM of glucose were tested, lactic acid production after 24 hours of fermentation reached 8.73 mM, yield (Yp/s) was 0.49 g/g, and productivity (Qp) was 0.033 g.l-1.h (Figure 1c). Control of pH had a positive effect on lactic acid production and on kinetic parameters compared to fermentation without pH control. The test with controlled pH during fermentation produced 6.5 times more lactic acid (57.44 mM) after 48 hours than did the test under the same conditions of fermentation without pH control. Yield was 0.86 g/g (~1.7 times greater), and productivity was 0.108 g.l-1.h (~3.3 times greater). Consumption of the substrate was also greater (Figure 2b).
BM supplementation with manganese chloride and YE (1 g.l-1) also significantly increased synthesis of lactic acid. These results were superior to those obtained in fermentation without addition of manganese (Figure 2b), and productivity and the amount of substrate consumed also increased.
In the experiment with the addition of 0.1 g.l-1 of manganese chloride, lactic acid production and productivity were statistically different from those in the tests without manganese. Tests with YE, with and without Mn+2, resulted in values close to, but statistically different than, those reported in the experiments with peptone and trypticase (Figure 2b). The conclusion drawn was that control of pH during fermentation and adding Mn+2 are key factors in the process of lactic acid production. Since the addition of Mn+2 ions has a positive effect on the formation of lactic acid by Thermoanaerobacter sp. USBA-018, additions of different concentrations of manganese chloride to the BM with glucose and yeast extract were evaluated through batch fermentations with pH control to identify optimal concentrations of manganese for the synthesis of lactic acid. Manganese is an essential growth factor for lactic acid producing microorganisms such as L. casei since it is a constituent of lactate dehydrogenase (de Angelis & Gobbetti, 1999) and many other enzymes.
The results show that there was no statistically significant effect (p = 0.078) on any kinetic parameter of fermentation (Figure 2c) in the range of 0.05 g.l-1 to 0.25 g.l-1. Nevertheless, the highest lactic acid concentration of 34.61 mM was obtained at a concentration of 0.1 g.l-1 of chloride manganese. The yield and productivity remained constant with respect to the concentration of MnCl2.
The results indicate that the factors that influence the production of lactic acid were the following: (1) initial culture pH (7.1-7.2) and pH control during fermentation, (2) addition of organic nitrogen (yeast extract) and (3) addition of manganese chloride (0.1 g.l-1). Based on this, the experiments explained below evaluated these factors in a bioreactor with an effective working volume (EWV) of 1 L and controlled fermentation conditions including temperature, agitation, and automatic pH control.
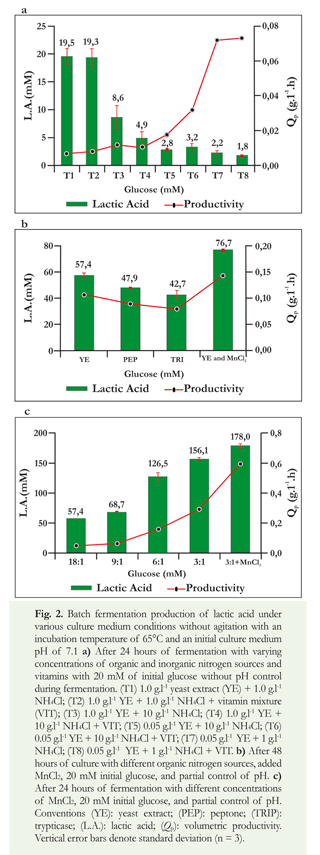
Effect of carbon source: organic nitrogen source ratio on lactic acid production in batch fermentations
Different formulations of BM were used to obtain varying ratios of glucose, the carbon source (CS) and YE, the organic nitrogen source (NS). The glucose concentration was kept constant at 100 mM, and CS:NS was varied by changing the concentration of YE. Duplicate batch fermentations in the bioreactor under the conditions previously described were evaluated. The results were compared with those obtained in a control fermentation which had a CS:NS ratio of 3:1 and which had 0.1 g.l-1 of manganese chloride added.
The results showed that the largest proportion of the metabolites produced in all fermentations performed was lactic acid, that smaller amounts of ethanol and acetic acid were produced, and only traces of succinic acid were produced (data not shown). Results also show that the CS:NS proportion has a significant effect on lactic acid production in the bioreactor and that the amounts of lactic acid produced in each of the experiments were statistically different. Larger CS:NS ratios resulted in larger final concentrations of lactic acid. At a CS:NS of 3:1 a final output of 156 mM with a yield (Yp/s) of 0.84 g/g and productivity of 0.293 g.l-1.h was produced (Figure 3a). CS:NS also had a significant effect on productivity: the highest productivity of 0.294 g.l-1.h was obtained at a ratio of 3:1 (Figure 3a). At different CS:NS ratios similar product yields were obtained in the substrate: 0.79 g/g at a ratio of 6:1 and 0.84 g/g at a ratio of 3:1 (Figure 3a).
The effect of the addition of manganese was checked again to compare the various different experiments with manganese supplements that had resulted in increases in the kinetic parameters (Figure 3a). It was observed that addition of manganese to fermentation resulted in reductions of the time required for consumption of the added glucose (100 mM). At a CS:NS ratio of 3:1, twenty-six hours were required for consumption of the glucose when manganese was added, but 48 hours were required when it was absent. At a CS:NS of 6:1, 72 hours were required, and at CS:NS of 9:1 and CS:NS of 18:1, 96 hours were required. Under those conditions, the percentages of glucose consumed were 56% and 62% respectively. This indicates that, despite control of pH during fermentation, the organism was not able to transform all of the glucose added to the medium. Possibly, this was due to the fact that the proportion of YE was low compared to the proportion of glucose which is a condition that prevents or limits the growth of these organisms.
When CS:NS ratios of 1:1, 1:2 and 1:3 were evaluated with the addition of 0.1 g.l-1, the lactic acid production decreased significantly (p = 0.015) at all three ratios tested. At CS:NS ratios of 1:1 and 1:2, although process productivity increased (Figure 3b). Under these conditions with the addition of Mn+2, the fermentation time of glucose decreased from 26 hours at a CS:NS ratio of 3:1 to 20 hours with a CS:NS ratio of 1:3, to 14 hours with a CS:NS ratio of 1:2, and to 12 hours with a CS:NS ratio of 1:1. However, these changes resulted in the formation of more ethanol and less lactic acid, as shown in Figure 3b. Yields of lactic acid in glucose were significantly lower than those reported in a CS:NS of 3:1 with 0.1 g.l-1 of manganese chloride (Figure 3b).
Because increases in lactic acid formation were not observed at CS:NS of 1:1, 1:2 and 1:3, fermentation in the bioreactor with an BM with glucose as the carbon source and yeast extract as the nitrogen source at CS:NS of 3:1, plus the addition of 0.1 gl-1 of manganese chloride and pH control, was evaluated. All of these had been previously identified as favorable conditions for lactic acid formation. Under these culture conditions, the yield (Yp/s) was 0.96 g/g with a productivity (Qp) of 0.62 g.l-1.h and a maximum concentration of 178 mM of lactic acid at 26 hours of fermentation. This was a significant increase in the process productivity, yields and production of lactic acid compared to the final results of the experiments performed earlier in this study.
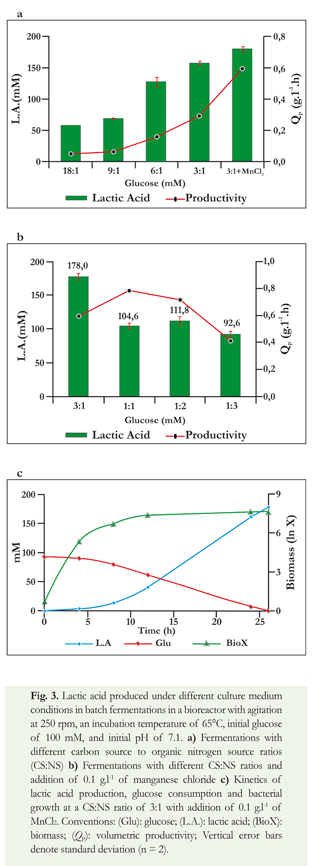
Evaluation of hydrolyzed Aloe vera extract as a culture medium for lactic acid production
Once favorable conditions for producing lactic acid through growing Thermoanaerobacter sp. strain USBA-018 had been identified using a chemically defined medium, AHE was evaluated as alternative substrate. First, the growth of the bacterial strain was evaluated on this substrate. Thermoanaerobacter sp. USBA-018 was cultured with AHE as its sole source of nutrients resulting primarily in production of lactic acid with lesser amounts of ethanol and acetic acid (Figure 4). During fermentation of AHE, glucose was so rapidly consumed that after 24 hours no residual glucose was found. This is in contrast to what happened to the cellobiose and mannose which had been identified in AHE (data not shown). Mannose was consumed more slowly, with residual concentrations after 48 hours of the process. The cellobiose was not consumed at all by the microorganism, and a constant concentration was maintained throughout the process. Aside from glucose, mannose, and cellobiose, the sugars identified in AHE, other sugars such as xylose, galactose and mannan are likely to be present in leaves of Aloe, but strain USBA-018 is capable of fermenting all of them to lactic acid.
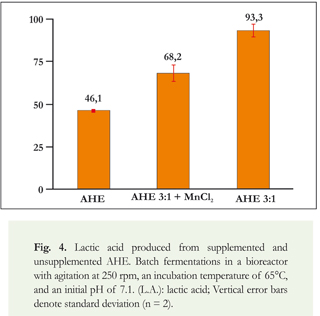
When AHE was supplemented with YE, a final lactic acid concentration of 93 mM was observed (Figure 4). This concentration was higher than the concentration of 46 mM found when AHE was used alone. However, the addition of 0.1 g.l-1 of MnCl2 to the AHE together with the addition of YE did not increase the production of lactic acid. It resulted in a final concentration of 68 mM. Bromatological analyses of AHE (Giraldo-Estrada & Ramirez, 2010) have shown ~20 ppm of Mn+2 which is equivalent to half of the moles of Mn+2 that had been added to the BM (0.1 g.l-1 of MnCl2). This could indicate that adding manganese chloride results in exceeding the required concentration of Mn+2 which could in turn result in inhibition of the organism. Furthermore, it is possible that a crossover effect may be generated between this element and other components of AHE.
Finally, the results obtained show that the production of lactic acid with AHE, with and without the addition of YE, and with and without the addition of manganese (Figure 4), are statistically different (p = 0.001). The use of AHE supplemented with YE at a CS:NS of 3:1 without the addition of manganese resulted in a greater production of lactic acid (93.3 mM) and a productivity of 0.175 g.l-1.h.
Discussion
To date, there have been very few studies of thermophilic anaerobic bacteria that produce lactic acid. Those studies that have been done focus primarily on thermophilic organisms of the Bacillus genus (Meng etaL, 2012, Tongpim etaL, 2013, Ma et al., 2014, Xu & Xu, 2014) which have high efficiencies but depend on the oxygen supply during fermentation. The strain USBA-018 grew at an optimum temperature of 65°C which considerably limits the risk of contamination during fermentation of sugars.
Among the most important factors that influence production of acid is pH which plays an important role because of the catalytic activity of enzymes and because the metabolic activity of these microorganisms depends on extracellular pH (Mussatto et al., 2008). The pH of fermentation can be set at the start, but will diminish over time because of the production of lactic acid. In the specific experiments conducted, the fermentation of glucose to lactic acid led to decreases of the pH of the culture medium after 24 hours of fermentation that ranged between 3.9 and 5.1 (data not shown). These pH values outside the range of growth probably inhibited the growth of the microorganism by limiting consumption of available glucose. Furthermore, the productivity, yield and final concentration of lactic acid fermentations were observed to be lower in fermentations with initial pH values of 6.1 and 6.5. The initial pH of the culture medium can modify the profile of metabolites produced from the carbon source, as shown by Combet-Blanc et al. (1995) who evaluated the effect of pH on glucose fermentation using Bacillus thermoamylovorans. This thermophilic organism consumes large quantities of glucose at neutral pH and primarily produces acetate, ethanol and formate. However, when the pH is either acidic or alkaline, the rate of glucose consumption decreases, and the organism's primary product is lactate. This phenomenon did not occur with the Thermoanaerobacter sp. strain USBA-018 because, regardless of the pH at the start of glucose fermentation, the major metabolite produced was always lactic acid.
Possibly, lactic acid produced by fermentation decreased the pH to a level that inhibited the growth of Thermoanaerobacter sp. USBA-018 revealing low strain tolerance to acidic environmental conditions. This has been observed in LAB in which the more the pH of the medium decreases, the more the undissociated lactic acid proves toxic to the organisms (Hofvendahl et. al., 1999). Among the alternatives developed to control this phenomenon is the addition of neutralizing agents such as sodium, potassium, calcium, calcium carbonate and ammonia solution that partially control this inhibition and improve the efficiency of fermentation.
The nitrogen source is another factor that has a great influence on the production of lactic acid. Although yeast extract is the primary nitrogen source used for the production of this acid, its high cost is disadvantageous for an industrial process. Nitrogen-rich substances such as peptone, trypticase, ammonium sulfate, casein hydrolyzate, urea and substrates from industrial waste products have been studied for possible cost reductions in the production process (Cook et al., 1996, Nancib et al., 2001, Nancib et al., 2005, Altaf et al., 2006, Altaf et al., 2007, Yu et al., 2008, Liu et al., 2010). The experiments conducted in this study show that the three organic nitrogen sources evaluated were assimilated by the microorganism, and that, at higher concentrations, they increased substrate consumption and production of lactic acid production. Nevertheless, in all the experiments conducted, some glucose remained at the end of the process. Possibly, this is related to cell inhibition by culture medium acidification. A comparison of the three sources of organic nitrogen shows that 30 g.l-1 of trypticase results in the highest concentration of lactic acid and in greater productivity. However, the trypticase concentration required was very high and would not be economically feasible. YE was used as organic nitrogen source for the production of lactic acid in subsequent experiments because the concentration of YE needed to produce similar concentrations of lactic acid (5 g.l-1) is less than the concentration of PEP that is needed to achieve the same results. In addition, YE's productivity is similar, but its yield is higher.
On the other hand, the factorial design for evaluation of the influence of vitamins (thiamine, riboflavin, pantothenic acid, pyridoxine and biotin), yeast extract (the organic nitrogen source) and ammonium chloride (the inorganic nitrogen source) on the production of lactic acid showed that the organic nitrogen source (YE) could not be replaced with NHtCl. Although in some cases the addition of vitamins to the medium has been observed to replace yeast extract and improve the production process (Yoo et al., 1997), this was not observed in this study. As some authors have suggested, the YE added to the culture medium may provide important compounds such as purines, pyrimidines and B vitamins that contribute to the growth of the organism (Nancib et al., 2005, Altaf et al., 2007).
Control of pH and the addition of manganese chloride had increased production of lactic acid and kinetic parameters over that achieved through fermentation without pH control. These results are comparable to other studies (Mussatto et al., 2008, de Lima etal., 2010) which have found that pH control generates significant increases in lactic acid production, the consumption of the substrate and productivity. In addition, because of its role as a constituent of many enzymes, but especially lactate dehydrogenase, manganese is an essential growth factor for some lactic acid bacteria such as L. casei, (de Angelis & Gobbetti, 1999, FitzPatrick et al, 2010). Since previous studies had identified the effect of metal ions such as Mn+2 on the activity of various enzymes involved in degradation of sugars such as L-arabinose isomerase from T. mathranii and xylose isomerase from T. ethanolicus (Fan et al., 2011, Liang et al., 2012), the effect of this ion in the degradation of glucose was evaluated.
Our results show a dependence on the proportion of CS:NS for the production of lactic acid as a major metabolite. The carbon source should have a higher concentration than the organic nitrogen source. The results show that this may condition metabolic pathways used by the organisms for oxidation of the carbon source. Nevertheless, it is important to note that when there is a high concentration of YE with respect to that of glucose, the YE may also provide carbon to the organisms which could explain the production of ethanol instead of the production of lactic acid.
A comparison of our final result with the results of the previous experiments in this study showed a significant increase in process productivity, yields and production of lactic acid. These results were comparable to those obtained using Bacillus sp. strain WL-20 in fed-batch fermentation under alkaliphilic conditions although the lactic acid concentration was significantly higher (Meng et al., 2012).
This study determined that Thermoanaerobacter sp. strain USBA-018 produces lactic acid as its major metabolic degradation product from glucose. Its kinetic performance value of Yp/s was 0.96 g/g, and its productivity was 0.62 g.l-1.h. The values found in this study are comparable to those obtained using strains of Lactobacillus delbrueckii subsp. delbrueckii mutant strains ATCC 9649 and Lactobacillus delbrueckii DP3, which exhibited productivity values of 0.72 and 1.2 g.l-1.h respectively in Batch fermentations using glucose as the carbon source (Demirci & Pometto, 1992). An additional advantage of strain USBA-018 over lactic acid bacteria is its utilization of a wide variety of substrates including pentoses, hexoses and polysaccharides in thermophilic conditions for production of organic lactic acid. Nevertheless, microorganisms that have been genetically engineered for increased output that are used industrially for biological synthesis of lactic acid have higher productivity values using different substrates. Glucose is the carbon source that has been studied the most. A recent study by Subramanian et al. (2014) evaluated lactic acid production from the homofermentative strain of Enterococcus faecalis CBRD01. It obtained volumetric productivity of 3.10 g.l-1.h in fed-batch fermentations at mesophilic temperatures and under microaerophilic conditions using glucose as the carbon source. The results of our study leave open the possibility of further developments in optimizing the production of lactic acid with the heterolactic fermentation Thermoanaerobacter sp. strain USBA-018.
Evaluation of hydrolyzed Aloe vera peel extract as a culture medium for lactic acid production
Fresh A. vera leaves contain carbohydrates such as cellulose and hemicellulose in their cell walls while the pulp mostly contains water and abundant polysaccharides such as glucomannan, glucogalactomannan, arabinomannan and acetylated mannan (Reynolds, 2004; Moghaddasi & Kumar, 2011). Leaves produce exudates when the gel is removed. These exudates contain phenolic compounds of which most are chromone, anthraquinone or anthrone derivatives (Rodríguez et al., 2010). Various studies have reported that the most abundant sugars in Aloe leaves are mannose and glucose, but the proportion of each is species dependent with ratios varying from 6:1 to 10:1. Trace concentrations of arabinose, galactose and xylose are also found (Eshun & He, 2004, Reynolds, 2004 Nandal & Bhardwaj, 2012). Aloe vera has high contents of pectic acid (70% to 85%), and in less proportion galactan, glucomannan and arabinan. The pectic acid fraction contains mainly galacturonic acid and galactose as well as traces of glucose and arabinose (Reynolds, 2004, Hamman, 2008). Based on these characteristics of the plant, hydrolyzed A. vera leaf extract (AHE), an Aloe processing by-product of a Colombian cosmetics company, was selected as the substrate for the fermentation of sugars and production of lactic acid. Although AHE can be used as the sole energy source for synthesis of lactic acid with Thermoanaerobacter sp. USBA-018, using it together with yeast extract in a proportion of three parts AHE and one part YE produces twice the final concentration of lactic acid with better batch fermentation productivity after 48 hours of the fermentation process.
One advantage of thermophilic fermentation of sugars using Thermoanaerobacter sp. strain USBA-018 is the reduction of costs associated with cooling the culture medium after sterilization. The use of AHE directly after enzymatic hydrolysis at 50°C eliminates the need for sterile media. Another important advantage is that AHE is easy to obtain in Colombia since there are approximately 400 hectares of A. vera in the country, of which 100 are already mature. This area alone could produce 500 metric tons of leaves per month (6,000 metric tons of leaves per year) which could produce an average of 3,000 tons of fermentable products each year since the product is about 50% of weight of the whole leaves (Gomez, 2013). Additional studies focused on optimizing the fermentation of AHE with Thermoanaerobacter sp. USBA-018 are needed. It may also be a good idea to study other agricultural products which are rich in fermentable sugars.
Conclusions
This study evaluated the capacity of Thermoanaerobacter sp. strain USBA-018 to transform simple and complex sugars into L-lactic acid, a metabolite of industrial interest, under thermophilic anaerobic conditions. Determination of factors that influence the production of lactic acid, allowed for increases in the formation, yield (Yp/s) and productivity (Qp) of this acid. Our results open the door for further increases in values using various culturing strategies. The factors which influenced production of lactic acid in a chemically defined base medium were related to the initial pH of the culture medium and control of pH during fermentation. The addition of organic nitrogen and manganese chloride (0.1 g.l-1) also had positive influences on the production of lactic acid.
Acknowledgments
The authors extend their thanks and appreciation to Angela Cantillo Gonzalez, an industrial microbiologist, for her valuable support, and Ana Maria Gutierrez.
Financing
This research was conducted with financial support from Colciencias, Pontificia Universidad Javeriana, EAFIT and Agrobamboo de Colombia (Research project 120348925362; ID 3676), and had permit No. 33 2009 of the Ministry of Environment and Sustainable Development of Colombia to access genetic resources.
Conflicts of interest
The authors state that their sole interest in the results of this research is scientific.
References
Abdel-Rahman MA, Tashiro Y & Sonomoto K (2013) Recent advances in lactic acid production by microbial fermentation processes. Biotechnology Advances 31: 877902. doi:10.1016/j.biotechadv.2013.04.002. [ Links ]
Akerberg C & Zacchi G (2000) An economic evaluation of the fermentative production of lactic acid from wheat flour. Bioresource Technology 75: 119-126. doi:10.1016/ S0960-8524(00)00057-2. [ Links ]
Altaf M, Naveena BJ, Venkateshwar M, Kumar EV & Reddy G (2006) Single step fermentation of starch to lactic acid by Lactobacillus amylophilus GV6 in SSF using inexpensive nitrogen sources to replace peptone and yeast extract - Optimization by RSM. Process Biochemistry 41: 465-472. doi:10.1016/j. procbio.2005.07.011. [ Links ]
Altaf M, Naveena BJ & Reddy G (2007) Use of inexpensive nitrogen sources and starch for L(+) lactic acid production in anaerobic submerged fermentation. Bioresource Technology 98: 498-503. doi:10.1016/j. biortech.2006.02.013. [ Links ]
Balch WE, Fox GE, Magrum LJ, Woese CR & Wolfe RS (1979) Methanogens: reevaluation of a unique biological group. Microbiological Reviews 43:260-296. doi:0146-0749/79/02-0260/37$02.00/0. [ Links ]
Carlier JP, Bonne I & Bedora-Faure M (2006) Isolation from canned foods of a novel Thermoanaerobacter species phylogenetically related to Thermoanaerobacter mathranii (Larsen 1997): Emendation of the species description and proposal of Thermoanaerobacter mathranii subsp. Alimentarius subsp. Nov. Anaerobe 12: 153-159. doi:10.1016/j.anaerobe.2006.03.003. [ Links ]
Combet-Blanc Y, Kalamba KK & Kergoat PY (1995) Effect of pH on Bacillus thermoamylovorans Growth and Glucose Fermentation. Applied and Environmental Microbiology 61: 656-659. [ Links ]
Cook GM, Rainey FA, Patel BKC & Morgan HW (1996) Characterization of a New Obligately Anaerobic Thermophile, Thermoanaerobacter wiegelii sp. nov International Journal of Systematic Bacteriology 46: 123-127. [ Links ]
Corma A, Iborra S & Velty A (2007) Chemical Routes for the Transformation of Biomass into Chemicals. Chemical Reviews 107: 2411-2502. doi:10.1021/cr050989d. [ Links ]
de Angelis M & Gobbetti M (1999) Lactobacillus sanfranciscensis CB1: manganese, oxygen, superoxide dismutase and metabolism. Applied Microbiology and Biotechnology51: 358363. doi:10.1007/s002530051402. [ Links ]
de Lima CJB, Coelho L, F. , Gervasio PDS, Alvarez Georgina LM & Contiero J (2010) L(+) Lactic Acid Production by New Lactobacillus Rhamnosus B 103. Journal of Microbial Biochemical Technology 2:064-069 doi:10.4172/1948-5948.1000025. [ Links ]
de Vos WM & Hugenholtz J (2004) Engineering metabolic highways in Lactococci and other lactic acid bacteria. Trends in Biotechnology 22: 72-79. doi: 10.1016/j.tibtech.2003.11.011. [ Links ]
Demirci A & Pometto A, III (1992) Enhanced production of D (-) lactic acid by mutants of Lactobacillus delbrueckii ATCC 9649. Journal of Industrial Microbiology& Biotechnology 11:23-28. doi: 10.1007/BF01583728. [ Links ]
DuBois M, Gilles KA, Hamilton JK, Rebers PA & Smith F (1956) Colorimetric Method for Determination of Sugars and Related Substances. Analytical Chemistry 28: 350-356. doi:10.1021/ac60111a017. [ Links ]
Eiteman M.A & Ramalingam S (2015) Microbial Production of Lactic Acid. Biotechnology Letters 37: 955 - 972. doi:10.1007/s10529-015-1769-5. [ Links ]
Eshun K & He Q (2004) Aloe vera: A Valuable Ingredient for the Food, Pharmaceutical and Cosmetic Industries, A Review. Critical Reviews in Food Science and Nutrition 44: 91-96. doi:10.1080/10408690490424694. [ Links ]
Fan L, Zhang Y, Qu W Wang J & Shao W (2011) Cloning and analysis of the xylAB operon and characterization of xylose isomerase from Thermoanaerobacter ethanolicus. Biotechnology Letters 33: 593-598. doi:10.1007/s10529-010-0463-x. [ Links ]
Fitz Patrick M, Champagne P, Cunningham MF & Whitney RA (2010) A biorefinery processing perspective: Tre atment of lignoc ellulosic materials for the production of value-added products. Bioresource Technology 101: 8915-8922. doi:10.1016/j.biortech.2010.06.125. [ Links ]
Gao C, Ma C & Xu P (2011) Biotechnological routes based on lactic acid production from biomass. Biotechnology Advances 29: 930-939. doi: 10.1016/j.biotechadv.2011.07.022. [ Links ]
Giraldo-Estrada C & Ramírez Y (2010) Evaluación de la hidrólisis de biomasa para la obtención de azúcares fermentables. Informe interno de Investigación. 118 p. Universidad EAFIT, Medellin, Colombia. [ Links ]
Gomez J (2013) Evaluación de la Producción de Ácido Láctico por Thermoanaerobacter sp. USBA 018 a Partir de Subproductos del Procesamiento de Aloe barbadensis Mill (Aloe vera). Trabajo de Grado Maestría. Pontificia Universidad Javeriana. [ Links ]
Hamman J (2008) Composition and Applications of Aloe vera Leaf Gel. Molecules 13: 1599-1616. doi:10.3390/ molecules13081599. [ Links ]
Hofvendahl K, van Niel EWJ & Hahn-Hágerdal B (1999) Effect of temperature and pH on growth and product formation of Lactococcus lactis ssp. lactis ATCC 19435 growing on maltose. Applied Microbiology and Biotechnology 51: 669-672. doi:10.1007/s002530051449. [ Links ]
Jin B, Huang LP & Lant P (2003) Rhnzppus arrhizus - a producer for simultaneous saccharification and fermentation of starch waste materials to L(+)-lactic acid. Biotechnology Letters 25: 1983-1987. doi:10.1023/ B:BILE.0000004389.53388.d0. [ Links ]
LeBlanc JG, Laiño JE, del Valle MJ, Vannini V, van Sinderen D, Taranto MP, de Valdez GF, de Giori GS & Sesma F (2011) B-Group vitamin production by lactic acid bacteria - current knowledge and potential applications. Journal of Applied Microbiology 111: 12971309. doi:10.1111/j.1365-2672.2011.05157.x. [ Links ]
Lee JW Kim HU, Choi S, Yi J & Lee SY (2011) Microbial production of building block chemicals and polymers. Current Opinion in Biotechnology22: 758-767. doi:10.1016/j.copbio.2011.02.011. [ Links ]
Liang M, Chen M, Liu X, Zhai Y, Liu X-w, Zhang H, Xiao M & Wang P (2012) Bioconversion of D-galactose to D-tagatose: continuous packed bed reaction with an immobilized thermostable L-arabinose isomerase and efficient purification by selective microbial degradation. Applied Microbiology and Biotechnology93: 1469-1474. doi: 10.1007/s00253-011-3638-z. [ Links ]
Liu B, Yang M, Qi B, Chen X, Su Z & Wan Y (2010) Optimizing L-(+) -lactic acid production by thermophile Lactobacillus plantarum As.1.3 using alternative nitrogen sources with response surface method. Biochemical Engineering Journal 52: 212-219. doi:10.1016/j. bej.2010.08.013. [ Links ]
Ma K, Maeda T, You H & Shirai Y (2014) Open fermentative production of l-lactic acid with high optical purity by thermophilic Bacillus coagulans using excess sludge as nutrient. Bioresource Technology 151: 28-35. doi:10.1016/j. biortech.2013.10.022. [ Links ]
Maas RW, Bakker R Eggink G & Weusthuis R (2006) Lactic acid production from xylose by the fungus Rhizopus oryzae. Appl Microbiol Biotechnol 72: 861-868. doi:10.1007/s00253-006-0379-5. [ Links ]
Meng Y, Xue Y, Yu B, Gao C & Ma Y (2012) Efficient production of l-lactic acid with high optical purity by alkaliphilic Bacillus sp. WL-S20. Bioresource Technology 116: 334-339. doi:10.1016/j.biortech.2012.03.103. [ Links ]
Miller GL (1959) Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Analytical Chemistry 31: 426-428. doi:10.1021/ac60147a030. [ Links ]
Moghaddasi SM & Kumar SV (2011) Aloe vera their chemicals composition and applications: A review. International Journal of Biological and Medical Research 2: 446 - 471. [ Links ]
Mussatto SI, Fernandes M, Mancilha IM & Roberto IC (2008) Effects of medium supplementation and pH control on lactic acid production from brewer's spent grain. Biochemical Engineering Journal 40: 437-444. doi: 10.1016/j.bej.2008.01.013. [ Links ]
Nancib N, Nancib A, Boudjelal A, Benslimane C, Blanchard F & Boudrant J (2001) The effect of supplementation by different nitrogen sources on the production of lactic acid from date juice by Lactobacillus casei subsp. rhamnosus. Bioresource Technology 78: 149-153. doi:10.1016/S0960-8524(01)00009-8. [ Links ]
Nancib A, Nancib N, Meziane-Cherif D, Boubendir A, Fick M & Boudrant J (2005) Joint effect of nitrogen sources and B vitamin supplementation of date juice on lactic acid production by Lactobacillus casei subsp. rhamnosus. Bioresource Technology 96: 63-67. doi:10.1016/j. biortech.2003.09.018. [ Links ]
Nandal U & Bhardwaj RL (2012) Aloe vera for human nutrition, health and cosmetic use -A review. International Research Journal of Plant Science 3: 038 -046. [ Links ]
NTC 4657 (1999) Alimento para animales. Determinación del contenido de nitrógeno y cálculo del contenido de proteína cruda. Metodo Kjeldahl ( ISO 5983). [ Links ]
NTC 5151 (2003) Alimentos para animales. Determinación de los contenidos de calcio, cobre, hierro, magnesio, manganeso, potasio, sodio y zinc. Metodo usando espectrometría de absorción atómica. [ Links ]
Payot T, Chemaly Z & Fick M (1999) Lactic acid production by Bacillus coagulans - kinetic studies and optimization of culture medium for batch and continuous fermentations. Enzyme and Microbial Technology 24: 191-199. doi:10.1016/S0141-0229(98)00098-2. [ Links ]
Qin J, Zhao B, Wang X, Wang L, Yu B, Ma Y, Ma C, Tang H, Sun J & Xu P (2009) Non-Sterilized Fermentative Production of Polymer-Grade L-Lactic Acid by a Newly Isolated Thermophilic Strain Bacillus sp. 2 - 6. Plos One 4:e4359 doi:10.1371/journal.pone.0004359. [ Links ]
Reynolds T (2004) Medicinal and Aromatic Plants -Industrial Profiles. Aloes: The Genus Aloe, (Reynolds T, ed.) Taylor & Francis Group. [ Links ]
Rodríguez ER, Martín JD & Romero CD (2010) Aloe vera as a Functional Ingredient in Foods. Critical Reviews in Food Science and Nutrition 50: 305-326. doi:10.1080/10408390802544454. [ Links ]
Romani A, Yáñez R, Garrote G & Alonso JL (2008) SSF production of lactic acid from cellulosic biosludges. Bioresource Technology 99: 4247-4254. doi:10.1016/j.biortech.2007.08.051. [ Links ]
Silveira MS, Fontes CPML, Guilherme AA, Fernandes FAN & Rodrigues S (2012) Cashew Apple Juice as Substrate for Lactic Acid Production. Food and Bioprocess Technology 5: 947-953. doi:10.1007/s11947-010-0382-9. [ Links ]
Subramanian MR, Talluri S, & Christopher LP (2014) Production of lactic acid using a new homofermentative Enterococcus faecalis isolate. Microbial Biotechnology 8(2), 221-229 doi:10.1111/1751-7915.12133. [ Links ]
Tongpim S, Meidong R, Poudel P, Yoshino S, Okugawa Y, Tashiro Y, Taniguchi M & Sakai K (2013) Isolation of thermophilic l-lactic acid producing bacteria showing homo-fermentative manner under high aeration condition. Journal of Bioscience and Bioengineering. doi:10.1016/j.jbiosc.2013.08.017. [ Links ]
Wasewar KL (2005) Separation of Lactic Acid: Recent Advances. Chemical and Biochemical Engineering Quartery19: 159 - 172. [ Links ]
Wee YJ & Ryu HW (2009) Lactic acid production by Lactobacillus sp. RKY2 in a cell-recycle continuous fermentation using lignocellulosic hydrolyzates as inexpensive raw materials. Bioresource Technology 100: 4262-4270. doi:10.1016/j.biortech.2009.03.074. [ Links ]
Wee YJ, Kim JN & Ryu HW (2006) Biotechnological production of lactic acid and its recent applications. Food Technology and Biotechnology 44: 163-172. [ Links ]
Xu K & Xu P (2014) Efficient production of 1-lactic acid using co-feeding strategy based on cane molasses/ glucose carbon sources. Bioresource Technology 153: 23-29. doi:10.1016/j.biortech.2013.11.057. [ Links ]
Yoo IK, Chang HN, Lee EG, Chang YK & Moon SH (1997) Effect of B vitamin supplementation on lactic acid production by Lactobacillus casei. Journal of Fermentation and Bioengineering 84: 172-175. doi:10.1016/ S0922-338X(97)82551-2. [ Links ]
Yu L, Lei T, Ren X, Pei X & Feng Y (2008) Response surface optimization of L-(+)-lactic acid production using corn steep liquor as an alternative nitrogen source by Lactobacillus rhamnosus CGMCC 1466. Biochemical Engineering Journal 39: 496-502. doi:10.1016/j. bej.2007.11.008. [ Links ]













