Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Universitas Scientiarum
Print version ISSN 0122-7483
Univ. Sci. vol.21 no.1 Bogotá Jan./Apr. 2016
https://doi.org/10.11144/Javenana.SC21-1.feoe
Fishing effects on elasmobranchs from the Pacific Coast of Colombia
Efectos de pesca sobre elasmobranquios en el Pacífico colombiano
Efeitos sobre a pesca nos elasmobrânquios no Pacífico colombiano
Andres Felipe Navia1*, Paola Andrea Mejía-Falla1
Edited by Juan Carlos Salcedo-Reyes (salcedo.juan@javeriana.edu.co) & Alberto Acosta (laacosta@javeriana.edu.co)
1Fundación colombiana para la investigación y conservación de tiburones y rayas SQUALUS. Carrera 60A No.11-39, Cali, Colombia
*anavia@squalus.org
Andrés Felipe Navia
His main research interest is directed to the evaluation of the ecological function of elasmobranchs in marine ecosystems, and the determination of the relationship between life history characteristics of these species and their vulnerability, with emphasis on reproduction and age. He recently initiated studies on the essential habitats of elasmobranchs and ecological processes that determine their richness and distribution.
Paola Andrea Mejía-Falla
Her research focused on the life history strategies and demographics of elasmobranchs, as well as to assess the effect of fishing on such population parameters. She has conducted research on reproduction, age and growth of various species of elasmobranchs, both marine and freshwater. She has initiated studies on essential habitats of elasmobranches and on the macro-ecological processes that determine the distribution of these species.
Funding: N/A
Electronic supplementary material: N/A
Received: 11-02-2015 Accepted: 13-11-2015 Published on line: 20-01-2016
Para citar este artículo / To cite this article
Navia AF, Mejia-Falla PA. Fishing effects on elasmobranchs from the Pacific Coast of Colombia, Universitas Scientiarum, 21 (1): 9-22, 2016. doi: http://dx.doi.org/10.11144/Javenana.SC21-1.feoe
Abstract
During 1995, 2001, 2003, 2004 and 2007; we studied the temporal variation in the structure of the elasmobranch assemblage along the Colombian Pacific coast using: the community index of diversity, heterogeneity, equitability, species composition, average catch sizes, and mean trophic levels. A total of 1711 specimens from 19 species (7 sharks and 12 rays) were collected during the 90 trawling operations. The number of species captured varied between 7 (1995) and 12 (2007) demonstrating a trend towards an imbalance in the assemblage attributes. In 1995, the mean trophic level (TLm) of the assemblage was 3.60, but in 2007 it decreased to 3.55 when the functional level of large predators was absent (TL > 4). These results suggest changes in species composition, structural attributes, and a reduction of the highest functional level. Alterations to the catch proportions were also found: i.e. a greater abundance of rays of lower trophic levels. This study suggests an effect of trawling on the stability of this tropical coastal ecosystem.
Keywords: sharks; rays; fisheries; trophic levels; community structure
Resumen
Durante 1995, 2001, 2003, 2004 y 2007 estudiamos la variación temporal en la estructura de un ensamblaje de elasmobranquios a lo largo de la costa pacífica colombiana. Para ello utilizamos los índices de diversidad, heterogeneidad, equitatividad y composición de especies, así como el tamaño promedio de capturas y los niveles tróficos promedio. Se colectó un total de 1711 especímenes de 19 especies (7 tiburones y 12 rayas) durante 90 operaciones de pesca de arrastre. El número de especies capturadas varió entre 7 (1995) y 12 (2007), lo cual demuestra una tendencia hacia un desbalance en los atributos del ensamblaje. En 1995 el nivel trófico promedio (TLm) del ensamblaje fue de 3.60, pero en 2001 disminuyó a 3.55, cuando estuvo ausente el nivel funcional de grandes predadores (TL > 4). Estos resultados sugieren cambios en la composición de especies y en los atributos estructurales, y una reducción del nivel funcional superior. Se encontraron también alteraciones en las proporciones de captura, como una mayor abundancia de rayas de niveles tróficos inferiores. Este estudio sugiere un efecto de la pesca de arrastre en la estabilidad de este ecosistema tropical costero.
Palabras clave: tiburones; rayas; pesquerías; niveles tróficos; estructura comunitaria
Resumo
Durante 1995, 2001, 2003, 2004 e 2007 estudamos a variatilo temporal na estrutura da comunidade de elasmobranquios ao longo da costa pacífica colombiana usando o índice comunitário de diversidade, heterogeneidade, equidade, composic. So de espécies, tamanho médio das capturas e níveis tróficos médios. Um total de 1711 espécimes de 19 espécies (7 tubarões e 12 raias) foram coletadas durante as 90 operações de pescas de arrasto. O número de espécies capturadas variou entre 7 (1995) e 12 (2007), demonstrando uma tendencia ao desequilibrio nos atributos da comunidade. Em 1995, o nível trófico médio (TLm) da comunidade era de 3,60, mas em 2007 decresceu para 3,55 quando o nível funcional de grandes predadores estava ausente (TL ≥ 4). Estes resultados sugerem alterações na compostilo das espécies, em atributos estruturais e uma redução do mais alto nível funcional. Também foram encontradas alterações na propongo de capturas, ou seja, uma maior abundancia de raias de níveis tróficos mais baixos. Este estudo sugere um efeito da pesca de arrasto na estabilidade de este ecossistema costeiro tropical.
Palavras-chave: tubarões; raias; pesca; níveis tróficos; estrutura da comunidade
Introduction
Over the last 50 years, sustained fishing activity worldwide and the steady degradation of habitats have had a wide range of impacts on ecosystems; reflecting in changes of abundance, spatial distribution, productivity, and structure of those communities under exploitation (Hall 1999, Jackson et al. 2001, Myers & Worm 2005, Lotze et al. 2006, Navia et al. 2012). This impact on the structure and function of communities has been widely documented, quantified, and found to impair the maintenance of goods and services that ecosystems provide to humans (Worm et al. 2006, Branch et al. 2010, Lotze et al. 2011).
There are ecological limits (especially related to the energetic balance within food webs) that explain the low number of top predators that may be extracted from a marine environment (Pérez-España et al. 2006). Regardless of known implications, most fisheries tend to focus on these top predators (Branch et al. 2010). Pauly et al. (1998) documented the results of such extractive activities as "fishing down marine food webs": a gradual change in landings from long-lived, upper trophic level species to short-lived, lower trophic species. This phenomenon was observed at both a global (Pauly et al. 1998) and local scale in Thailand (Christensen 1998), Canada (Pauly et al. 2001), the Mediterranean Sea (Pinnegar et al. 2003), Chile (Arancibia & Neira 2005), Uruguay and Argentina (Jaureguizar & Milessi, 2008), India (Bhathal & Pauly 2008), and Brazil (Freire & Pauly 2010). Conversely, some authors suggest that the decline in mean trophic levels of fisheries landings is due to increased exploitation of the lower trophic levels of marine food webs (Essington et al. 2006). This effect, called "fishing through the food web", has been recorded in North Pacific fisheries (Litzow & Urban 2009).
Sharks and rays are associated with many fisheries worldwide and more than half of the annual catch is through by-catch (Bonfil 1994, Stevens et al. 2000). Consequently, these species are listed as highly vulnerable to exploitation (Holden 1974, Walker 1998) and considered to be among the most endangered vertebrate groups in the world (Stevens et al. 2000, Dulvy & Reynolds 2002, Dulvy et al. 2014). Numerous studies have recorded high reduction rates of elasmobranchs over the past 50 years in many fisheries (e.g. Graham et al. 2001, Coelho et al. 2003, Baum et al. 2003, Myers & Worm 2005). Moreover, it has been proposed that the reduction or disappearance of sharks considered as top predators may have strong effects on the function and structure of the marine food webs (Stevens et al. 2000, Myers et al. 2005, Navia et al. 2010, Navia 2013).
The central and southern Pacific coast of Colombia have been key areas to the domestic shrimp industry since 1960 (de la Pava & Mosquera 2001). Several species of sharks and rays are caught as by-catch and some are commercially exploited (Mejia-Falla & Navia 2011). Despite the history of the shrimp industry in this area, official records of catch efforts and landings are poor (Rueda et al. 2006, Mejia-Falla & Navia 2011). As a result, data required for the design of an appropriate fishery management plan is lacking. Furthermore, trawling impacts and changes to the fish assemblages over time in this area have not been assessed. Based on industrial shrimp trawling fleet data, the goal of our research is to evaluate the changes in the elasmobranch assemblage structure between 1995 and 2007 and determine possible effects of trawling on the assemblage.
Materials and methods
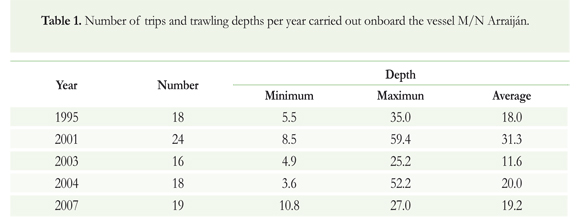
Monitoring of onboard shrimp vessels trawling between Golfo de Tortugas and Ensenada de Guapi (Colombian Pacific coast) was conducted every October in 1995, 2001, 2003, 2004 and 2007 (Figure 1). To standardize efforts, all fishing operations were carried out by the same vessel (M/N Arraijan) using the same fishing gear (trawl nets; each 27 m in length, mesh size 2 %"); between 11.6 and 31.0 m deep (Table 1), in the same area (Figure 1), and under procedures traditionally used by commercial fishing vessels: i.e. trawling three hours during the day and six hours at night. Following each haul, sharks and rays were identified and recorded to species level; sex, size (total length, LT in cm), weight (g), and number of specimens per species.
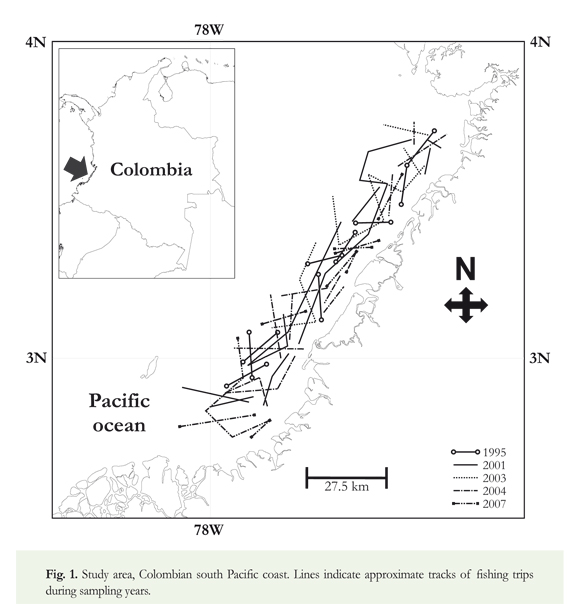
Relative abundance (catch per unit effort) was calculated by dividing the total number of specimens of each species by the total number of hours per haul in the sampling year. Temporal variations in these relative abundances were analyzed using ANOVA (Kruskal-Wallis test, Zar 1999) and differences in species richness over time were evaluated using Cochran's Q test (Zar 1999). Differences in species catch size (Lt) between sampling years were evaluated with a nonparametric ANOVA (Zar 1999).
To analyze changes in the elasmobranch assemblage attributed over time, we calculated the indices of heterogeneity, diversity, and equitability (Krebs 1999); using the software Ecological Methodology (Krebs 2001). The nonparametric Simpson's index (D) was used to compare heterogeneity, and respectively, the complement index (1-D) towards diversity ranging from 0 (low diversity) to 1 (maximum diversity). Shannon diversity index (H') was also used to measure diversity. Equitability was quantified using Simpson's measure (Evd), which ranges from 0 to 1 and is not affected by rare species (Krebs 1999). These indices accurately describe the structural and functional attributes of fish communities (Piet & Jennings 2005) and there is available theoretical and empirical evidence that they can measure the impact of fishing (Rochet & Trenkel 2003).
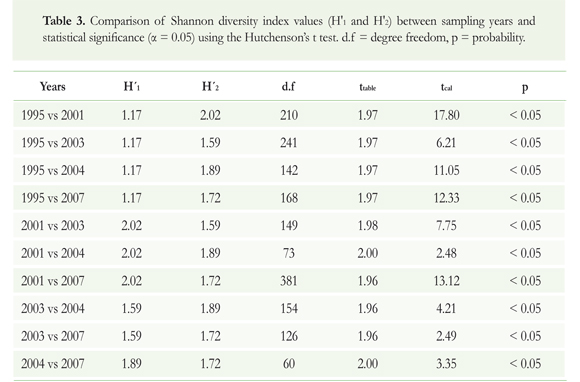
Significant differences (a = 0.05) between the attributes of the assemblage were evaluated by applying the Hutchenson's t test (1970) to the Shannon diversity index values, under the null hypothesis that the diversity of the samples were not different. This test was calculated as t = (H'1 - H'2)x(Var H'1 + Var H'2)-05, where H'1 and H'2 are the Shannon diversity indices of samples 1 and 2, respectively, and Var H'1 and Var H'2 are the variances of each of the indices (Magurran 1988).
To evaluate the effects of trawling on the trophic structure of the ecosystem in question, the historical changes of the mean trophic level (TLm) of the elasmobranch assemblage were analyzed. To quantify the trophic level of the species, studies on feeding habits carried out in the same studied area were used (Gómez et al. 2003, Mejía-Falla et al. 2006, Navia et al. 2006, 2007, 2011, López-García et al. 2012, Navia 2013). For those species whose diet has not been studied in this area, we used studies conducted in the Eastern Tropical Pacific (ETP) (Valadez-González 2007). Literature on the diet of Carcharhinus leucas and C. porosus in the ETP was not available. Thus, we used trophic level values calculated by Cortés (1999): TL = 4.3 for C. leucas and TL = 4.1 for C. porosus. Trophic levels (TLk) were quantified by applying the formula proposed by Cortés (1999) and reviewed by Ebert & Bizarro (2007).
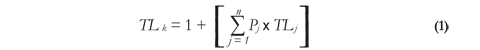
where Pj is the fraction of prey i in the diet of predatorj; TLj is the trophic level of prey i in the diet of predator j. We used the reference values of trophic levels for prey suggested by Cortés (1999), Ebert & Bizarro (2007) and López-García et al. (2012).
The mean trophic level (TLm) of the assemblage for each sampling year was calculated according to Pauly & Palomares (2005):
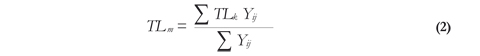
Where TLk, is the mean trophic level of the species or group k, and Yij is the catch of species or group i in year j.
Results
A total of 90 net hauls and 396 hours of fishing were monitored; a total of 1711 specimens of 19 species (7 sharks and 12 rays) were captured (Table 2).
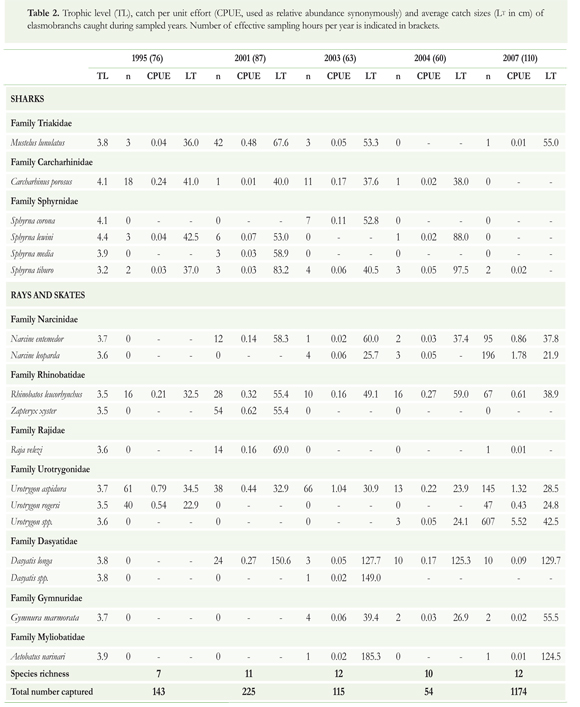
The number of species varied between 7 (1995) and 12 (2003, 2007). No significant differences were found in occurrence (Cochran, Q = 4.30, df = 4, p = 0.367) but there were significant differences in relative abundance (CPUE) between studied years (Kruskal-Wallis test, H = 3.01, df = 4, p = 0.045). For example, in sharks, the relative abundance of C. porosus changed from 0.24 in 1995 to 0.01 in 2001, and Mustelus lunulatus changed from 0.48 in 2001 to 0.01 in 2007. Average catch size decreased during these years from 67.6 to 55.0 cm Lt. Regarding batoids, Dasyatis longa changed from a relative abundance of 0.27 and average catch size of 150.6 cm Lt in 2001 to 0.09 and 129.7 cm Lt, respectively, in 2007. On the other hand, the index of relative abundance of some small sized rays with no commercial value, such as Narcine entemedor, N. leoparda and Urotrygon aspidura increased; while the catch sizes declined (Table 2).
There was no clear trend in equitability and heterogeneity over time. Between 1995 and 2001, these attributes increased, whereas between 2001 and 2007, they decreased. Either way, this suggests an alteration of the species richness and abundance during the research period. Species richness of elasmobranchs differed significantly between all sampled years (Table 3), indicating considerable variation in the assemblage composition during the studied period.
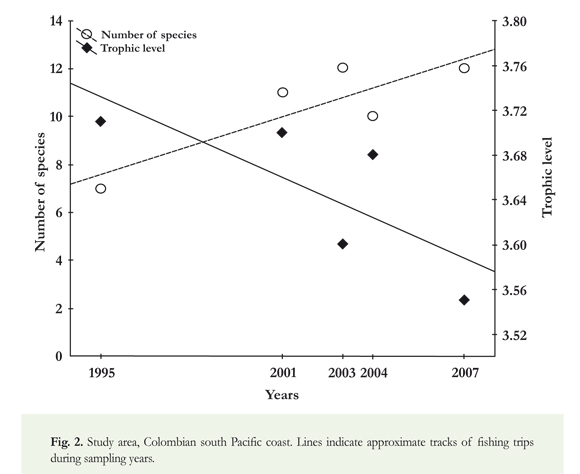
Over the years studied, the number of species increased (7 to 12) while the TLm decreased slightly (Figure 2). As an exception to this trend, 11 species were collected in 2001 and the TLm was the second highest of all sampled years. This was due to the catch of Zapteryx xyster and Raja velezi, resulting in a TL higher than the other rays in the study, and only occurred in that year. The TLm of the assemblage ranged from 3.61 in 1995 to 3.55 in 2007. Species from the top predator functional level (TL ≥ 4.0), such as those from the Sphyrnidae and Carcharhinidae families, were not recorded during any of the fishing trips in 2007.
Discussion
Changes in the elasmobranch assemblage detected during this study included alteration of assemblage attributes (heterogeneity, diversity, and equitability), modification of the relative abundance of most species, a slight reduction in mean trophic level, reduction in average catch size, and a substantial reduction in upper trophic level predators. For example, M. lunulatus and D. longa, considered the most important commercial elasmobranch species in the late 1990s on the Pacific coast (Zapata et al. 1999), showed a significant downward trend in abundance and average catch size. According to Piet & Jennings (2005), this effect on average catch size and species composition is directly linked to changes in the trophic structure of the community.
Thirty of the 48 largest marine ecosystems on the planet have shown reductions in trophic levels (Essington et al. 2006). Although the results of this study do not demonstrate the removal of top predators from the studied area, they do show that species with TL ≥ 4 decreased significantly. In contrast, the intermediate trophic level species increased in abundance over the same period. These results are consistent with fisheries statistics for the Colombian Pacific coast (Mejia-Falla & Navia, 2011), which showed a reduction in average catch sizes and an increase in abundance of juvenile and intermediate TL species. This suggests a significant reduction or even possible loss of the ecological function of top predators, especially those at the adult stage, which could impact, in the short or medium term, the structure of the food web in the study area. This is especially important considering top predators were identified as the most important species in keeping the balance of an ecosystem's structure (Navia et al. 2010, 2012, Navia 2013).
The rate of decline of trophic levels in this assemblage (0.0035 year-1) is lower than those reported in other studies (e.g. Pauly et al. 1998, Pinnegar et al. 2003, Milessi et al. 2005; Jaureguizar & Milessi 2008). However, it is worth noting that in just 5 years of sampling, the TLm of the assemblage showed a reduction. This might reflect the significant impact that trawling has had on the species within the studied area even though this fishery does not catch high trophic level shark species on a regular basis.
The increase of species richness and evenness between 1995 and 2007 suggests the occurrence of additional elasmobranchs species that were not associated with the shrimp trawl fishery in earlier years. This change in the assemblage composition is the result of increased catch frequency of intermediate or lower trophic level species including N. leoparda, N. entemedor, Rhinobatos leucorhynchus, and U. aspidura (TL < 3.7).
According to Piet & Jennings (2005), the assumption that assemblages have had an integrated response to fishing pressures implies that all fish community indicators should decrease. This has been observed in shallow water fisheries of the North Sea (Piet & Jennings 2005) and coastal shrimp fisheries along the Pacific coast of Colombia in the latter years of this study. It has been argued that community indices and trophic levels calculated from landings in some countries are biased by economic interests towards targeted species (Bianchi et al. 2000, Essington et al. 2006). Consequently, changes in community indices and trophic levels are not reliable indicators of the impact on fisheries. This work, however, was based on direct monitoring onboard fishing vessels and assessed all the elasmobranch species captured (regardless of their economic value). Therefore, this study provides accurate and reliable information on the performance of both indicators and assemblage attributes.
The changes in the assemblage found in this study; i.e. reduction in mean trophic level, increased richness of small species, and changes in their abundance; suggests fishing effects some of the structural and functional parameters of the trophic networks: proportion of top predators, links density, and predatory function. If this trend continues and extends to other functional groups, the upper trophic levels of the food web within the study area will experience overexploitation. Consequently, the network structure will be simplified and the productivity and biomass will decrease, as has been recorded within many other ecosystems highly impacted by fishing (Coll et al. 2008, 2009a, Barausse et al. 2009, Morissette et al. 2009, Navia et al. 2012). Essington et al. (2006) suggests that the reduction observed in trophic levels of an ecosystem can be influenced by the depletion of upper trophic levels (e.g. fish) and the creation of alternative fisheries of lower trophic levels (e.g. invertebrates). However, this is not the case in Colombian Pacific fisheries because despite the reduction in yields from traditional fisheries, no new alternative fisheries have been developed.
In the Pacific Region, the shrimp fishery began in the 1960s and peaked in 1974 when there were 138 boats in this fishery (de la Pava & Mosquera, 2001). In the mid-1980s, the shrimp crisis began, which led to the continuous reduction of vessels until 2007 when there were only 29 active (Navia et al. 2008). Despite this drastic reduction in fishing activity, the proportion of by-catch (including elasmobranchs) versus shrimp in the Colombian Pacific Ocean between 2004 and 2005 was around 14:1 kg (Rueda et al. 2006), which was well above the overall average of 10:1 kg in other world tropical fisheries (Hall et al. 2000, Lewinson et al. 2004).
Conclusion
Our results suggest that trawling has possibly impacted the structure and composition of the elasmobranch assemblage in the Colombian Pacific coastal waters. This has led to the reduction of individuals in upper trophic levels, potentially contributing to instability of the food web and/or alteration of ecosystem control mechanisms. Impacts on the elasmobranch assemblage may also trigger gradual changes that could lead to a new organization of the food web as has been suggested for various marine ecosystems (Chen et al. 2008, Coll et al. 2009a, 2009b, Duan et al. 2009, Andersen & Pedersen 2010, Scheffer 2010, Lotze et al. 2011). These changes to the Colombian Pacific ecosystems could make this food web particularly sensitive to the loss of top predators, as suggested by Navia et al. (2012).
Acknowledgements
The authors wish to thank A. Martínez and his crew for facilitating onboard monitoring from the vessel "Arraiján". They also thank A. Tobón, LF. Payán and S. Hleap for their assistance conducting field work. They thank V Ramírez-Luna and P. Sargent for translating the manuscript into English. This project was supported by Conservación Internacional and WWF Colombia. Idea Wild donated equipment for field and laboratory work.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
Andersen KH, Pedersen M (2010) Damped trophic cascades driven by fishing in model marine ecosystems. Philosophical Transactions Royal Society Ijondon B 277:795-802 doi: 10.1098/rspb.2009.1512 [ Links ]
Arancibia H, Neira S (2005) Long-term changes in the mean trophic level of Central Chile fishery landings. Scientia Marina 69:295-300. [ Links ]
Barausse A, Ducci A, Mazzoldi C, Artoli Y, Palmeri L (2009) Trophic network model of the Northern Adriatic Sea: Analysis of an exploited and eutrophic ecosystem. Estuarine Coastal and Shelf Science 83:577-590 doi: 10.1016/j.ecss.2009.05.003 [ Links ]
Baum JK, Myers RA, Kehler DG, Worm B, Harley SJ, Doherty PA (2003) Collapse and conservation of shark populations in the Northwest Atlantic. Science 299: 289-392 doi: 10.1126/science.1079777 [ Links ]
Bhathal B, Pauly D (2008) 'Fishing down marine food webs' and spatial expansion of coastal fisheries in India, 1950-2000. Fisheries Research 91:26-34 doi: 10.1016/j.fishres.2007.10.022 [ Links ]
Bianchi G, Gislason H, Graham K, Hill L, Jin X, et al. (2000) Impact of fishing on size composition and diversity of demersal fish communities. ICES Journal of Marine Science 57:558-571 doi: 10.1006/jmsc.2000.0727 [ Links ]
Bonfil R (1994) Overview of world elasmobranch fisheries, FAO Fisheries technical paper No 341. FAO Roma, Italy. [ Links ]
Branch AT, Watson R, Fulton EA, Jennings S, McGilliard CR, et al. (2010) The trophic fingerprint of marine fisheries. Nature 468:431-435 doi: 10.1038/nature09528 [ Links ]
Chen Z, Qiu Y, Jia X, Xu S (2008) Using an ecosystem modeling approach to explore possible ecosystem impacts of fishing in the Beibu Gulf, Northern South China sea. Ecosystems 11: 1318-1334 doi: 10.1007/s10021-008-9200-x [ Links ]
Christensen V (1998) Fishery-induced changes in the marine ecosystem: insights from models of the Gulf of Thailand. Journal of Fish Biology 53:128-142. [ Links ]
Coelho R, Bentes L, Goncalves JM, Lino PG, Ribeiro J, et al. (2003) Reduction of elasmobranch by-catch in the hake semipelagic nera-bottom longline fishery in the Algarve (Southern Portugal). Fisheries Science 69:293-299 doi: 10.1046/j.1444-2906.2003.00620.x [ Links ]
Coll M, Lotze HK, Romanuk TN (2008) Structural degradation in Mediterranean sea food webs: testing ecological hypotheses using stochastic and mass-balance modeling. Ecosystems 11:939-960 doi: 10.1007/s10021-008-9171-y [ Links ]
Coll M, Palomera I, Tudela S (2009a) Decadal changes in a NW Mediterranean Sea food web in relation to fishing explotation. Ecological Modelling 220:2088-2102 doi: 10.1016/j.ecolmodel.2009.04.049 [ Links ]
Coll M, Santojanni A, Palomera I, Arneri E (2009b) Food web changes in the Adriatic Sea over the last three decades. Marine Ecology Progress Series 381: 17-37 doi: 10.3354/meps07944 [ Links ]
Cortés, E., 1999. Standarized diet compositions and trophic levels of sharks. ICES Journal of Marine Science 56:707-717. [ Links ]
De la Pava ML, Mosquera C (2001) Diagnóstico regional de la cadena camarón de pesca en el Pacífico colombiano. ACODIARPE, Buenaventura. [ Links ]
Duan LJ, Li SY, Liu Y, Moreau J, Christensen V (2009) Modeling changes in the coastal ecosystem of the Pearl River Estuary from 1981 to 1998. Ecological Modelling 220:2802-2818 doi: 10.1016/j.ecolmodel.2009.07.016 [ Links ]
Dulvy NK, Fowler SL, Musick JA, Cavanagh RD, Kyne PM, et al. (2014) Extinction risk and conservation of the world's sharks and rays. Elife 3: e00590 doi: org/10.7554/eLife.00590.002 [ Links ]
Dulvy NK, Reynolds JD (2002) Predicting extinction vulnerability in skates. Conservation Biology 16:440-450. [ Links ]
Ebert D, Bizarro JJ (2007) Standardized diet compositions and trophic levels of skates (Chondrichthyes: Rajiformes: Rajoidei). Environmental Biology of Fishes 80:221-237 doi: 10.1007/978-1-4020-9703-4_8 [ Links ]
Essington TE, Beaudreau AH, Wiedenmann J (2006) Fishing through marine food webs. Proceedings of the National Academy of Science USA 103:3171-3175 doi: 10.1073/pnas.0510964103 [ Links ]
Freire KMF, Pauly D (2010) Fishing down Brazilian marine food webs, with emphasis on the east Brazil large marine ecosystem. Fisheries Research 105:57-62 doi: 10.1016/j.fishres.2010.02.008 [ Links ]
Gómez G, Zapata L, Franke R, Ramos G (2003) Hábitos alimentarios de Mustelus lunulatus y M. henlei (Pisces: Triakidae) colectados en el Parque Nacional Natural Gorgona, Pacífico colombiano. Boletín de Investigaciones Marinas y Costeras 32:219-231. [ Links ]
Graham KJ, Andrew NL, Hodgson KE (2001) Changes in relative abundance of sharks and rays on Australian South East Fishery trawl grounds after twenty years of fishing. Marine and Freshwater Research 52:549-561 doi: 10.1071/MF99174 [ Links ]
Hall SJ (1999) The effects of fishing on marine ecosystems and communities. Blackwell Science, Oxford. [ Links ]
Hall MA, Alverson DL, Metuzals KI (2000) By-catch: problems and solutions. Marine Pollution Bulktin 41:204-219. [ Links ]
Holden MJ (1974) Problems in the rational exploitation of elasmobranch populations and some suggested solutions. In: Harden-Jones, F.R. (Ed.). Sea Fisheries Research. John Wiley and Sons, New York, pp. 117-137. [ Links ]
Hutchenson K (1970) A test for comparing diversities based on the Shannon formula. Journal of Theoretical Biology 29:151-154. [ Links ]
Jackson JBC, Kirby MX, Berger, WH, Bjorndal, KA, Botsford, LW et al. (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629-638. [ Links ]
Jaureguizar AJ, Milessi AC (2008) Assessing the sources of the fishing down marine food web process in the Argentinean-Uruguayan common fishing zone. Scientia Marina 72:25-36. [ Links ]
Krebs CJ (1999) Ecological Methodology 5.1. Department of zoology, University of British Columbia, Vancouver. [ Links ]
Krebs CJ (2001) Ecology: The Experimental Analysis of Distribution and Abundance. Benjamin Cummings, San Francisco. [ Links ]
Lewinson RL, Crowder LB, Read AJ, Freeman SA (2004) Understanding impacts of fisheries bycatch on marine mega fauna. Trends in Ecology and Evolution 19:598-604. [ Links ]
Litzow MA, Urban D (2009) Fishing through (and up) Alaskan food webs. Canadian Journal Fisheries and Aquatic Science 66:201-211. [ Links ]
López-García J, Navia AF, Mejía-Falla PA, Rubio EA (2012) Feeding habits of Dasyatis longa (Elasmobranchii: Myliobatiformes): sexual, temporal and ontogenetic effects. Journal of Fish Biology 80:1563-1579 doi: 10.1111/j.1095-8649.2012.03239.x [ Links ]
Lotze HK, Lenihan HS, Bourque BJ, Bradbury RH, Cooke RG, et al. (2006) Depletion, degradation and recovery potential of estuaries and coastal seas. Science 312:1806-1809 doi: 10.1126/science.1128035 [ Links ]
Lotze HK, Coll M, Dunne JA (2011) Historical changes in marine resources, food web structure and ecosystem functioning in the Adriatic Sea, Mediterranean. Ecosystems 14:198-222 doi: 10.1007/s10021-010-9404-8 [ Links ]
Magurran AE (1988) Ecological diversity and its measurement. Croom-Helm, London. [ Links ]
Mejía-Falla PA, Navia AF, Giraldo A (2006) Notas biológicas de la raya ocelada (Zapteryx xyster) en la zona central de pesca del Pacífico colombiano. Investigaciones Marinas 34:181-185. [ Links ]
Mejía-Falla PA, Navia AF (2011) Estadísticas pesqueras de tiburones y rayas en el Pacífico colombiano. Documento técnico Fundación SQUALUS No FS0111. 70 pp. [ Links ]
Milessi AC, Arancibia H, Neira S, Defeo O (2005) The mean trophic level of Uruguayan landings during period 1990-2001. Fisheries Research 74: 223-231 doi: 10.1016/j.fishres.2005.02.002 [ Links ]
Morissette L, Pedersen T, Nielsen M (2009) Comparing pristine and depleted ecosystems: the Sofjord, Norway versus the Gulf of St. Lawrence, Canada. Effects of intense fisheries on marine ecosystems. Progress in Oceanography 81:174-187. [ Links ]
Myers RA, Worm B (2005) Extinction, survival or recovery of large predatory fishes. Philosophical Transactions of the Royal Society B. 360:13-20 doi: 10.1098/rstb.2004.1573 [ Links ]
Navia AF, Giraldo A, Mejía-Falla PA (2006) Notas sobre la biología y dieta del toyo vieja {Mustelus lunulatus) de la zona central de pesca del Pacífico colombiano. Investigaciones Marinas 34:217-222. [ Links ]
Navia AF, Mejía-Falla PA, Giraldo A (2007) Feeding ecology of elasmobranch fishes in coastal waters of the Colombian Eastern Tropical Pacific. BMC Ecology 7:8 doi: 10.1186/1472-6785-7-8 [ Links ]
Navia AF, Cortés E, Mejía-Falla PA (2010) Topological analysis of the ecological importance of elasmobranch fishes: A food web study on the Gulf of Tortugas, Colombia. Ecological Modelling 221:2918-2926 doi: 10.1016/j.ecolmodel.2010.09.006 [ Links ]
Navia AF, Torres A, Mejía-Falla PA, Giraldo A (2011) Sexual, ontogenetic, temporal and spatial effects in the feeding ecology of Urotrygon rogersi in the Colombian Pacific Ocean. Journal of Fish Biology 78:1213-1224 doi: 10.1111/j.1095-8649.2011.02931.x [ Links ]
Navia AF, Cortés E, Jordán F, Crúz-Escalona VH, Mejía-Falla PA (2012) Changes to marine trophic networks caused by fishing. In: Mahamane A (ed) Diversity of Ecosystems. Intech, Croatia, pp. 417-452 [ Links ]
Navia AF (2013) Función ecológica de tiburones y rayas en un ecosistema costero tropical del Pacífico colombiano. Doctorate thesis. Centro Interdisciplinario de Ciencias Marinas, México. [ Links ]
Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F (1998) Fishing down marine food webs. Science 279:860-863 doi: 10.1126/science.279.5352.860 [ Links ]
Pauly D, Palomares ML, Froese R, Sa-a P, Vakily M, et al. (2001) Fishing down Canadian aquatic food webs. Canadian Journal Fisheries Aquatic Science 58:51-62. [ Links ]
Pauly D, Palomares ML (2005) Fishing down marine food web: it is far more pervasive than we thought. Bulletin of Marine Science 76:197-212. [ Links ]
Pérez-España H, Abarca-Arenas LG, Jiménez-Badillo ML (2006) Is fishing down trophic web a generalized phenomenon? The case of Mexican fisheries. Fisheries Research 79:349-352 doi: 10.1016/j.fishres.2006.03.027 [ Links ]
Piet GJ, Jennings S (2005) Response of potential fish community indicators to fishing. ICES Journal of Marine Science 62:214-225 doi: 10.1016/j.icesjms.2004.09.007 [ Links ]
Pinnegar JK, Polunin NVC, Badalamenti F (2003) Long term changes in the trophic level of western Mediterranean fishery and aquaculture landings. Canadian Journal Fisheries and Aquatic Science 60:222-235. [ Links ]
Rochet MJ, Trenkel VM (2003) Which community indicators can measure the impact of fishing? A review and proposals. Canadian Journal Fisheries and Aquatic Science 60:86-99. [ Links ]
Rueda M, Angulo A, Madrid N, Rico F, Girón A (2006) La pesca industrial de arrastre de camarón de aguas someras del Pacífico colombiano: su evolución, problemática y perspectivas hacia una pesca responsable. Instituto de Investigaciones Marinas y Costeras INVEMAR. Santa Marta. [ Links ]
Scheffer M (2010) Alternative states in ecosystems. In: Terborgh J, Estes JA (eds) Trophic cascades predators, prey, and the changing dynamics of nature. Island Press, Washington, pp. 287-298 Universitas Scientiarum Vol. 21 (1): 9-22 http://ciencias.javeriana.edu.co/investigacion/universitas-scientiarium [ Links ]
Stevens JD, Bonfil R, Dulvy K, Walker PA (2000) The effects of fishing on sharks, rays and chimaeras (Chondrichthyes) and the implications for marine ecosystems. ICES Journal of Marine Science 57:476-494. [ Links ]
Valadez-González, C (2007) Distribución, abundancia y alimentación de las rayas bentónicas de la costa de Jalisco y Colima, México. Doctorate thesis, Centro Interdisciplinario de Ciencias Marinas, México. [ Links ]
Walker TI (1998) Can shark resources be harvested sustainably? A question revisited with a review of shark fisheries. Marine and Freshwater Research 49: 553-572. [ Links ]
Worm B, Barbier E, Beaumont N, Duffy JE, Folke C et al. (2006) Impacts on biodiversity loss on ocean ecosystem services. Science 314:787-90 doi: 10.1126/science.1132294 [ Links ]
Zapata LA, Rodríguez G, Beltrán B, Gómez G, Cediel A, et al. (1999) Evaluación de recursos demersales por el método de área barrida en el Pacífico colombiano. Boletín Científico INPA 6:177-226. [ Links ]
Zar JH. (1999). Biostatistical analysis. Fourth edition. Prentice Hall, Englewood Cliffs, New Jersey. 663 p. [ Links ]














