Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Universitas Scientiarum
versão impressa ISSN 0122-7483
Univ. Sci. vol.21 no.1 Bogotá jan./abr. 2016
https://doi.org/10.11144/laveriana.SC21-1.naas
Nursery area and size structure of the lemon shark population, Negaprion brevirostris (Poey, 1868), in Los Roques Archipelago National Park, Venezuela
Área de criadero y estructura de tallas de la población del tiburón limón, Negaprion brevirostris (Poey, 1868), en el Archipiélago Los Roques, Venezuela
Área de berçário e estrutura de comprimentos da população do tubarão limão, Negaprion brevirostris (Poey, 1868), no Arquipélago de Los Roques, Venezuela
Rafael Tavares123 *, Jon Paul Rodriguez13, Misael Morales3
Edited by Juan Carlos Salcedo-Reyes (salcedo.juan@javeriana.edu.co) & Andrés Felipe Navia (anavia@squalus.org)
1Centro de Ecología, Instituto Venezolano de Investigaciones Científicas (IVIC), Apdo. 20632, Caracas 1020-A, Venezuela.
2Instituto Nacional de Investigaciones Agrícolas (INIA), La Asunción 6311, Isla de Margarita, Venezuela.
3Centro para la Investigación de Tiburones (CIT), Av. Don Bosco, Qta ABC, No. 10, La Florida, Caracas 1050, Venezuela.
*rtavares@inia.gob.ve
Rafael Tavares
Is a scientific researcher in the Instituto Nacional de Investigaciones Agrícolas (INIA), Venezuela. He received a deegre in biology from University of Lisbon (1997), Portugal, and a M.Sc. in marine sciences from Instituto Oceanografico de Venezuela (2005). Currently, he is a Ph.D. candidate in ecology from Instituto Venezolano de Investigaciones Científicas (IVIC). His professional interests focus on the fishery biology, ecology and stock assessment of sharks and rays.
Jon Paul Rodriguez
Is a Professor of Ecology at the Venezuelan Institute for Scientific Investigation. He holds a degree in biology from Universidad Central de Venezuela (1991), and a PhD in ecology and evolutionary biology from Princeton University (1999). He works on understanding patterns in the spatial distribution of threatened species and ecosystems, as well as the underlying causes of these patterns, and the development of policy guidelines for biodiversity conservation.
Misael Morales
Misael Morales is a Marine Biologist who has been a member of the Centro para la Investigacion de Tiburones (CIT), Venezuela, and its shark tagging program since 2005. He holds a degree in marine biology from Universidad de Oriente (2007), Venezuela, and a M.Sc. in Marine Biology Sciences from Université de Brest (2010), France. His professional interest has been focused on the fishery biology and stock assessment.
Funding: N/A
Electronic supplementary material: N/A
Received: 11-04-2015 Accepted: 21-01-2016 Published on line: 24-02-2016
Para citar este artículo / To cite this article
Tavares R, Rodriguez JP, Morales M. Nursery area and size structure of the lemon shark population, Negaprion brevirostris (Poey, 1868), in Los Roques Archipelago National Park, Venezuela, Universitas Scientiarum, 21 (1): 33-52, 2016. doi: http://dx.doi.org/10.11144/laveriana.SC21-1.naas
Abstract
The protection of the habitats used by juvenile sharks is a management strategy that has recently caught the attention of fishery biologists. In the present study, we evaluated the population of the lemon shark (Negaprion brevirostris) from Los Roques Archipelago in order to identify the nursery area, describe the size composition, and examine the variation in nocturnal activity of the juvenile individuals. The data analysed came from three different sources: commercial shark fishery, tag-recapture sampling, and visual records. A total of 375 lemon sharks with total lengths between 55 and 281 cm were recorded during the study period. Overall data showed that the area occupied by juvenile lemon sharks was clearly partitioned into primary and secondary nurseries. Additionally, nighttime activity seemed to change according to the size of sharks in the primary nursery, suggesting a reduction of time activity overlapping among juveniles of distinct size/age. Results suggest that the strategy of utilization of the primary nurseries by the lemon shark may lead to important ecological benefits by reducing the competition, predation and natural mortality.
Keywords: Caribbean Sea, conservation, ecology, nursery, sharks, tagging.
Resumen
La protección de los hábitats utilizados por tiburones juveniles es una estrategia de manejo que ha capturado recientemente la atención de los biólogos de pesquerías. En el presente estudio se evaluó la población del tiburón limón (Negaprion brevirostris) del Archipiélago Los Roques con el fin de identificar su zona de cría, describir su composición de tamaño y examinar la variación en la actividad nocturna de los juveniles. Los datos analizados provienen de 3 fuentes distintas: pesquería comercial de tiburones, muestreo de captura-marcaje-recaptura y registros visuales. Durante el período de estudio se registró un total de 375 tiburones limón, con longitudes totales entre 55 y 281 cm. En general, los datos mostraron que el área ocupada por los tiburones limón estaba claramente dividida entre zonas de cría primarias y secundarias. Adicionalmente, la actividad nocturna parecía cambiar de acuerdo con el tamaño de los tiburones en la zona de cría primaria, lo cual sugiere una reducción de la coincidencia entre juveniles de distinto tamaño/edad. Los resultados sugieren que la estrategia de utilización de la zona de cría primaria por el tiburón limón puede conducir a importantes beneficios ecológicos por la reducción de competencia, predación y mortalidad natural.
Palabras clave: Mar Caribe, conservación, ecología, zona de cría, tiburones, marcaje.
Resumo
A protecao dos habitats utilizados pelos tubaroes jovens é uma estratégia de manejo que recentemente tem chamado á atenção dos biólogos pesqueiros. No presente estudo avaliamos a populacao do tubarao limao (Negaprion brevirostris) no Arquipélago de Los Roques, com o propósito de identificar a área de berçário, descrever a composição de tamanhos, e examinar a variação da atividade noturna dos individuos jovens. Os dados analisados foram originados a partir de tres fontes diferentes: Foram analisados dados da pescaría comercial de tubaroes, trabalhos de marcação e recaptura, e registos visuais. Um total de 375 tubaroes limáo com comprimento total entre 55 e 281 cm foi registado durante o período de estudo. Os dados mostram que a zona ocupada pelos tubaroes limao jovens se encontra claramente dividida em áreas de berçários primaria e secundária. Adicionalmente, a atividade noturna dentro da área de berçário primaria pareceu mudar de acordo com o tamanho dos tubaroes, sugerindo uma separação desta atividade por grupos de idade. Os resultados sugerem que a estratégia de utilizacao da área de berçário primaria do tubarao limao poderia oferecer importantes benefícios ecológicos, como a reducao da competicao, predacao e mortalidade natural.
Palavras-chave: Mar do Caribe, conservacao, ecologia, área de berçário, tubaroes, marcacao.
Introduction
Despite the commercial and ecological importance of sharks, their populations have been severely altered due to overfishing, habitat destruction and pollution (Ferretti et al. 2010, Dulvy et al. 2014). Sharks are among the most threatened groups of marine animals worldwide (Lucifora et al. 2011). Owing to the difficulty of managing shark populations by conventional strategies (e.g. fishing gear regulations, catch quotas, size limits, fishing closures), a management strategy that has recently caught the attention of fishery biologists is the protection of the habitats used by neonate and juvenile sharks (Heithaus 2007). Studies based on demographic modelling have indicated that the survival of juvenile sharks is more important for population maintenance than the survival of adult stages (Cortés 1999, Simpfendorfer 1999, Gallucci et al. 2006). The creation of marine protected areas (MPAs) and other kinds of time-area closure that include marine zones inhabited by juvenile sharks (i.e. nurseries) suggests that this measure is potentially beneficial for the conservation of shark populations (Heupel & Simpfendorfer 2005, Garla et al. 2006, Knip et al. 2012).
Shark nursery areas are typically characterized by the occurrence and abundance of neonate and juvenile individuals. Springer (1967) noted that shark nurseries correspond to coastal zones not usually inhabited by adults, except females laying eggs or giving birth to young. Most coastal shark species have a geographically discrete nursery located in highly productive shallow waters, such as coastal marshes, reef lagoons and estuaries, where the young can find abundant food and shelter from predation (Branstetter 1990, Castro 1993). Shark nurseries can be identified in the field by the capture of neonates, juveniles or gravid females with embryos in advanced stages of development (Castro 1993). However, it is necessary to take into account other elements in order to characterize shark nursery areas. Heupel et al. (2007) proposed that nurseries for sharks have to be defined on the basis of three primary criteria: species abundance, degree of site attachment and inter-annual use of the area. Also within the nurseries, distribution of juvenile sharks can vary spatially according to age or size. Bass (1978) classified shark nursery areas into two types: primary and secondary. Primary nurseries are those in which parturition occurs and where the young spend the first stages of life, while secondary nurseries are those in which the juveniles live after leaving the primary nursery and before reaching maturity. The utilization of two separate nurseries (primary and secondary) by young sharks may be an advantageous strategy for reducing predation and competition, mainly during the earlier life stages.
The lemon shark, Negaprion brevirostris, is a coastal tropical shark of the continental and insular shelves that occasionally ventures into the open ocean for migration purposes (Compagno 2002). In the western Atlantic, it ranges from New Jersey (USA) to southern Brazil, including the Gulf of Mexico and Caribbean Sea (Compagno 2002). In the Venezuelan Caribbean, this species appears to be associated with groups of oceanic islands such as Los Roques and Las Aves archipelagos, which are characterized by the predominance of significant coral reef formations (Tavares 2005, 2009). During the last thirty years, the lemon shark has been extensively studied within their nurseries in the Bimini Islands, Bahamas (Gruber 1982, Gruber et al. 1988, Morrissey & Gruber 1993a, Gruber et al. 2001, Feldheim et al. 2002, Jennings et al. 2008, Newman et al. 2010). Nursery areas for this species have been also characterized at the Atol das Rocas, Brazil (Freitas et al. 2006), US Virgin Islands (De Angelis et al. 2008), Cape Canaveral, Florida, USA (Reyier et al. 2008), Fernando de Noronha, Brazil (Garla et al. 2009), and Turks and Caicos Islands, British West Indies (Henderson et al. 2010). Although most of the biological knowledge on the lemon shark comes from the Bahamian population, essential habitats of this species, such as nursery grounds, continue to be poorly studied in areas of the southern Caribbean Sea and elsewhere.
The Los Roques Archipelago is a tropical oceanic platform that is notable for its well conserved coral reef ecosystems and high marine diversity. Previous studies carried out in this area revealed that the archipelago provides habitat for 21 species of sharks, with important nursery areas for the blacktip (Carcharhinus limbatus) and Caribbean reef (C.perezi) sharks (Tavares 2008, 2009). Findings of these studies also suggest that tropical oceanic islands may be an efficient recruitment source for the recovery of shark populations. Due to the importance of nursery areas for sharks' life history, the characterization of these essential habitats becomes necessary to improve conservation and management plans. The purpose of this study was (1) to identify the nursery area of lemon shark based on the occurrence of new-born and juvenile individuals, (2) to describe their size and sex composition, and (3) to examine the variation in nighttime activity, with emphasis on the juvenile population of the lemon shark from Los Roques Archipelago.
Materials and methods
Study site
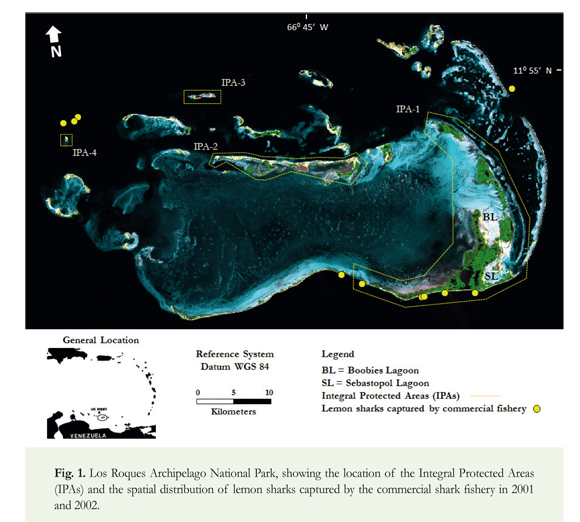
Los Roques Archipelago National Park is a tropical oceanic complex located in the Caribbean Sea, 160 km north of the central coast of Venezuela (11°43'-11°58' N/66°35'-66°57' W; Figure 1). It comprises more than 40 small and low islands arranged around a main central lagoon. Water temperature ranges between 25 and 30 °C with the minimum values occurring in January-February and maximum values in July-August. Los Roques contains diverse coral forms, such as dense and diffuse patch reefs, fringing reefs, and one major barrier reef 30 km in length that is located along the eastern edge of the archipelago (Baamonde 2003). The management plan of the Los Roques Archipelago includes four Integral Protected Areas (IPAs; Figure 1) that are closed to recreational and commercial fishing, transport boats and tourism activities. Commercial fishing of sharks has been prohibited in Los Roques since 2012, with the exception of an incidental catch quota of five sharks by other fishing activities conducted by local fishers (Gaceta Oficial de la República Bolivariana de Venezuela 2012). Most of the field work was concentrated in the eastern archipelago within the main Integral Protected Area (IPA-1; Figure 1). The IPA-1 encompasses a group of islands with a significant coverage of mangrove formations, and two major semi-enclosed lagoons: Sebastopol and Boobies. These shallow-water lagoons (maximum depth of 2.5 m) are typically characterized by sandy and seagrass habitats, and are almost completely bordered by mangroves (mainly red mangrove Rhizophora mangle). Macro-benthic invertebrate fauna within the lagoons is dominated by the queen conch (Strombus gigas), spiny lobster (Panulirus argus) and blue crab (Callinectes sapidus). In this study, tagging sampling of sharks constituted the primary source of data and was mainly conducted in the Sebastopol Lagoon, previously reported by local fishermen as a location with high abundance of juvenile lemon sharks.
Sampling
The first data set used in this study came from monitoring of the commercial shark fishery in the Los Roques Archipelago during 2001 and 2002. The fishing vessels comprised mainly small wooden boats (6-8 m long) equipped with outboard motors (40-48 hp), and the fishing gear used was bottom longline (100-400 hooks; size number 3-5) with a total of 194 longline sets (32,728 hooks deployed). A total of 803 captured sharks belonging to 14 species were recorded. Fishing zones comprised both internal shallow waters (< 20 m) and bordering deep zones (20-80 m) of the archipelago. Detailed information of this shark fishery (e.g. effort, fishing operations, species composition, and spatial distribution) is given by Tavares (2009). The data recorded for each lemon shark caught were: location, pre-caudal length (PCL), total length (TL), weight (W) and sex. Sexual maturity was assessed by the general development and characteristics of the reproductive organs in both sexes (Castro 1996).
A tagging procedure targeting juvenile lemon sharks was applied in Sebastopol Lagoon during 2001-2002 (preliminary sampling) and 2005-2014 (general sampling). Data were obtained through a total of 34 field surveys in the study area. The surveys were carried out in different months every year but combined data covered all seasons of the year. Lemon sharks were captured with nylon multi-filament gillnets (n = 4-6; 25-100 m long; 1.0-1.5 m deep; 8.9-11.5 cm square mesh) that were generally set perpendicular to the shoreline. In 2001-2002, gillnets were continuously set for 12 hours, between 18:00 and 06:00 h. Based on preliminary results of the night catch frequencies such as that lemon sharks appeared to be more active during the first hours after sunset, swimming in areas close to the mangrove fringes, gillnets were set between 18:00 and 24:00 h during 2005-2014. Accumulated throughout the study period, fishing sites covered the vast majority of the internal coastline of Sebastopol Lagoon. Only a few shore areas with depths over 1.5 m were not explored using gillnets. To avoid the death of sharks, nets were continuously monitored by checking their float lines approximately every 10 minutes. Captured lemon sharks were measured (PCL, TL), weighed (W), sexed, their umbilical scar state examined, and tagged with a conventional external tag. Then, individuals were held in a marine corral with the purpose of monitoring their health status. Despite this, a few individuals died possibly due to stress related to capture and handling before release. Neonates were identified by the presence of an open or partially closed umbilical scar. Date, location and time of capture were also recorded for each shark. Throughout the sampling periods, biometric measurements (PCL, TL and W) were always recorded by the same researcher in order to minimize measurement errors. Three kinds of external shark tags were used during the study: M-type dart tag, plastic dart tag and rototag. The M-type dart tag was typically used with adult sharks. To avoid injury and additional stress on captured juveniles, the dart head of the tag was inserted between the skin and the muscle through an incision with a surgical scalpel following Tavares (2008). The M-type and plastic dart tags were provided by the Cooperative Shark Tagging Program/National Marine Fisheries Service (USA), and the rototags with the contact information pointed by the Instituto Nacional de Investigaciones Agrícolas (Venezuela) were manufactured by ©Premier1Supplies (USA). Also, the study included educational activities for the fishing community of Los Roques Archipelago; the fishermen were trained in the collection of basic data (PCL, TL and date and location of recapture) in case of eventual recaptures of tagged sharks.
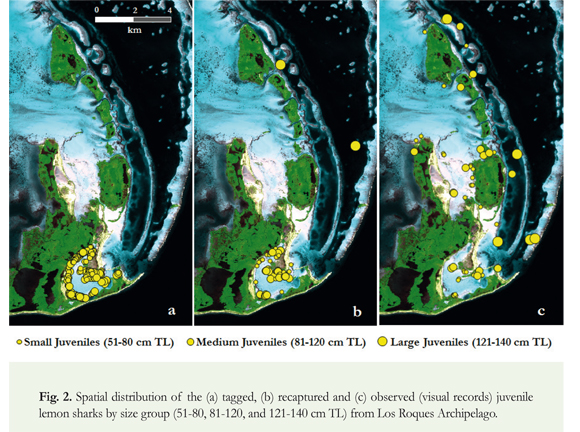
Juvenile lemon sharks are occasionally observed from boats during diurnal navigation in areas within and around the eastern IPA-1 of the Los Roques Archipelago. With the purpose of complementing information on the spatial distribution, locations of sightings were recorded during 2010-2013. Furthermore, TL of the sighted sharks in the Los Roques was visually estimated from the boats, and individuals were classified into three general length classes: 51-80 cm TL (small juveniles), 81-120 cm TL (medium juveniles) and 121-140 cm TL (large juveniles) in order to analyse their spatial distribution. Seawater clarity and transparency in Los Roques Archipelago facilitated the size estimation of lemon sharks seen from the boat.
Data analysis
The spatial distribution and size structure of lemon shark in the Los Roques Archipelago were described based on all three data sources (commercial fishery, tagging sampling, and visual records). This information was vital to identify and describe the areas occupied by juvenile lemon sharks. A SPOT satellite image of the Los Roques Archipelago (source: Centro de Procesamiento Digital de Imágenes, CPDI) and the Quantum GIS 2.6.0 software (QGIS Development Team 2014) were used in the spatial analysis.
The sex and size structure of the juvenile lemon sharks captured during the tagging effort in the Sebastopol Lagoon were examined in detail. A chi-square (x2) test was used to evaluate the expected sex ratio (1:1) of captured individuals. The length composition of tagged sharks by sex was graphically described by constructing a length frequency histogram and grouping individuals within size classes of 5 cm TL. To compare size composition, length data were log-transformed and then differences between mean values by sex were tested with an analysis of variance (ANOVA). The mean birth size of lemon sharks in the study area was estimated from the lengths of all neonates having an open umbilical scar.
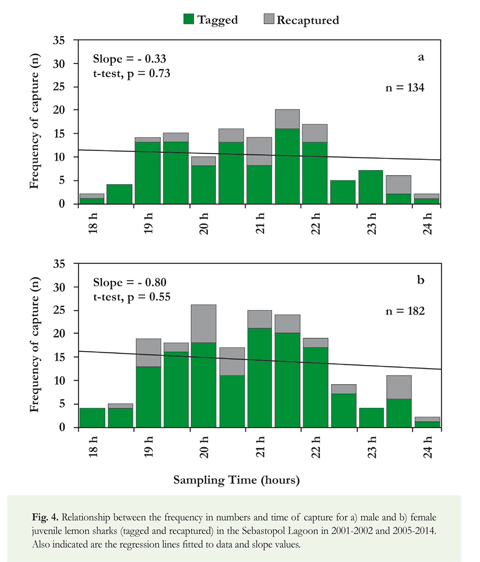
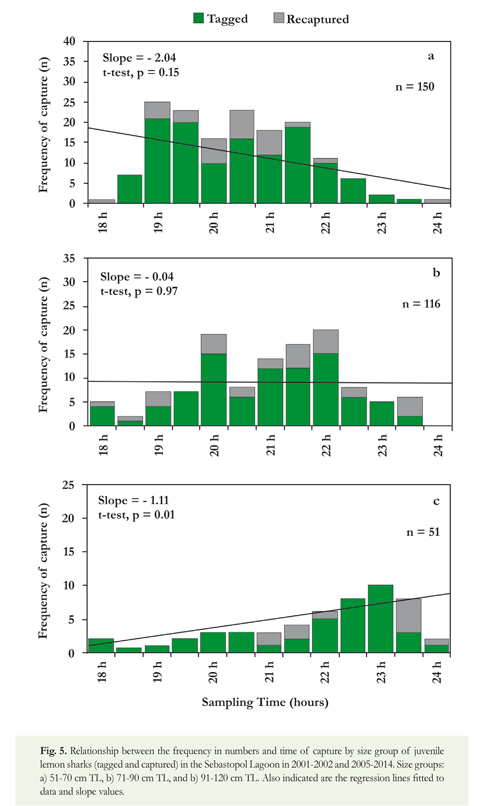
Additionally, variation in nocturnal activity by sex and by size of the juvenile lemon sharks (tagged and recaptured) within the Sebastopol Lagoon was examined from the relationship between the frequency in numbers and time of capture (period 18:0024:00 h). Taking into account the distribution of lengths observed in the Sebastopol Lagoon, juveniles were classified into three size groups: 51-70 cm TL, 71-90 cm TL, and 91-120 cm TL. Subsequently, a linear regression model was fitted to the data (frequency numbers vs. time of capture), and then slopes obtained were compared between sex and size groups by using an F-test. The following expression (Equation 1) was used to calculate the experimental value of the Fisher distribution:

where, RSSc is the "common" residual sum of squares, RSSp is the "pooled" residual sum of squares, DFp is the "pooled" residual degrees of freedom, and k is the number of models.
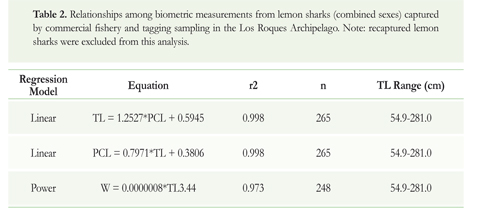
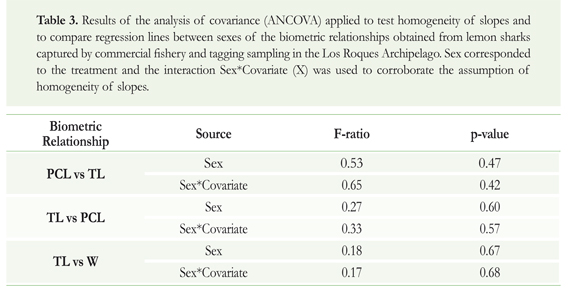
In order to establish comparisons with other studies, biometric relationships (TL vs. PCL, PCL vs. TL, and TL vs. W) were applied using regression procedures. An analysis of covariance (ANCOVA) tested differences between line regressions by sex, and the homogeneity of slopes was evaluated a priori through the interaction of the treatment (sex) with the covariate (X, independent variable). Failure to meet the assumption of the homogeneity of slopes implies that there is an interaction between the covariate and the treatment (i.e. significant interaction). As the relationship between TL and W is typically a non-linear function, data were log-transformed in order to linearize the relationship prior to the ANCOVA. All statistical analyses (a = 0.05) applied in this study are described in Zar (1996).
Results
During monitoring of local shark fishing in 2001 and 2002, a total of 11 lemon sharks (6 males and 5 females) were captured in zones bordering the insular platform of the Los Roques Archipelago, at depths between 20 and 80 m (Figure 1). Assessment of the reproductive condition showed that 3 males between 210 and 270 cm TL, and 2 females between 204 and 281 cm TL were sexually mature. The remaining individuals, ranging in size from 142 to 193 cm TL, had not reached maturity and were classified as sub-adults. This group of immature sharks but with lengths near the size of maturity was observed in areas of the southern external edge of the archipelago but close to the IPA-1, suggesting that these individuals do not venture far from nursery areas.
In the Sebastopol Lagoon, 254 juvenile lemon sharks were captured and tagged during the study period (2001-2002 and 2005-2014). Overall information related to field work, such as number of surveys, sampling days, tagged sharks, incidental deaths and recaptures by year is shown in Table 1. The annual proportion of mortality due to the stress of capture and handling varied between 0 and 16.7%, with an average value of 4.7%. The spatial catch distribution showed that juvenile lemon sharks were predominantly distributed near the mangrove shoreline (Figure 2). A high proportion of the individuals (71.3%) were caught in the first 15 meters of the gillnet lines, close to the shore. No other species of sharks were captured in the Sebastopol Lagoon. Out of the 254 lemon sharks captured, 107 were males and 147 were females; a proportion not significantly different from the expected ratio of 1:1 (x2 = 2.48, p = 0.12).
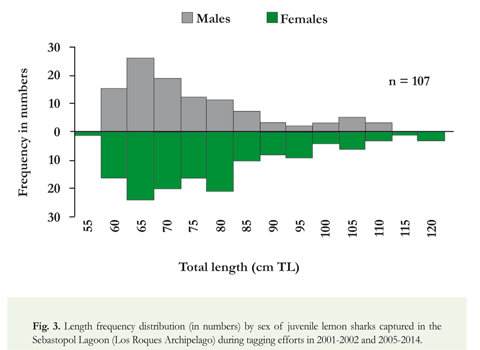
The analysis of the size composition of juvenile lemon sharks caught in the Sebastopol Lagoon indicated that males measured between 55.4 and 110.0 cm TL (mean: 71.4 ± 12.6 SD), and females measured between 54.9 and 118.0 cm TL (mean: 75.7 ± 15.0 SD). Difference in mean length between sexes was significant (ANOVA; F = 5.38, p = 0.02). Juvenile lemon sharks remain in the Sebastopol Lagoon until they reach a size of approximately 120 cm TL. Furthermore, the length frequency distribution by sex showed dominance of the females among individuals of 80-120 cm TL (Figure 3). A significant difference in sex ratio (x2 = 13.92,p < 0.01) was found for individuals with sizes between 80 and 120 cm TL (21 males and 46 females). Neonates (n = 31, 54.9-65.9 cm TL) were captured only between June and August, indicating that this is the birth season at the study area. Mean birth size estimated from neonate lemon sharks was 59.5 cm TL (± 2.4 SD).
Recaptures of lemon sharks were recorded only during 2005-2014. In total, 56 juveniles were recaptured on one or several occasions, resulting in a general recapture rate of 23.8%. All recaptures occurred in the Sebastopol Lagoon, except for two individuals that were recorded by the local fishery in the eastern border of the IPA-1 (Figure 2). Time at liberty ranged from 3 to 739 days (mean: 219.8 ± 192.3 SD). Recaptures from the Sebastopol Lagoon comprised 25 males with a size range of 57.5108.5 cm TL (mean: 75.8 ± 14.4 SD), and 29 females with a size range of 61.0-106.5 cm TL (mean: 79.0 ± 12.8 SD). The individuals recaptured out of Sebastopol Lagoon were two females that measured 123.2 and 140.0 cm TL. No significant difference in sex ratio (x2 = 1.15, p = 0.28) was found for the group of recaptured lemon sharks. In addition, 78.6% of the juvenile lemon sharks were recaptured in areas near the tagging location (< 180 m), indicating a high degree of site attachment.
The relationship between the frequency and time of capture in the Sebastopol Lagoon indicated that juvenile lemon sharks of both sexes appeared to be more active between 19:00 and 22:00 h (Figure 4). Comparison of the slopes between males and females was not significant (F-test; F = 1.70, p = 0.21), suggesting no variation in nighttime activity by sex. In the case of the analysis by size groups, the periods of major nighttime activity appeared to change according to the size of sharks (Figure 5). Juveniles of 51-70 cm TL were more active during 19:00-21:30 h, juveniles of 71-90 cm TL during 20:00-22:00 h, and juveniles of 91-120 cm TL during 22:00-23:30 h. Statistical analysis indicated a significant difference of the slopes by size groups (F-test; F = 6.49, p = 0.01).
In 2010-2013, a total of 54 lemon sharks were visually recorded from boats on twenty-four occasions during diurnal navigation. Of these, 32 were classified as small juveniles (51-80 cm TL), 17 corresponded to medium juveniles (81-120 cm TL), and most individuals of these two groups (81.6%) were observed on the most internal zones of the IPA-1, such as the Sebastopol and Boobies lagoons (Figure 2). The other five lemon sharks were large juveniles (121-140 cm TL), which were recorded in zones surrounding the IPA-1. Size distribution data revealed that lemon sharks in semi-enclosed lagoons were predominantly small and medium, with a clear predominance of individuals measuring between 51 and 120 cm TL.
Conversion equations resulting from the relationships among the biometric variables are shown in Table 2. The analyses for testing homogeneity of slopes as well as differences between line regressions by sex were not significant in any relationship (TL vs. PCL, PCL vs. TL, TL vs. W; ANCOVA;p > 0.05; Table 3).
Discussion
While monitoring shark fishing in 2001-2002, only 11 lemon sharks were captured in areas bordering the insular platform of Los Roques Archipelago. The contribution of lemon sharks to the total catch was 1.9%, compared with 47.9% for the blacktip shark, and 37.7% for the Caribbean reef shark (Tavares 2009). The low numbers of captured lemon sharks during that period can be explained by the fact that fishermen avoided fishing practices near or within the IPA-1, which is a primary habitat for this species in the study area. The sizes of adult lemon sharks observed in the present study were around the mean size of maturity (230 cm TL) previously reported (Brown & Gruber 1988). The areas of the archipelago where sub-adult and adult lemon sharks were caught reflect the type of habitat used by these ontogenetic stages. Juveniles commonly occur in shallow water lagoons, but as they grow into sub-adults and adults they gradually occupy offshore reefs and deeper waters (Gruber et al. 1988).
Regarding the spatial distribution of lemon sharks by size, the habitat occupied by juveniles is clearly partitioned into primary and secondary nurseries. Primary nurseries include the Sebastopol and Boobies lagoons, where lemon sharks are born and remain during their first years of life. Although complementary research needs to be carried out in the Boobies Lagoon, the size structure of juvenile lemon sharks appears to be similar in both lagoons. Secondary nurseries were adjacent to primary nurseries, and involved the western and eastern edges of the IPA-1. However, large juveniles in Los Roques Archipelago seemed to occur in a large area as they gradually reached maturity. Similar results were found in Bimini Islands, Bahamas, where three distinct zones were used by lemon sharks: a nursery characterized by shallow water and mangrove fringes, a zone occupied by large juveniles (4-10 years), and a more extensive area for sub-adults and mature individuals (Gruber et al. 1988). Furthermore, the absence of other shark species in the Sebastopol Lagoon indicates that this area constitutes a monospecific primary nursery for N. brevirostris. Most studies have reported that nursery areas are utilized simultaneously by several species of sharks, usually referred to as multispecies or communal nurseries (Bass 1978, Castro 1993, Simpfendorfer & Milward 1993, Yokota & Lessa 2006, Tavares & Sanchez 2012). In the case of lemon shark, other studies carried out in the central western Atlantic have found that juveniles of this species may share nursery areas with the nurse shark Ginglymostoma cirratum (Gruber et al. 1988, De Angelis et al. 2008, Reyier et al. 2008, Garla et al. 2009, Guttridge et al. 2009). The nurse shark commonly occurs in the Los Roques Archipelago (Tavares 2009); however, this species has not been captured or observed in the Sebastopol and Boobies lagoons. Even though our fishing method was restricted to gillnets, there is no evidence of the presence of nurse sharks in the lagoons (i.e. observations from boats and snorkelling activities during almost 15 years of work). This discrepancy between nurseries could be due to differences in the marine environment, composition and abundance of shark species, and the availability of prey in each study site. In any case, juvenile lemon and nurse sharks have differing daily activity budgets and social behaviour within the nursery, which may lead to decreased competition between these species (Guttridge et al. 2009).
The absence of other shark species in the nurseries has several ecological benefits. It could lead to increased availability of food and to reduced competition, and hence positively influence feeding efficiency, survivorship and growth of juvenile individuals (Werner et al. 1983, Simpfendorfer & Milward 1993). A previous study conducted in Los Roques Archipelago reported a monospecific nursery for the blacktip shark (Tavares 2008), also revealing that juveniles of blacktip shark have a rapid growth and sexual maturation, compared with populations of the same species located in other regions of the Atlantic Ocean. Preliminary growth data of the lemon sharks from Los Roques indicated that juveniles had high growth rates (~29.0 cm TL/year; Tavares 2010). Freitas et al. (2006) found similar estimates (25.0 cm TL/year) for juveniles in Atol das Rocas, which is a tropical oceanic island similar to Los Roques Archipelago. Barker et al. (2005) also reported relatively high growth rates (~20.0 cm TL/year) for juveniles of this species in Marquesas Keys (Florida), although the sample size of these authors was very small. In contrast, Henningsen & Gruber (1985), and Barker et al. (2005) reported lower values of early growth rates i.e. around 8.0 cm TL/year for the lemon shark in Bimini islands. This group of Bahamian islands, located in a subtropical region, has been under strong human influence, leading to degradation of the mangrove and seagrass ecosystems and pollution of the seawater. These threats, together with differences in temperature, may negatively affect the growth of juvenile sharks, as well as their survival and recruitment (Barker et al. 2005, Jennings et al. 2008, DiBattista et al. 2011). In general, the rapid growth exhibited by juvenile sharks in Los Roques Archipelago may be attributable to the high temperature of the seawater together with its comparatively slight seasonal variation, abundance of prey, low levels of competition, and well-conserved marine ecosystems.
In the Sebastopol Lagoon (a primary nursery), juvenile lemon sharks were mostly captured near mangrove fringes. This may be related to the high productivity of mangrove ecosystems, which provides a reliable source of food to juvenile sharks, but they may also remain in these areas in order to reduce predation risk. Newman et al. (2010) found a high overlap between shark diet and mangrove communities in Bimini nurseries, showing the importance of mangroves to lemon sharks and their prey. Conversely, other studies suggested that mangrove fringes and other shallow water areas within nurseries are used by juvenile lemon sharks as a means of predator avoidance (Morrissey & Gruber 1993b, Guttridge et al. 2012). Heupel & Hueter (2002) found that predator avoidance may be more important in the use of nursery grounds by young blacktip sharks (C. limbatus) than prey abundance. Intra-specific predation or cannibalism of juvenile lemon sharks by larger conspecifics has been previously documented in Bimini nurseries (Morrissey & Gruber 1993a). Then, in the case of lemon shark, predator avoidance may be the main factor in determining spatial distribution and habitat use by juveniles within nurseries.
Analysis of the length composition showed that juvenile lemon sharks of both sexes remain in the Sebastopol Lagoon until they reach approximately 120 cm TL. As mentioned above, a similar pattern in the length structure was also observed for juvenile lemon sharks in the Boobies Lagoon, indicating that this semi-enclosed lagoon may constitute another primary nursery for the species in the study area. The very low numbers of juveniles captured near the entrance of the Sebastopol Lagoon, together with its high degree of site attachment, suggests the absence of movements between these two primary nursery areas. Other studies conducted in the Bimini Islands also showed no permanent migration between the two main primary nurseries used by lemon sharks (Morrissey & Gruber 1993a, Franks 2007), which can be attributed to the high degree of site attachment observed in lemon sharks during their first years of life (Morrissey & Gruber 1993a; Freitas et al. 2006).
The overall sex ratio of the juveniles captured in the Sebastopol Lagoon did not vary significantly from the expected ratio (1:1); however, the length frequencies by sex showed a predominance of the females at sizes between 80 and 120 cm TL.
Males appeared to grow at higher rates than females with growth rates by sex: 30.9 and 28.0 cm TL/year, respectively (Tavares 2010). If males do indeed grow faster than females, then they will be the first ones to reach the maximum sizes observed in the Sebastopol Lagoon, and hence may have their home range expanded. If this were the case, it would explain the predominance of females among the larger individuals recorded, between 80 and 120 cm PCL. In relation to the neonate lemon sharks, the mean birth size estimated in the present study was 58.8 cm TL. This birth size agrees with those reported for the lemon shark in other regions of the western Atlantic (Barker et al. 2005, Freitas et al. 2006, Reyier et al. 2008, Garla et al. 2009). The capture of neonates having an open or partially closed umbilical scar indicated that the birth season in the Los Roques Archipelago is from June to August. Seasonal variation in the period of birth among geographic regions appears to be influenced by water temperatures. For example, births of lemon sharks occur between April and July in the north western Atlantic (Gruber & Stout 1983) and between January and April in the south western Atlantic (Freitas et al. 2006, Oliveira et al. 2011). In subtropical and temperate regions, births during spring and summer months will allow neonates to remain within the nurseries for a period of several months characterized by warm water. Castro (1993) stated that in temperate zones, winter temperatures force young sharks out from the nurseries into deeper waters or to other areas. This could increase the fishing and natural mortality rates of the juvenile populations.
During sampling of juvenile sharks, some individuals can die due to the stress of capture and handling. In this study, lemon sharks deaths were observed in gillnets or within the marine corral. The mean proportion of mortality reported for Los Roques was 4.7%, a higher value than that reported for lemon sharks in Bimini, Bahamas (1.7%, Gruber et al. 2001). This discrepancy could be explained by the number of collaborators or volunteers who participated and assisted during the monitoring of gillnets, but also by the higher mean temperature found in the study area. Hueter & Manire (1994) found that mortality from gillnet captures increased with water temperature. Warmer water can increase the physiological stress of capture in sharks due to hypoxia and respiratory and/or metabolic acidosis, among other causes (Manire et al. 2001). The recapture rate obtained was 23.8%, which is very similar to the value (22.3%) reported by Freitas et al. (2006) in Atol das Rocas, northeastern Brazil. However, Gruber et al. (1988) reported a recapture rate of 35.8% in Bimini Islands, and De Angelis et al. (2008) reported 29.0% in US Virgin Islands, both in the Caribbean Sea. In general, these high recapture rates can be attributed to the high degree of site attachment and limited home ranges displayed by juvenile lemon sharks within their nurseries. This behaviour has also been documented with ultrasonic telemetry studies conducted with lemon sharks (Morrissey & Gruber 1993a, Wetherbee et al. 2007, Murchie et al. 2010), as well as with other species of coastal sharks (McKibben & Nelson 1986, Holland et al. 1993, Heupel et al. 2004, Garla et al. 2006). In such cases, predator avoidance and survivorship of young sharks could also favour a high degree of site fidelity and preference for shallow habitats (Heupel & Hueter 2002, Wetherbee et al. 2007). In the same manner as with the juvenile lemon sharks captured and tagged in Sebastopol Lagoon, the group of recaptured individuals did not show significant difference in the observed sex ratio, an observation that would confirm the absence of segregation by sex within their primary nursery.
Based on telemetry studies, lemon sharks appear to increase their activity during crepuscular and/or nocturnal hours (Gruber 1984, Sundstrom et al. 2001). Our catch data results showed that juvenile lemon sharks of both sexes in the Sebastopol Lagoon were more active during the period between 19:00 and 22:00 h. However, nighttime activity seems to change according to the size of sharks, suggesting a reduction of overlap in the time activity among juveniles of distinct size/age groups. Although juvenile lemon sharks have overlapping home ranges and are social (Gruber et al. 1988, Morrissey & Gruber 1993a), the variation in nighttime activity by size found in the present study could be a strategy used to avoid or reduce intra-specific competition. During the tagging study period, small lemon sharks with bite wounds probably made by larger conspecifics were observed on several occasions (R. Tavares, pers. obs.). This finding agrees with previous observations that confirm the cannibalistic behaviour of the lemon shark (Morrissey & Gruber 1993a). No evidence of the presence of other shark species exists, at least, for the Sebastopol Lagoon; therefore we propose that avoidance of competition and predation through the partitioning of time activity could have significant benefits for juvenile lemon sharks by increasing availability of food and survivorship.
Conclusion
The nursery area of the lemon shark at Los Roques Archipelago was characterized on the basis of the occurrence and distribution of neonate and juvenile individuals, the degree of site attachment, and the inter-annual use of the habitat. The nurseries (partitioned into primary and secondary) occupy an important extension of islands and shallow lagoons in the south eastern archipelago, with predominance of mangrove ecosystems. Our results showed that the strategy of nursery utilization by the lemon shark in the study area may provide several ecological benefits. Strategies that lead to reduction of stress by competition and predation, and to increased survival, are expected to maximize reproductive success and overall fitness. Additionally, the biotic and abiotic characteristics of tropical oceanic islands, such as Los Roques Archipelago, may be important for the development of juvenile sharks and their population recruitment in the Caribbean Sea. Therefore, the protection of essential habitats is critical to the conservation of this ecologically important species. Further research is necessary to identify and protect shark nursery areas located in other Venezuelan oceanic islands.
Acknowledgements
Financial support was provided by the Oficina Nacional de Diversidad Biológica, Organización Ecochallenge de Venezuela, Centro para la Investigación de Tiburones, Pew Environment Group, Fondo Nacional de Ciencia, Tecnología e Innovación, Instituto Nacional de Investigaciones Agrícolas, Instituto Venezolano de Investigaciones Científicas and Provita. The study was made possible by logistical support from Aquatic Dive Center, Eco-buzos, Arrecife Dive, Aquarena, La Chuchera, Línea Turística Aerotuy, Oscar Shop, Aguasal and Estación de Guarda Costas Los Roques, among others. The shark tags used at the beginning of the study were provided by the Cooperative Shark Tagging Program/ National Marine Fisheries Service of the USA. We thank the many volunteers and students who participated in field surveys and helped to collect the data analysed in this paper. Research permits were provided by the Instituto Nacional de Parques and Instituto Socialista de Pescay Acuicultura. We also thank the local community and artisanal fishermen of Los Roques Archipelago.
Conflicts of interest
We declare no conflict of interest regarding the results published in this work.
References
Baamonde JM. Origen y formación del archipiélago. In: Zamarro J, (ed) Parque Nacional Archipiélago Los Roques. Agencia Española de Cooperación Internacional, Caracas, pp 85-98, 2003. [ Links ]
Barker MJ, Gruber SH, Newman SP, Schluessel V Spatial and ontogenetic variation in growth of nursery-bound juvenile lemon sharks, Negaprion brevirostris: A comparison of two age-assessing techniques. Environmental Biology of Fishes, 72: 343-355, 2005. doi: 10.1007/s10641-004-2584-3 [ Links ]
Bass AJ. Problems in studies of sharks in the Southwest Indian Ocean. In: Hodgson ES, Mathewson RF (eds) Sensory biology of sharks, skates and rays. Office of Naval Research, Department of the Navy, Arlington, VA, USA, pp 545-594, 1978 [ Links ]
Branstetter S. Early life-history implications of selected carcharhinoid and lamnoid sharks of the northwest Atlantic. In: Pratt HL Jr, Gruber SH, Taniuchi T (eds) Elasmobranchs as living resources: advances in biology, ecology, systematics and the status of the fisheries. NOAA Technical Report 90, National Marine Fisheries Service, Silver Spring, MD, pp 17-28, 1990. [ Links ]
Brown CA, Gruber SH. Age assessment of the lemon shark, Negaprion brevirostris, using tetracycline validated vertebral centra. Copeia, 1988: 747-753, 1988. Retrieved from: http://wwwjstor.org/stable/1445397 [ Links ]
Castro JI. The shark nursery of Bull Bay, South Carolina, with a review of the shark nurseries of the southeastern coast of the United States. Environmental Biology of Fishes, 38: 37-48, 1993. doi: 10.1007/BF00842902 [ Links ]
Castro JI. The biology of the blacktip shark, Carcharhinus limbatus, off the southeastern United States. Bulletin of Marine Science, 59: 508-522, 1996. [ Links ]
Compagno LJV Sharks. In: Carpenter K.E. (ed.) The living marine resources of the Western Central Atlantic: Species identification guide for fishery purposes, FAO Special Publication, 5: 358-505, 2002. [ Links ]
Cortés E. A stochastic stage-based population model of the sandbar shark in the western north Atlantic. In: Musick JA (ed.) Life in the slow lane: ecology and conservation of long lived marine animals. American Fisheries Society Symposium 23, Bethesda, M.D, pp 115-136, 1999. [ Links ]
De Angelis BM, McCandless CT, Kohler NE, Recksiek CW, Skomal GB. First characterization of shark nursery habitat in the United States Virgin Islands: evidence of habitat partitioning by two shark species, Marine Ecology Progress Series, 358: 257-271, 2008. doi: 10.3354/meps07308 [ Links ]
DiBattista JD, Feldheim KA, Garant D, Gruber SH, Hendry AP. Anthropogenic disturbance and evolutionary parameters: a lemon shark population experiencing habitat loss. Evolutionary Applications 4: 1-17, 2011. doi: 10.1111/j.1752-4571.2010.00125.x [ Links ]
Dulvy NK, Fowler SL, Musick JA, Cavanagh RD, Kyne PM et al. Extinction risk and conservation of the world's sharks and rays, eLIFE, 3: 1-34, 2014. doi: 10.7554/eLife.00590 [ Links ]
Feldheim KA, Gruber SH, Ashley MV The breeding biology of lemon sharks at a tropical nursery lagoon, Proceedings of Biological Science, 269: 1655-1661, 2002. doi: 10.1098/rspb.2002.2051 [ Links ]
Ferretti F, Worm G, Britten GL, Heithaus MR, Lotze HK. Patterns and ecosystem consequences of shark declines in the ocean, Ecology Letters, 13: 1055-107, 2010. doi: 10.1111/j.1461-0248.2010.01489.x [ Links ]
Franks BR. The spatial ecology and resource selection of juvenile lemon sharks (Negaprion brevirostris) in their primary nursery areas, Doctorate Thesis, Drexel University, USA, 2007. Retrieve from: https://wwwiresearchgate.net/publication/28675852 [ Links ]
Freitas RHA, Rosa RS, Gruber SH, Wetherbee BM. Early growth and juvenile population structure of lemon sharks, Negaprion brevirostris, in the Atol das Rocas Biological Reserve, off north-east Brazil, Journal of Fish Biology, 68: 1319-1332, 2006. doi: 10.1111/j.0022-1112.2006.00999.x [ Links ]
Gaceta Oficial de la República Bolivariana de Venezuela. Resolución mediante la cual se dictan las normas técnicas de ordenamiento para regular la captura, intercambio, distribución, comercio y transporte de tiburones. Caracas, martes 19 de junio de 2012, No. 39.947. 2012. Retrieved from: http://historico.tsj.gob.ve/gaceta/junio/1962012/1962012-3457.pdf [ Links ]
Gallucci VF, Taylor IG, Erzini K. Conservation and management of exploited shark populations based on reproductive value, Canadian Journal of Fishery and Aquatic Science, 63: 931-942, 2006. doi: 10.1139/F05-267 [ Links ]
Garla RC, Chapman DD, Wetherbee BM, Shivji MS. Movement patterns of young Caribbean reef sharks, Carcharhinus perei, at Fernando de Noronha Archipelago, Brazil: the potential of marine protected areas for conservation of a nursery ground, Marine Biology, 149: 189-199, 2006. doi: 10.1007/s00227-005-0201-4 [ Links ]
Garla RC, Garcia J Jr, Veras LB, Lopes NP. Fernando de Noronha as an insular nursery area for lemon sharks, Negaprion brevirostris, and nurse sharks, Ginglymostoma cirratum, in the equatorial western Atlantic Ocean, Marine Biodiversity Records 2: 1-4, 2009. Retrieved from: http://connection.ebscohost.com/c/articles/57425030 [ Links ]
Guttridge TL, Gruber SH, Gledhill KS, Croft DP, Sims DW, Krause J. Social preferences of juvenile lemon sharks, Negaprion brevirostris, Animal Behavior, 78: 543-548, 2009. doi: 10.1016/j.anbehav.2009.06.009 [ Links ]
Guttridge TL, Gruber SH, Franks BR, Kessel ST, Gledhill KS, Uphill J, Krause J, Sims DW Deep danger: intra-specific predation risk influences habitat use and aggregation formation of juvenile lemon sharks Negaprion brevirostris, Marine Ecology Progress Series, 445: 279-291, 2012. doi: 10.3354/meps09423 [ Links ]
Gruber SH. Role of the lemon shark, Negaprion brevirostris (Poey), as a predator in the tropical marine environment: A multidisciplinary study, Florida Scientist, 45: 46-75, 1982. [ Links ]
Gruber SH. Bioenergetics of the captive and free-ranging lemon shark. AAZPA, Annual Conference Proceedings, 340-373, 1984. [ Links ]
Gruber SH, Stout RG. Biological materials for the study of age and growth in a tropical marine elasmobranch, the lemon shark, Negaprion brevirostris (Poey), NOAA Technical Report NMRS, 8: 193-205, 1983. [ Links ]
Gruber SH, Nelson DR, Morrissey JF. Patterns of activity and space utilization of juvenile lemon sharks, Negaprion brevirostris, in a shallow Bahamian lagoon, Bulletin of Marine Science, 43 :61-76, 1988. https://www.researchgate.net/publication/233525742 [ Links ]
Gruber SH, Marignac JRC, Hoenig JM. Survival of juvenile lemon sharks at Bimini, Bahamas, estimated by mark-depletion experiments, Transactions of the American Fishery Society, 130: 376-384, 2001. doi: 10.1577/1548-8659(2001)130<0376:SOJLSA>2.0.CO;2 [ Links ]
Heithaus MR. Nursery areas as essential shark habitats: A theoretical perspective, American Fishery Society Symposium, 50: 3-13, 2007. https://www.researchgate.net/publication/235662090 [ Links ]
Henderson AC, McClellan K, Calosso M. Preliminary assessment of a possible lemon shark nursery in the Turks & Caicos Islands, British West Indies, Caribbean Journal of Science, 46: 29-38, 2010. doi: 10.18475/cjos.v46i1.a5 [ Links ]
Henningsen AD, Gruber SH. Assessment of two lemon shark, Negaprion brevirostris populations, by multiple mark procedures, Florida Scientist, 48: 32, 1985. [ Links ]
Heupel MR, Hueter RE. Importance of prey density in relation to the movement patterns of juvenile blacktip sharks (Carcharhinus limbatus) within a coastal nursery area, Marine and Freshwater Research, 53: 543-550, 2002 [ Links ]
Heupel MR, Simpfendorfer CA, Hueter RE. Estimation of shark home ranges using passive monitoring techniques, Environmental Biology of Fishes, 71: 135-142, 2004. doi: 10.1023/B:EBFI.0000045710.18997.f7 [ Links ]
Heupel MR, Simpfendorfer CA. Using acoustic monitoring to evaluate MPAs for shark nursery areas: the importance of long-term data, Marine Technology Society Journal, 39: 10-18, 2005. [ Links ]
Heupel MR Carlson JK, Simpfendorfer CA. Shark nursery areas: concepts, definition, characterization and assumptions, Marine Ecology Series Progress, 337: 287-297, 2007. doi: 10.3354/meps337287 [ Links ]
Holland KN, Wetherbee BM, Peterson JD, Lowe CG. Movement and distribution of hammerhead shark pups on their natal grounds, Copeia, 2: 495-502, 1993. doi: 10.2307/1447150 [ Links ]
Hueter RE, Manire CA. Bycatch and catch-release mortality of small sharks in Gulf Coast nursery grounds of Tampa Bay and Charlotte Harbor. Mote Marine Technical Report 368 (Final report to NOAA/NMFS MARFIN Project NA 17 FF0378-01), Mote Marine Laboratory, Sarasota, Florida, USA. 1994. [ Links ]
Jennings DE, Gruber SH, Franks BR, Kessel ST, Robertson AL. Effects of large-scale anthropogenic development on juvenile lemon shark (Negaprion brevirostris) populations of Bimini, Bahamas, Environmental Biology of Fishes, 83: 369-377, 2008. [ Links ]
Knip DM, Heupel MR, Simpfendorfer CA. Evaluating marine protected areas for the conservation of tropical coastal sharks, Biological Conservation, 148: 200-209, 2012. doi: 10.1016/j.biocon.2012.01.008 [ Links ]
Lucifora LO, Garcia VB, Worm B. Global diversity hotspots and conservation priorities for sharks, PLoS ONE, 6: 1-7, 2011. doi: 10.1371/journal.pone.0019356 [ Links ]
Manire C, Hull E, Spiller R. Serological changes associated with gillnet capture and restraint in three species of sharks. Transactions of the American Fisheries Society, 130: 1038-1048, 2001. doi: 10.1577/1548-8659(2001)130<1038:SCAWGN>2.0.CO;2 [ Links ]
McKibben JN, Nelson DR. Patterns of movement and grouping of gray reef sharks, Carcharhinus amblyrhynchos, at Enewetak, Marshall Islands, Bulletin of Marine Science, 1: 89-110, 1986. [ Links ]
Morrissey JF, Gruber SH. Home range of juvenile lemon sharks, Negaprion ¡brevirostris, Copeia, 1993: 425-434, 1993a. [ Links ]
Morrissey JF, Gruber SH. Habitat selection by juvenile lemon sharks, Negaprion brevirostris, Environmental Biology of Fishes, 38: 311-319, 1993b. doi: 10.1007/BF00007524 [ Links ]
Murchie KJ, Schwager E, Cooke SJ, Danylchuk AJ, Danylchuk SE et al. Spatial ecology of juvenile lemon sharks (Negaprion brevirostris) in tidal creeks and coastal waters of Eleuthera, The Bahamas, Environmental Biology of Fishes, 89: 95-104, 2010. doi: 10.1007/s10641-010-9693-y [ Links ]
Newman SP, Handy RD, Gruber SH. Diet and prey preference of juvenile lemon sharks, Negaprion brevirostris, Marine Ecology Progress Series, 398: 221-234, 2010. doi: 10.3354/meps08334 [ Links ]
Oliveira PGV, de Oliveira DS, Pinheiro PB, Hazin FH, Carvalho FC, Veras DP, Silva MB. Population structure and growth of young lemon shark, Negaprion brevirostris (Poey, 1868), at the Atol das Rocas Biological Reserve, Brazil, Journal of Integrated Costal Zone Management, 11: 389-395, 2011. [ Links ]
Reyier EA, Adams DH, Lowers RH. First evidence of a high density nursery ground for the lemon shark, Negaprion brevirostris, near Cape Canaveral, Florida, Florida Scientist, 71: 134-148, 2008. [ Links ]
Simpfendorfer CA. Demographic analysis of the dusky shark fishery in southwestern Australia. In: Musick JA (ed.) Life in the slow lane: ecology and conservation of long lived marine animals. American Fisheries Society Symposium 23, Bethesda, M.D., pp 149-160, 1999. [ Links ]
Simpfendorfer CA, Milward NE. Utilization of a tropical bay as a nursery area by sharks of the families Carcharhinidae and Sphyrinidae, Environmental Biology of Fishes, 37: 337-345, 1993. [ Links ]
Springer S. Social organization of shark populations. In: Gilbert PW, Matheson RF, Rall D (eds) Sharks, skates and rays. John Hopkins Press, Baltimore, pp 149-174, 1967. [ Links ]
Sundström LF, Gruber SH, Clermont SM, Correia JPS, de Marignac JRC et al. Review of elasmobranch behavioral studies using ultrasonic telemetry with special reference to the lemon shark, Negaprion brevirostris, around Bimini Islands, Bahamas, Environmental Biology of Fishes, 60: 225-250, 2001. doi: 10.1023/A:1007657505099 [ Links ]
Tavares R. Abundance and distribution of sharks in Los Roques Archipelago National Park and other Venezuelan oceanic islands, 1997-1998, Ciencias Marinas, 31: 441-454, 2005. [ Links ]
Tavares R. Occurrence, diet and growth of juvenile blacktip sharks, Carcharhinus limbatus, from Los Roques Archipelago National Park, Venezuela, Caribbean Journal of Science, 44: 291-302, 2008. [ Links ]
Tavares R. Análisis de la abundancia, distribución y tallas de tiburones capturados por la pesca artesanal en el Parque Nacional Archipiélago Los Roques, Venezuela, Interciencia, 34: 463-470, 2009. [ Links ]
Tavares R Sánchez L. Áreas de cría de tiburones en el Golfo de Venezuela, Ciencia, 20: 116-124, 2012. [ Links ]
Tavares R. Preliminary results from tag-recapture procedures applied to lemon sharks, Negaprion brevirostris (Poey 1868), at Los Roques Archipelago, Venezuela. Gulf and Caribbean Fisheries Institute, 62: 450-454, 2010. [ Links ]
Werner EE, Gilliam JF, Hall DJ, Mittelbach GG. An experimental test of the effects of predation risk on habitat use in fish, Ecology, 64: 1540-1548, 1983. http://www.jstor.org/stable/1937508 [ Links ]
Wetherbee BM, Gruber SH, Rosa S. Movement patterns of juvenile lemon sharks within Atol das Rocas, Brazil: a nursery characterized by tidal extremes, Marine Ecology Progress Series, 343: 283-293, 2007. doi: 10.3354/meps06920 [ Links ]
Yokota L, Lessa RP. A nursery area for sharks and rays in Northeastern Brazil, Environmental Biology of Fishes, 75: 349-360, 2006. doi: 10.1007/s10641-006-0038-9 [ Links ]
Zar J. Biostatistical analysis. Prentice Hall, New Jersey, USA. 1996. [ Links ]














