Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Universitas Scientiarum
versión impresa ISSN 0122-7483
Univ. Sci. vol.21 no.1 Bogotá ene./abr. 2016
https://doi.org/10.11144/Javeriana.SC21-1.eaol
Ecological aspects of Lebiasina erythrinoides (Characiformes: Lebiasinidae) from an andean piedmont stream in Colombia
Aspectos ecológicos de Lebiasina erythrinoides (Characiformes: Lebiasinidae) de una quebrada del piedemonte andino en Colombia
Aspetos ecológicos de Lebiasina erythrinoides (Characiformes: Lebiasinidae) de um riacho do piedemonte andino na Colômbia
Alexander Urbano-Bonilla1, Jhon Zamudio1, Javier Alejandro Maldonado-Ocampo1*
Edited by Juan Carlos Salcedo-Reyes (salcedo.juan@javeriana.edu.co)
1Laboratorio de Ictiología, Unidad de Ecología y Sistemática (UNESIS), Departamento de Biología, Facultad de Ciencias, Pontificia Universidad Javeriana, Carrera 7 N° 43-82, Bogotá D.C., Colombia.
*maldonadoj@javeriana.edu.co
Alexander Urbano-Bonilla
Is a Biologist from the UNITROPICO. He obtained his Masters in Biological Sciences at the Pontificia Universidad Javeriana. Currently he is a researcher at the Alexander von Humboldt Institute and the Laboratory of ichthyology at the Universidad Javeriana. His research interests are focused on ecology, taxonomy, systematics and biogeography of freshwater fishes. Recently in the assessment and monitoring of aquatic ecosystems, he has performed studies in biotic integrity index for piedmont rivers in the Colombian Orinoco basin. .
Jhon Zamudio
Is a Biologist from the UNITROPICO. Currently is involved as a master student of the Conservation and Use of Biodiversity program at the Pontificia Universidad Javeriana. His research focus on ecology, taxonomy and biogeo-graphy and it has especially developed in the Colombian llanos ecosystems (Orinoco basin), a region with high leves of richness and endemism of fish. Actually is associate researcher at the Laboratory of Ichthyology, Departamento de Biolog'a, Pontificia Universidad Javeriana and at the Fundación Reserva Natural La Palmita in Bogotá.
Javier A. Maldonado-Ocampo
Is an evolutionary biologist. Much of his work is highly collaborative, his research program is broad, combining systematics, taxonomy and ecology to explore basic questions of biodiversity relate to patterns of diversity, distribution and biogeography. My preferred study organisms are freshwater fishes of the Neotropical region, especially from northwest South America. Also his have been extensively involved in collection-based research and curation.
Funding: N/A
Electronic supplementary material: N/A
Received: 11-02-2015 Accepted: 12-02-2016 Published on line: 11-03-2016
Para citar este artículo / To cite this article
Urbano-Bonilla A, Zamudio J, Maldonado-Ocampo JA. Ecological Aspects of Lebiasina erythrinoides (Characiformes: Lebiasinidae) from an Andean piedmont stream in Colombia, Universitas Scientiarum, 21 (1): 83-97, 2016. doi: http://dx.doi.org/10.11144/Javeriana.SC21-1.eaol
Abstract
The present study describes ecological aspects of Lebiasina erythrinoides; 200 individuals were sampled throughout an annual hydrological cycle from October 2008 to September 2009 in La Calaboza stream, a Piedmont tributary of the Rio Cravo Sur. The studied population had more females than males (1.5:1) and preferred habitats dominated by shoreline vegetation and rocky substrates. Prior to reproductive events, individuals presented an increase in the condition factor (K) and the gonosomatic index (GSI). On average, 648.8 oocytes are discharged twice a year: at the beginning of the rainy season, and during falling water phase. The mean sizes at sexual maturity found for females were 73.5 mm standard length (SL) and for males 70 mm SL. Rounding up, an average minimum size of capture of 75 mm SL is proposed. Most specimens of the population (65 %) were found to be maturing or mature (stages II and III), 28.5 % immature (stage I), 5.0 % in post reproduction stage V, and 1.5 % in reproductive phase (IV). Results suggest this species is omnivorous with a preference for invertebrates (IRI = 41.2 %) and vegetal material (IRI = 27.8 %), but also includes a variety of other items. The values of the diet are correlated with hydrological cycle and size.
Keywords: Lebiasininae; diet; reproduction; hydrological cycle; Orinoco Basin.
Resumen
El presente estudio describe aspectos ecológicos de Lebiasina erythrinoides. Se muestrearon 200 individuos a lo largo del ciclo hidrológico anual, de Octubre de 2008 a Septiembre de 2009, en la quebrada La Calaboza, un tributario de piedemonte del Río Cravo Sur. La población estudiada presentó más hembras que machos (1.5:1) y prefirió hábitats dominados por vegetación riparia y sustratos rocosos. Antes de los eventos reproductivos, los individuos presentaron un incremento en el factor de condición (K) y en el índice gonadosomático (GSI). En promedio, se descargaron 648.8 oocitos dos veces en el año: al inicio de la estación lluviosa y durante la fase de descenso de las aguas. Los tamaños promedio de las hembras en madurez sexual fueron de 73.5 mm de longitud estándar (SL), y de los machos, 70 mm SL. De acuerdo con estas cifras, se propone un tamaño mínimo de captura de 75 mm SL. La mayor parte de especímenes de la población (65 %) se encontró en proceso de maduración o de madurez (estadios II y III), 28.5 % en estado inmaduro (estadio I), 5.0 % en estado post-reproductivo (V) y 1.5 % en fase reproductiva (V). Los resultados sugieren que esta especie es omnívora: prefiere invertebrados (IRI = 41.2 %) y material vegetal (IRI = 27.8 %), pero su dieta incluye también variedad de otros ítems. Los valores de la dieta se correlacionan con el ciclo hidrológico y con el tamaño.
Palabras clave: Lebiasininae; dieta; reproducción; ciclo hidrológico; cuenca del río Orinoco.
Resumo
O presente estudo descreve aspectos ecológicos de Lebiasina erythrinoides. Foram coletados 200 indivíduos ao longo de um ciclo hidrológico anual, entre Outubro de 2008 e Setembro de 2009 no riacho La Calaboza, um afluente do rio Cravo Sur. A populacho estudada possuía mais fèmeas que machos (1.5:1) e preferiam habitats dominados por vegetalo costeira e substratos rochosos. Prèvio aos eventos reprodutivos, os individuos presentaram um aumento do fator de condicio (K) e do índice gonadosomatico (GSI). Em mèdia, 648.8 ovócitos são desovados duas vezes ao ano: no inicio do período chuvoso, e durante a descida das águas. O tamanho mèdio encontrado para as fèmeas na maturidade sexual foi de 73.5 mm comprimento padrão (SL), e para os machos foi de 70 mm SL. Considerando os valores encontrados, se propoe que o tamanho mínimo de captura seja de 75 mm SL. A maioria dos espècimes da populacho estudada (65 %) se encontrava nos estágios de maturação ou maduros (Estágios II e III), 28.5 % de imaturos (I), 5.0 % em estágio pós-reprodução (V) e 1.5 % em fase reprodutiva (IV). Os resultados sugerem que a espécie é omnívora, com uma preferencia por invertebrados (IRI = 41.2 %) e material vegetal (IRI = 27.8 %), mas também se inclui uma variedade de outros itens. Os valores da dieta estão correlacionados com o ciclo hidrológico e tamanho.
Palavras-chave: Lebiasininae; dieta; reprodução, ciclo hidrológico, bacia do rio Orinoco.
Introduction
Species of the genus Lebiasina belong to the subfamily Lebiasininae (Netto-Ferreira et al. 2011), and are found widely distributed in drainages of Central America (Costa Rica, Panama), northern South America (Colombia, Ecuador, Venezuela) and the Orinoco and Amazon River Basins. Currently, 27 nominal species have been described in this genus (Netto-Ferreira 2012, Netto-Ferreira et al. 2013). Ten have been found in Colombia, in streams of the Caribbean, Pacific, Magdalena-Cauca, Amazonas and Orinoco hydrographic zones (Maldonado-Ocampo et al. 2008).
Some of the Lebiasina species found in Colombia prefer small stream habitats with clear water and abundant shore vegetation that provides them food and shelter from predators (Ardila-Rodríguez 2004, 2008a, 2008b, Román-Valencia & Vélez 1986, Román-Valencia 1996, 2004). Lebiasina erythrinoides (Valenciennes 1850) is the only species of the genus found in Andean piedmont streams of the Orinoco River Basin in Colombia (Maldonado-Ocampo et al. 2008). This species is abundant in most of the drainages of the piedmont region of Colombia's Casanare department (Urbano-Bonilla et al. 2009). Aspects of the biology of L erythrinoides are known mostly from previous studies in Venezuela (Machado-Allison 1974, Taphorn & Lilyestrom 1980, Sette 1991, 1993). Whereas in Colombia, only preliminary information about its geographic distribution and habitat characterization is available (Maldonado-Ocampo et al. 2005).
The piedmont region in the Colombian Orinoco, where L. erythrinoides and around 465 freshwater fish species are distributed, has been characterized in recent years by a landscape transformation (terrestrial and aquatic ecosystems); mainly due to deforestation, cattle, mining, material extraction along the river banks, and oil production (Machado-Allison et al. 2010, Urbano-Bonilla et al. 2014). To contrast this trend in the transformation along this region, new protected areas focusing on aquatic ecosystem have been proposed (Machado-Allison et al. 2010). Nonetheless, this strategy has to be associated with a better knowledge of the life history of the fish distributed there. Without this information, monitoring how the new propose conservation areas are helping to preserve ecosystem functioning and biodiversity will be difficult. Herein the length/weight ratio, mean size at sexual maturity, condition factor K, gonadal development, gonosomatic index and fecundity, diet and feeding habits of L. erythrinoides are described.
Materials and methods
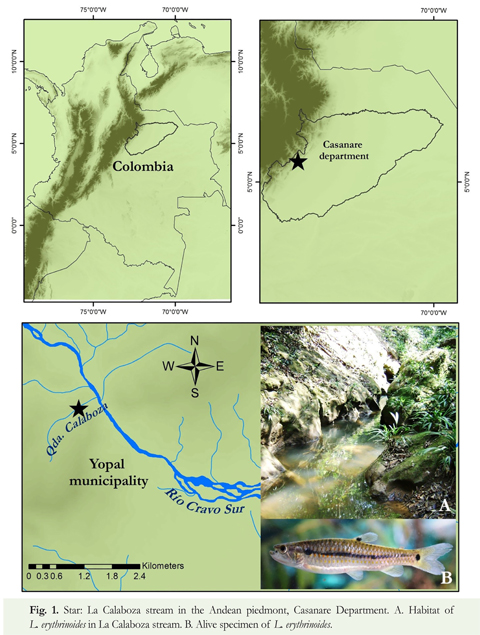
Study Area: The Andean piedmont in Casanare Department extends along the foothills of the eastern branch of the Andes from 06° to 04° north latitude and 73° to 71° west longitude, between 350 and 1100 masl (Romero et al. 2004). This study was done in La Calaboza stream, a tributary of the Rio Cravo Sur, in the piedmont of the Yopal municipality, Casanare Department, Colombia, located at 5° 21' 9" North, 72° 25' 12" West. The average monthly precipitation for the region (1981 - 2010) is 199.2 mm with a maximum of 360.1 mm in May and a minimum of only 9.6 mm in January, with a total annual of 2 390.1 mm (IDEAM 2010).
Sampling: Monthly sampling in a 500 m length stretch was performed from October 2008 to September 2009 in effort to include the entire annual hydrological cycle. Time collecting effort in all sampling events took two hours; using a 3 m x 1.3 m seine with 3 mm mesh, and hook and line (hooks #2 and #3). All the individuals captured in each sample were collected and fixed. Once collected, the fish were anesthetized and slaughtered in a benzocaine solution and later fixed in 10 % formalin for examination in the lab. All specimens were deposited in the Reference Freshwater Fish Collection of the Department of Biology, Pontificia Universidad Javeriana, Bogotá, Colombia (MPUJ). During each collecting event, physicochemical parameters (temperature, conductivity, pH, dissolved oxygen using an Oakton-waterproof multiparameter meter) were recorded at one specific point along the 500 m stretch. Percentage substrate were estimated along the stretch transect, and riparian vegetation was characterized.
Data analysis: Ten size classes in 15 mm standard length (SL) intervals were established to estimate size class frequency (Sturges 1926). To calculate the length/ weight ratio, we recorded each fish's weight in grams and the SL in millimeters. The length/weight ratio permits the determination of the increase rate of weight with respect to length or vice versa, and can be used as an indirect way to analyze the growth rate of a population of fishes. It is expressed by equation 1 (Ricker 1971):
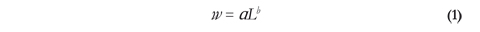
Where weight (W) is proportional to a certain power (b) of length (L), and b is an exponent with a value from 2 to 4; values close to 3 indicated isometric growth (Ricker 1971).
The condition factor was calculated with equation 2:
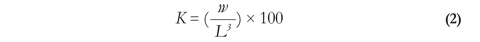
Where K is the condition factor, W is total weight of fish in grams and L is standard length in cm.
The condition factor estimates the nutritional state of the individual at a given moment during its development and records the environmental pressure exerted upon life conditions as related to sex and age of the fish (Vazzoler 1996), depending on the variations of growth patterns expressed along the three axes of the body (Laevastu 198°).
Proportion of the sexes was calculated with equation 3 (Vazzoler 1996):
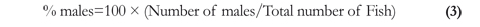
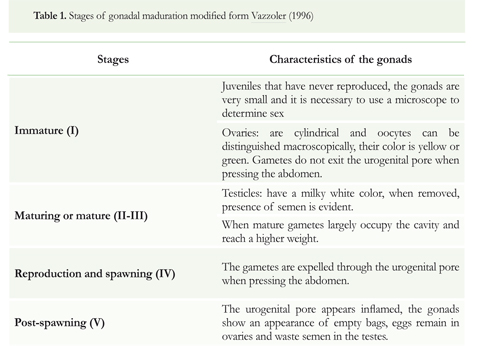
Sex ratio was determined using a Chi-square (X2) test (Sokal & Rohlf 1995). Software IBM SPSS 2° (Windows) was used for statistical processing. Maturation states of the gonads were determined by macroscopic inspection using a modified version of the scale proposed by Vazzoler (1996, Table 1). To determine the mean size at sexual maturity, the distribution of accumulated frequencies for mature fish was used following Vazzoler (1996).
The gonosomatic index was calculated using equation 4 (Vazzoler 1996):
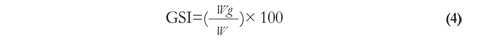
Where Wg is the weight of the gonad, and W is the total weight of the fish in grams.
Standard Deviation (SD) was calculated. The number of mature eggs was determined for individuals in stage IV. The gonads were preserved in 70 % alcohol for posterior analysis. To separate eggs from adjacent ovarian tissue, the ovaries were treated with a modified Gibson solution: 100 ml alcohol (60 %), 880 ml water, 15 ml nitric acid (80 %), 18 ml glacial acetic acid, and 70 mg mercury chloride. The number of eggs per stage IV was calculated by taking the weight of the dry gonad, removing a weighed subsample of the gonad to count the eggs, and then extrapolating the total (Cala 1971).
Stomach contents were examined under a stereoscope and items were separated, identified and grouped into five categories: 1) bird parts (feathers), 2) fish or fish parts, 3) detritus, 4) vegetal material and 5) invertebrates (Bouchard 2004, Wolff 2006). To estimate the importance of food categories, the Index of Relative Importance was calculated using equation 5 (Yáñez-Arancibia et al. 1985):
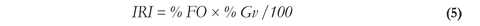
Where (% FO) is the frequency of occurrence of each food item in the total number of stomachs examined and (% Gv) is the weight percentage of each food item in the total weight of stomachs examined. Each food category was weighed using an analytical balance (Adventurer) with 0.0001 g precision. The coefficient of emptiness was evaluated (Number of Empty Stomachs/Total Number of Stomachs Analyzed * 100) (Windell 1971). All the measurements and analysis were performed after each collecting event at the laboratories of the Fundación Universitaria Internacional del Trópico Americano - UNITROPICO.
To verify compliance with the assumptions of normality, a Shapiro-Wilk test was performed (Ghasemi & Zahediasl 2012). Then, a Kruskal-Wallis test (H) was performed in order to evaluate monthly differences in dietary values and sex. In addition we estimated a linear model between standard length and diet. The software R (R Core Team 2014), along with the packages Rfit (Kloke & McKean 2012), rand-tests (Caeiro & Mateus 2014), and extreme values (Van der Loo 2010) were used for statistical analyses.
Results
Average water temperature in La Calaboza stream was 22 oC (SD : 0.8), conductivity was 27.2 uS/cm (SD : 30.7), pH 7.2 (SD : 0.3), and dissolved oxygen 1.25 mg/L (SD : 0.22). The current was moderate, water crystalline with substrate dominated by rocks (70 %), rolling stones (15 %), gravel, sand, and clay with 5 % each. Depth varied from 0.5-1.5 m, and width from 1-3 m. Vegetation associated with this type of water were rheophilic bryophytes and riparian plants such as Cyclanthaceae, Zyngiberaceae, Clusiaceae, Mimosaceae (Ingd), Arecaceae, Melastomataceae, and Rubiaceae. Lebiasina erythrinoides shares this habitat with species of the family Trichomycteridae (Trichomycterus knerii), Characidae (Creagrutus bolivari, C. melasma, C. sp., Hemibrycon metae), Loricariidae (Ancistrus triradiatus, Lasiancistrus tentaculatus, Chaetostoma dorsale), and Poeciliidae (Poecilia sp.).
A total of 200 specimens of L erythrinoides were collected along the hydrological cycle with standard length ranging from 20 to 160 mm (mean 76.4 mm SL; SD : 2.8). Females were more abundant, with 105 (52.5 %) individuals, whereas males corresponded to 34 % of the sample (68 individuals); the 27 remaining specimens were undetermined. The majority of the specimens pertain to the 70-85 mm SL class.
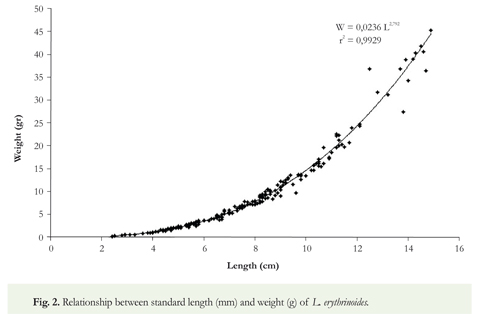
The length/weight ratio of L. erythrinoides was W = 0.0236 L2,79 where r = 0.992 for all individuals of both sexes. The value for b (2.79) indicates a nearly isometric growth (Figure 2). The lowest values of the condition factor (K) for males were April and June, and for females August. The highest values for both sexes were in February. When analyzed separately, males showed greater values of the condition factor (1.58) than females (1.53) throughout the year (Table 2).
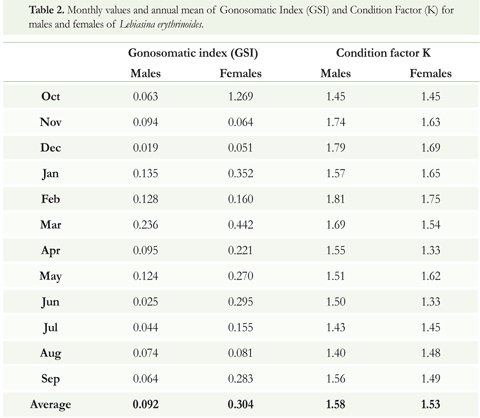
In this population of L erythrinoides, both immature (stage I) males and the females were in the same size of sexual maturity range of 24-56 mm SL.; for specimens in stages II and III, both sexes fell into the 57-147 mm SL class; for reproductively mature and post-spawning individuals (IV and V) of both sexes the SL was between 9°-149 mm. The distribution of accumulated frequencies for mature individuals showed that for males the mean size is 7° mm SL and for females 73.5 mm SL; whereas for both sexes combined the mean size of sexual maturity is 71 mm SL (Figure 3).
The sex ratio was 1:1.5 (68 males - 34 % vs. 105 females - 52.5 % / Chi-square test, X2 = 7.91, P = 0.005), indicating a population structure dominated by females during the entire annual hydrological cycle. Among captured specimens, 27 (13.5 %) did not have well differentiated gonads, and were thus classified as "undetermined."
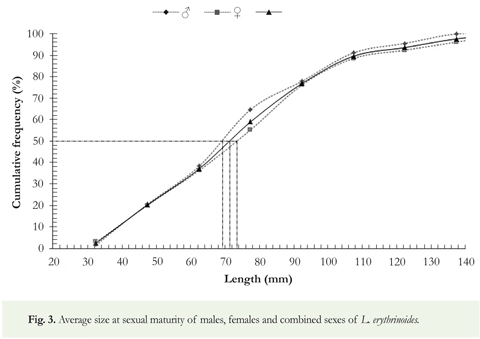
Most individuals of this population pertain to the stages II-III (65 %). This indicates a clear predominance of maturing or mature individuals. Stages I (28.5 %), IV (1.5 %) and V (5.° %) presented the lowest frequency values suggesting periodic reproduction coinciding with the beginning of rain (March-April) and falling water phase (September-October). Individuals of both sexes had mature gonads during those seasons (Figure 4).
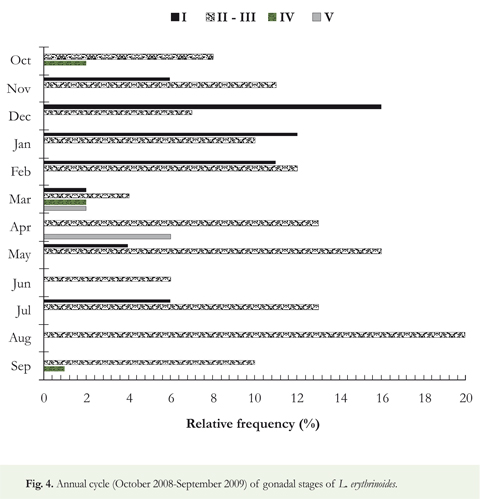
In general terms, the females showed a higher and more variable GSI (annual mean = 0.304) than the males (annual mean = 0.092). However, peaks in GSI were seen during the months of March (°.442) and October (1.269) (Table 2). For the L erythrinoides females analyzed, the counts revealed an average fecundity of 929.3 oocytes (SD : 732.4) during September-October and 399 oocytes (SD : 246.9) during March-April, for an overall annual mean of 648.8 oocytes (SD : 617.4).
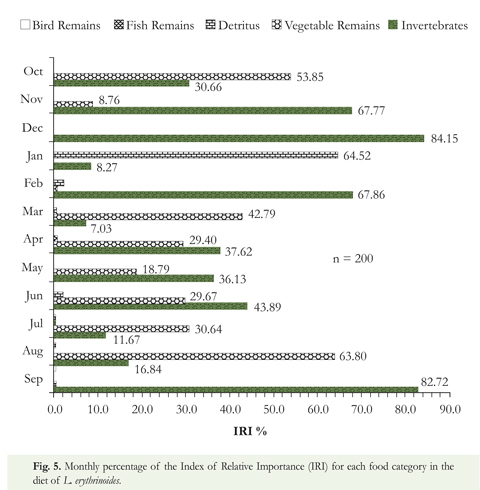
Of the 200 stomachs examined, 35 were empty (17.5 %). For each of the defined categories, the following items were found: a) bird remains: feathers; b) fish remains: scales and other body parts; c) detritus: sand, clay and loam particles; d) vegetal material: seeds and flowers from the families Mimosaceae (Inga spp.) and Clusiaceae, as well as roots and other elements that could not be identified because of the high degree of fragmentation; e) invertebrates: Araneae, Coleoptera (families Dytiscidae, Elmidae, Hydrophilidae and Staphylinidae), Ephemeroptera, Hemiptera (family Corixidae), Hymenoptera (family Formicidae, Atta spp., and species of Apoidea and Vespoidea), Mantodea, Plecoptera, Trichoptera, Odonata and Orthoptera (family Gryllotalpidae); as well as larvae of Diptera, Coleoptera, Lepidoptera and one species of leech (Hirudinea), and others of uncertain identification. Although this fish consumed a wide variety of food items, invertebrates are the most utilized food resource (IRI = 41.22 %) followed by vegetal material (IRI = 27.88 %), detritus (IRI = 11.64 %), bird remains (IRI = 0.23 %), and fish remains (IRI = 0.03 %) (Figure 5). Differences statistically significant in the values of the diet as a function of sizes and monthly variation were found (Kruskal-Wallis chi-squared = 42.8789, df = 11, p-value = 1.14e-05) [(Robust linear fit, intercept -0.00612928, slope 0.00165854, Multiple R-squared (Robust): 0.199417, p-value << No differences were found as a function of sexes (Kruskal-Wallis chi-squared = 0.0024, df = 1, p-value = 0.9613).
Discussion
This population of L. erythrinoides were observed in a habitat with abundant shoreline vegetation, and rocky substrates with little gravel, sand, or clay. This same type of habitat has been recorded for other species of Lebiasina present in the basins of Orinoco (Ardila-Rodríguez 2004, Netto-Ferreira et al. 2011), Magdalena-Cauca (Ardila-Rodríguez 2008a, Román-Valencia 1996), Sinú (Ardila-Rodríguez 2008b), San Juan (Román-Valencia & Vélez 1986) and Mazaruni river basin (Netto-Ferreira et al. 2013).
The length/weight ratio for both sexes was W = 0,0236 L2.79 (r2 = 0.992). The value for b (2.79) indicates a nearly isometric growth where individuals of L. erythrinoides gain weight in proportion to a corresponding increase in length (Figure 2). According to Lowe-McConell (1975), several studies of tropical fishes have demonstrated that when length and weight are highly correlated, the population is in good condition, and sexual maturity may be retarded. When the opposite is true, sexual maturity occurs at smaller sizes.
Lebiasina erythrinoides showed high values for the condition factor (K) for females (Table 2) prior to spawning indicating that they can ingest sufficient high-quality food (aquatic insects) in those periods (Figure 5). The rapid decline in condition factor coincides with reproduction expressed in the highest values of gonosomatic index for females in March and October (Table 2), suggesting that L erythrinoides reproduces twice per year and are closely related to the monomodal hydroclimate cycle of the Orinoco River Basin (with dry and rainy seasons) and r2 life history strategy (Winemiller & Taphorn 1989; Taphorn 2003). Variations in K could reflect gonad maturation or increased feeding intensity (Wootton 1990) meaning K values can be used as markers signaling reproduction of the species.
Although the majority of ecological life history studies of freshwater fish in Colombia are restricted to species of commercial importance (Maldonado-Ocampo & Usma-Oviedo 2006), description of aspects such as the minimum and average size at sexual maturity in species like L erythrinoides (Figure 3) are of ecological interest since it permits the establishment of minimum permissible sizes for harvest. That information ensures that individuals can be able to reproduce at least once before being extracted from the population. Given the mean size of sexual maturity, 71 mm SL for both sexes, a minimum capture size of 75 mm SL is proposed to guarantee a sustainable harvest of this species. Individuals smaller than the minimum capture size proposed herein are caught for local consumption, and juveniles as small as 7 mm SL are caught and sold as ornamental fishes. The number of individuals harvested for these purposes are unknown.
The population structure of L. erythrinoides observed in the piedmont streams of Casanare where sexually maturing or mature individuals seem to prevail (most of the individuals captured belonged to II-III stages) contrasts with that observed by Sierra & Pefaur (1998) who indicated a dominance of young individuals in the Venezuelan Piedmont Andes. Taphorn (2003) attributes such structure to a late sexual maturity (12 months) of the specimens in the Rio Apure. It has been reported that adult females of L erythrinoides can spawn up to 2700 eggs during the first or second month of the rainy season (Taphorn 2003) and that they may spawn twice per season (Winemiller & Taphorn 1989). A spawning average of 648.8 oocytes was observed during two seasons: at the beginning of the rainy season (March-April) and during descending waters (September-October) (Figure 4). During those seasons, the high values of the GSI reaffirm the advanced stage of gonadal development (Table 2). Differences in the reproductive aspects of L erythrinoides reported by Winemiller & Taphorn (1989) and Taphorn (2003) and our results could be due to differences between biotic (such as availability of food or microhabitat) or abiotic (such as temperature, dissolved oxygen, flow regime) variables present along the different rivers on the Andean Piedmont, that could affect the reproductive strategy of the species.
As presented in this study for L. erythrinoides, most Lebiasina species have similar omnivorous diets (Ardila-Rodríguez 2004, Román-Valencia & Vélez 1986, Román-Valencia 1996, 2004, Maldonado-Ocampo et al. 2005), whereas in Lebiasina Colombia only vegetal material has been reported (Ardila-Rodríguez 2008b). For L. erythrinoides, the strong dominance of invertebrates (IRI = 41.2 %) and vegetal materials (IRI = 27.8 %) observed were maintained throughout the hydrological cycle. Although these two categories present small variations, they are compensated by others items of less importance (Figure 5). In general terms, this species has an omnivorous diet with a preference for both aquatic and terrestrial insects, similar to that reported by Taphorn (2003) and Rodríguez-Olarte et al. (2007).
Statistical differences occurred in the values of the diet of L erythrinoides along the time span of this study. Seasonal changes in the diet of Neotropical freshwater fish have been reported. Such shifts seem to be caused by hydrological changes in streams, influencing the presence, abundance, and availability of food (Lowe-McConnell 1987, Winemiller et al. 2008). Similarly, statistical differences among values of the diet in relation to the sizes were found. Differences on food items consumed by fish along the species ontogeny (from larvae to adults), as presented in L. erythrinoides, has been reported for several Neotropical freshwater fish (Winemiller et al. 2008).
Conclusion
The rivers that flow along the Piedmont area of the Orinoco Basin in Colombia present particular biotic and abiotic conditions that provide specific habitat conditions to the species distributed there. L. erythrinoides takes advantage of these particular conditions, consuming food items from the surrounding riparian forest, and timing their life history cycle with the different food availability and favorable spawning conditions that coincide with the different seasons of the annual hydrological cycle. Habitat conservation for the entire fish assemblage, not just L erythrinoides, is vital due to the increase of anthropogenic impacts such as cattle, agriculture, mining, and the resulting deforestation and water pollution that produce detrimental changes on this particular landscape. Increasing our knowledge about the life history of fish species in this area is an important step to generate information that can provide data to formulate better conservation strategies that take into consideration the link between the terrestrial and aquatic ecosystems.
Acknowledgments
The authors express their thanks to Carlos Ardila Rodríguez, Gilberto Cortés Millán, and Gustavo A. Ballen for their collaboration with donation of literature. Biologists Alfredo Niño and Vicente Javier Preciado helped us with data processing and lab work. Thanks, also, to Dr. Donald Taphorn for English revision and corrections. We are also grateful to the Fundación Universitaria Internacional del Trópico Americano UNITROPICO, for the use of equipment, materials and labs.
Conflict of interest
This work does not present any conflicts of interest.
References
Ardila-Rodríguez CA. Lebiasina taphorni (Pisces: Characiformes: Lebiasinidae), una nueva especie, Dahlia (Revista Asociación Colombiana de Ictiólogos), 7: 57-65, 2004. [ Links ]
Ardila-Rodríguez CA. Lebiasina ortegai (Characiformes: Lebiasinidae), nueva especie, sistema río Cauca, Colombia, Dahlia (Revista Asociación Colombiana de Ictiólogos), 10: 17-25, 2008a. [ Links ]
Ardila-Rodríguez CA. Lebiasina Colombia (Characiformes: Lebiasinidae), nueva especie de la Cuenca Alta río Sinú. Colombia, Dahlia (Revista Asociación Colombiana de Ictiólogos), 10: 27-32, 2008b. [ Links ]
Bouchard RW Jr. Guide to aquatic invertebrates of the upper Midwest: Identification Manual for Students, Citizen Monitors, and Aquatic Resource Professionals. Water Resources Center, University of Minnesota, St. Paul, MN, 2004. [ Links ]
Caeiro F, Mateus A. Randtests: Testing randomness in R. R package version 1.0. URL: http://CRAN.R-project.org/package=randtests, 2014. [ Links ]
Cala P. Size and age at maturity, ripening and fecundity of the ide Idus idus (L.). Reports of the Institute of Fresh-water Research, Drottningholm 51: 31-46, 1971. [ Links ]
Ghasemi A, Zahediasl S. Normality tests for statistical analysis: a guide for non-statisticians. International journal of endocrinology and metabolism 10: 486-489, 2012. doi: 10.5812/ijem.3505 [ Links ]
IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp. [ Links ]
IDEAM - Instituto de Estudios Ambientales de Colombia. Estación Meteorológica (3521501)-Aeropuerto El Alcaraván, Yopal-Casanare. 2010. http://www.ideam.gov.co/web/tiempo-y-clima/clima [ Links ]
Kloke JD, Mckean JW Rfit: Rank-based estimation for linear models, The R Journal, 4, 57-64, 2012. [ Links ]
Laevastu T. Manual de métodos de Biología Pesquera. Ed Acribia Zaragoza, España. 1980. [ Links ]
Lowe-McConell R. Fish communities in tropical freshwater. Longman publishing, New York, USA, 1975. [ Links ]
Lowe-McConnell RH, Ecological studies in tropical fish communities. Cambridge University Press, Cambridge, UK, 1987. [ Links ]
Machado-Allison A. Etapas del desarrollo del pez Piabucina pleurotaenia Regan, 1903 (Characiformes Lebiasinidae), Acta Biológica Venezuelica, 8: 579-622, 1974. [ Links ]
Machado-Allison A, Lasso C, Usma JS, Sánchez-Duarte P, Lasso-Alcalá O. Peces. In: Lasso CA, Usma JS, Trujillo F, Rial A (eds) Biodiversidad de la cuenca del Orinoco: bases científicas para la identificación de áreas prioritarias para la conservación y uso sostenible de la biodiversidad. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, WWF Colombia, Fundación Omacha, Fundación La Salle e Instituto de Estudios de la Orinoquia (Universidad Nacional de Colombia). Bogotá D.C., Colombia, pp 217-257, 2010. [ Links ]
Maldonado-Ocampo JA, Ortega-Lara A, Usma JS, Galvis G, Villa-Navarro FA et al. Peces de los Andes de Colombia. Instituto de Investigación de Recursos Biológicos "Alexander von Humboldt", Bogotá D.C., Colombia, 2005. [ Links ]
Maldonado-Ocampo JA, Usma-Oviedo JS. Estado del conocimiento sobre peces dulceacuícolas en Colombia. In: Chaves ME, Santamaría M (Ed) Informe sobre el avance en el conocimiento y la información de la biodiversidad 1998-2004. Instituto de Investigación de Recursos Biológicos "Alexander von Humboldt" Tomo II, Bogotá D.C., Colombia, pp 174-194, 2006. [ Links ]
Maldonado-Ocampo JA, Vari R, Usma JS. Checklist of the Freshwater Fishes of Colombia, Biota Colombiana, 9: 143-237, 2008. [ Links ]
Netto-Ferreira AL. Oyakawa OT, Zuanon J, Nolasco JC. Lebiasinayepezi, a new Lebiasininae (Characiformes: Lebiasinidae) from the Serra Parima-Tapirapecó mountains, Neotropical Ichthyology, 9: 767-775, 2011. doi: 10.1590/S1679-62252011000400008 [ Links ]
Netto-Ferreira AL. Three new species of Lebiasina (Characiformes: Lebiasinidae) from the Brazilian Shield border at Serra do Cachimbo, Pará, Brazil. Neotropical Ichthyology 10: 487-498, 2012. doi: 10.1590/S1679-62252012000300002 [ Links ]
Netto-Ferreira AL, Lopez-Fernández H, Taphorn D, Liverpool AE. New species of Lebiasina (Ostariophysi: Characiformes: Lebiasinidae) from the upper Mazaruni River drainage, Guyana, Zootaxa, 3652: 562-568, 2013. doi: 10.11646/zootaxa.3652.5.5 [ Links ]
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2014. [ Links ]
Ricker W Methods for assessment of fish production in freshwater. Blackwell Cientific Publications, Oxford, UK, 1971 [ Links ]
Rodríguez-Olarte D, Coronel J, Amaro A, Taphorn D. Línea base para la estimación de la integridad en comunidades de peces en la cuenca del río Tocuyo, vertiente del Caribe, Venezuela, Memorias de la Fundación La Salle de Ciencias Naturales, 165: 63-81, 2007. [ Links ]
Román-Valencia C. Historia natural del Rollizo Piabucina sp. (Pisces: Lebiasinidae) en la cuenca del Río la Vieja, alto Cauca, Colombia, Revista de Biología Tropical, 45: 1255-1256, 1996. [ Links ]
Román-Valencia C. Sobre la bioecología de Lebiasina panamensis (Pisces: Lebiasinidae) en la Cuenca del río León, Caribe Colombiano, Dahlia (Revista Asociación Colombiana de Ictiólogos), 7: 33-35, 2004. [ Links ]
Román-Valencia C, Vélez MC. Estudio sobre las escamas del pez Rollizo Lebiasina cf. multimaculata (Boulenger, 1911) (Characiformes: Lebiasinidae), observaciones sobre su biología en el Quindío, Colombia, Revista Investigaciones Universidad del Quindío, 1: 1-24, 1986. [ Links ]
Romero M, Galindo G, Otero J, Armenteras D. Ecosistemas de la cuenca del Orinoco colombiano. Instituto de investigación de recursos biológicos Alexander von Humboldt, Bogotá D.C., Colombia. 2004. [ Links ]
Sette S. Características anatómicas de tres especies de peces en la cuenca alta del Río Uribante, en relación con su fisiología reproductiva, Veterinaria Tropical, 16: 69-110, 1991. [ Links ]
Sette S. Características biométricas del volador Lebiasina erythrinoides, Veterinaria Tropical, 18: 13-21, 1993. [ Links ]
Sierra N, Péfaur J. El volador, Lebiasina erythrinoides: Distribución y abundancia relativa en los Andes venezolanos, Veterinaria Tropical, 23: 127-145, 1998. [ Links ]
Sokal RR, Rohlf FJ. Biometry. WH Freemand, San Francisco, California, USA, 1995. [ Links ]
Sturges HA. The choice of a class interval. Journal of the American Statistical Association, 21: 65-66, 1926. [ Links ]
Taphorn D. Manual de identificación de los peces Characiformes del río Apure en Venezuela. Universidad Nacional Experimental de los llanos occidentales UNELLEZ. Biollania Edición Especial, 2003. [ Links ]
Taphorn D, Lilyestrom U. Piabucinapleurotaenia Regan, a synonym of P. erythrinoides Valenciennes (Pisces: Lebiasinidae): its distribution, diet and habitat in Lake Maracaibo basin, Venezuela, Copeia, 1980: 335-340, 1980. doi: 10.2307/1444011 [ Links ]
Urbano-Bonilla A, Zamudio J, Maldonado-Ocampo JÁ, Bogotá-Grégory JD, Cortes-Mllán G, López Y Peces del piedemonte del departamento de Casanare, Colombia, Biota Colombiana, 10: 149-162, 2009. [ Links ]
Urbano-Bonilla A, Prada-Pedreros S, Zapata A, Barrera-Cataño J, Moreno-Cárdena C. Composición y riqueza íctica en quebradas y ríos del piedemonte de la cuenca del río Cusiana (Orinoquia colombiana), Biota Colombiana, Especial Áreas de influencia de Ecopetrol, 15: 31-48, 2014. [ Links ]
Van der Loo MPJ. Extreme values, an R package for outlier detection in univariate data, R package version 2.1. 2010. [ Links ]
Vazzoler ADM. Biología da reprodujo de peixes teleósteos: teoría e práctica. Universidade Estadual de Maringa, Maringa, Brazil, 1996. [ Links ]
Windell JT. Food analysis and rate of digestion. In: Ricker WE (ed) Fish production in freshwaters. Blackwell Scientific Publications, Oxford, England, pp 215-226, 1971. [ Links ]
Winemiller K, Taphorn D. La evolución de las estrategias de vida en los peces de los llanos occidentales de Venezuela, Biollania, 6: 77-122, 1989. [ Links ]
Winemiller KO, Agostinho AA, Pellegrini A, Caramaschi E. Chapter 5: Fish ecology in tropical streams. In: Dudgeon D (ed) Tropical Stream Ecology. Elsevier/Academic Press, San Diego, CA, pp 107-146, 2008. [ Links ]
Wolff M. Insectos de Colombia, guía básica de familias. Laboratorio de Colecciones Entomológicas - GIEM (Grupo Interdisciplinario de Estudios Moleculares), Universidad de Antioquia. Medellín Colombia, 2006. [ Links ]
Wootton R. Ecology of teleost fishes (Vol. 1). Chapman and Hall Ltd. London, England, 1990. [ Links ]
Yáñez-Arancibia A, Lara-Domínguez AL, Aguirre-León A, Díaz-Ruiz S, Amezcua FL et al. Ecología de poblaciones de peces dominantes en estuarios tropicales: factores ambientales que regulan las estrategias biológicas y la reproducción. In: Yáñez-Arancibia A (ed) Fish Community Ecology in Estuaries and Coastal Lagoons: Towards an Ecosystem Integration, UNAM, México D.F., México, pp 311-366, 1985. [ Links ]














