Introduction
New encapsulation technologies of inoculants based on plant growth-promoting rhizobacteria-PGPR- offer improvements in other liquid formulations including stability, controlled release, environmental effects, biodegradation, profitability, and sustainability (Schoebitz et al. 2013, Arora et al. 2011). The use of encapsulated cells for release into the environment has several advantages over the formulations containing free cells including protection against biotic and abiotic stress (Smit et al. 1996). The microencapsulation is a promising process in which the cells are retained within an encapsulating matrix or membrane and their release occurs in controlled rates over prolonged periods of time (Champagne & Fustier 2007, Krasaekoopt et al. 2004, Park & Chang 2000). Furthermore, this process has been applied to enhance stability of the microorganisms (Lee & Heo 2000). For this, a technique known as spray drying is used for preservation and concentration of microorganisms (Maciel et al. 2014, To & Etzel 1997b). Previous spray-drying studies including those reported by Maciel et al. (2014), To & Etzel (1997b), and Mary et al. (1993) implied the description of the survival of Lactobacillus acidophilus, Brevibacterium, and Bradyrhipobium japonicum respectively. In addition, this technology has been applied to increase the viability of probiotic bacteria (Ozer et al. 2008).
Research about the microencapsulation process have focused on improving the coating of beads with polymers. This process has potential application in probiotic bacteria (Larisch et al. 1994). Motivated by these reports, this investigation includes the same concept of the covering process and the controlled release in the pharmaceutical industry, but applied to microorganisms of agricultural interest as an active ingredient. Given that the temperature causes a constant exposure in fluid bed, sucrose beads were used with the objective to protect and adhere to the bacteria. This approach would create a better covering of Rhipobium sp. with the polymers in the microparticles. Our hypothesis suggests that the entrapment of microorganism in the polymeric matrices together with the fluidized bed technique allows the controlled release of the bacteria without losing their symbiotic capabilities with cowpea. Therefore, the goal of this study was to evaluate the influence of polymeric materials on the entrapment and viability of cells of Rhipobium sp. G58 through the method of spray-drying and using sucrose beads as a vehicle. Furthermore, the objective was to demonstrate the efficacy of the solid-formulation on the properties of plant growth- promoting, as well as the ability to perform the symbiosis between Rhipobium-Cowpea under greenhouse conditions.
Materials and methods
1. Bacterial strain and polymeric materials
Rhipobium sp. G58, with accession number JQ771467, was provided by the Laboratorio de Microbiologia de Suelos of Corpoica. It was isolated from the nodules of cowpea (Vigna unguiculata L. Walp) in Guajira, Colombia and was selected based on its potential as plant growth-promoting bacteria-PGPB (Rivera et al. 2014). The strain was grown in a flask with Yeast Extract Mannitol (YEM) medium (Vincent 1970) through a process of batch fermentation at pH 6.8 and 28 ± 2 °C, with stirring it at 150 rpm for 24 h. High and low viscosity of sodium alginate polymer (KELTONE® HVCR and LVCR respectively, FMC Biopolymer, Ewing, USA) and hydroxypropyl methylcellulose- HPMC (METHOCEL™ K100M CR, Colorcon®, Harleysville, USA) were used in this study with concentrations of 0.5 %. To avoid agglomeration of microparticulates in the fluidized bed, the concentration was determined in accordance with the process conditions conducted in previous preformulation studies (Rivera et al. 2014).
Experimental set-up and conditions of entrapment of Rhizobium sp. G58 by fluidized bed
First, for the coating process in the fluidized bed reactor (Glatt, Colorcon®), an initial cell suspension was adjusted to an optical density (OD600: 0.500). In addition, the polymer was dispersed with a mechanical stirrer (Heidolph, RZR 2020) together with the bacterial suspension. After the dispersion of the materials, this mixture was injected into the fluid bed. This liquid formulation was atomized on a sucrose bead which was used as a coating vehicle to finally obtain the solid formulations. The conditions of process were evaluated as follows: (°Te) < 50 °C, (°Ts) < 35 °C, sucrose
bead size (18/20 mesh), bead load 500 g, a bacterial suspension load 500 mL for a 1:1, and an entrance flow rate (Qo) < 6 mL/min. A bottom-spray system was used for the atomization of the mixture (polymer + bacteria) on the sucrose beads. Three independent experiments were performed in triplicate. On the basis of the solid-formulation obtained, the cell viability was measured for three months (data not shown) using the microdrop technique. Thus, serial dilutions were prepared and 20 ¡iL of each dilution were plated on Yeast Extract-Mannitol (YEM) agar plates. For this, the solid-formulations were dissolved and diluted in a buffer solution by constant agitation. Plates were incubated under aerobic conditions at 28 ± 2 °C for 24 h. Cell counts for each solid-formulation, were performed in triplicate. Profiles of degradation of the microparticles and the controlled liberation of the Rhipobium sp. G58 were evaluated through the simulation in the soil within five days (data not shown).
2. Plant growth-promoting activities
Acetylene-reduction assay of symbiotic N2 fixation
Nodulated roots, previously inoculated with the solid formulation of Rhipobium sp. G58 were placed into a 280-mL container which was sealed immediately. In order to equalize the pressure to an atmospheric pressure, a syringe needle was used in the container. Next, 10 % of the atmosphere in the container was replaced with acetylene and the container was incubated for 1 hour at 28 ± 2 °C. The biological nitrogen fixation was estimated by using a Perkin Elmer gas chromatograph equipped with a flame ionization detector (FID) and a Poropak N 200/300 Mesh column measuring 6.0 ft (in diameter) by 3.0 mm in accordance with the methods described by Hardy et al. (1968).
Quantification of indolic compounds
Indolic compounds were estimated using the colorimetric assay described by Glickmann & Dessaux (1995). For the solid formulation of Rhipobium sp G58, K-lactate minimal medium with tryptophan at 100 ppm was employed. The incubation process was carried out for 72 h at 150 rpm in darkness. The reagent utilized here was the Pilet and Chollet (PC) which bases on the Salkowski reagent (12 g FeCh/L in 7.9 M H2SO4.). The reaction between PC reagent and culture supernatant was executed in relation 1:1. It was allowed to react for 30 min in darkness. Indolic compounds were examined spectrophotometrically at 540 nm. The concentration of the indolic compounds in the supernatant was determined using a calibration curve of pure IAA as a standard.
3. Greenhouse evaluation of the effect of solid-formulation on symbiotic activity in cowpea
The cowpea bean is a native species indigenous to Guajira-Colombia. The variety used was of black color, known as "black head", which is a warm- season legume. Following the protocol established by the Laboratorio de Microbiología de Suelos of Corpoica, the seeds were firstly sterilized for 30 s in 70 % ethanol and rinsed 3 times with sterile distilled water. Afterwards, the seeds were subsequently placed for 1 min in 2 % of sodium hypochlorite, rinsed 10 times with sterile distilled water, and then imbibed in distilled water for 1 h. After germination in darkness, 4-5 d-old seeds were seeded in the pots at the rate of seven cowpea seedlings per pot. Once grown, only two seedlings were kept in each pot. Seedlings were sown to a depth of 1 cm in plastic pots free-draining and filled with 2 Kg of vermiculite-sand sterilized ratio of 3:1 (w/w). During the experiment, the plants were watered every two days using the Hoagland nutrient solution described by Hoagland & Arnon (1950). Previous were applied without nitrogen and phosphorus. The polymeric treatments of the solid-formulation evaluated in plant were: high and low viscosity of sodium alginate 0.5 % and hydroxypropyl methylcellulose 0.5 %. In the case of the control treatment the polymer was not applied, but the same technological process was maintained. Finally, absolute control (uninoculated treatment) was used. At the time of sowing, each pot was inoculated with the solid formulation (containing 107 CFU/g). Plants were allowed to grow for 60 d under greenhouse conditions at a temperature of 30 ± 2 °C, a 70 % relative humidity and 14 h day: 10 h dark. Plants were irrigated regularly and the growth of the plants were observed daily. The nine variables evaluated were root length, shoot length, nodule number, acetylene reduction assay-ARA, nodule biomass, root fresh weight, shoot fresh weight, root dry weight, and shoot dry weight. They were analyzed by a multivariate analysis using principal components with six replicates per treatment and was performed with XLSTAT software.
4. Scanning electron microscopy (SEM) of microparticles
The samples of the best solid-formulation of Rhipobium sp. G58 were processed by metallization in a SDC-050 sputter (Balzers) following evacuation to <10-1 torr in argon on a gold-palladium (8:2) cathode plate. The processed sample was visualized in several fields using a scanning electron microscope (FEI Quanta 200, Netherlands) (Puente et al. 1999).
5. Transmission electron microscopy (TEM) of microparticles
The visualization in TEM of the bacteria was performed on a Hitachi HU-12A (Hitachi, Tokyo, Japan) The sample was centrifuged for 10 min at 10,000 rpm at 4 °C, fixed by immersion in 2.5 % glutaraldehyde in Sorensen buffer (pH 7.4) at 4 °C for 2 h, and postfixed in 1 % osmium tetroxide for 1 h. Then, it was dehydrated in a 50 - 100 % ethanol series and added to the resin. The resin was polymerized over 24 h at 60 °C. Ultrathin sections (0.5 mm thickness) were stained with toluidine blue. Ultrafine sections of 70 nm were doubly stained with uranyl acetate and lead citrate.
Results and discussion
Three prototypes of the solid-formulation were obtained by the fluidized bed technique. The results showed that there were no significant (p < 0.05) differences in the acetylene-reduction activity when sodium alginate polymers of high and low viscosity were used, respectively, in relation to the control (Table 1). However, the solid-formulation with HPMC showed a better biological nitrogen fixation than all the other treatments (Table 1). Authors such as Mary et al. (1993) have reported similar results, indicating that the spray-drying process using the fluidized bed does not induce changes in the ability of B. japonicum to form nodules and fix nitrogen.
Table 1 Evaluation of plant growth-promoting activities of solid-formulation of Rhizobium sp. G58. Each value represents the average of three replications. Different letters denote significant differences for activity (shown for column) according to the Tukey HSD test (p < 0.05). The symbol ± indicates the standard deviation. The values of moisture content of the prototypes were in averaged 2.25% measured on a balance halogen model (Radwag Mac 50/1). ** Acetylene reduction assay-ARA
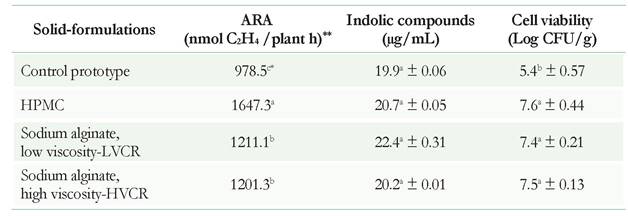
Neither the production of indole compounds nor percent humidity showed significant differences in any of the evaluated treatments (Table 1). In contrast, previous studies using polymers, such as alginate, demonstrated that among the growth-promoting rhizobacteria, the capacity to produce auxin hormones of the indole type or to produce siderophores during three years of storage was not affected in Pseudomonas (Trivedi & Pandey 2008).
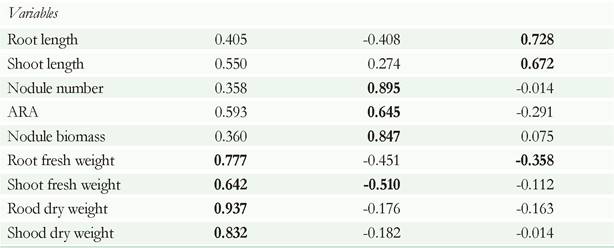
The effect of the solid-formulation of Rhipobium sp. G58 on the biological activity in cowpea was analyzed by using principal component multivariate analysis (Figure 1 A, B, C). The multivariate analysis explained 84.5 % of the experimental variation with three principal components. Of the variation, 40.67 % was explained by the first principal component (PC1), 30 % by the second (PC2), and 13.77 % by the third (PC3) (Table 2). The information contained in the nine original response variables could be reduced to three, which provided most of the experimental information. The factor loading analysis obtained from the principal component analysis (PCA) demonstrated the influence of each variable on the principal components. The analysis of factor loadings permitted the correlation of PC1 with the total biomass of the cowpea plant. PC2 indicated the symbiotic relationship between Rhipobium sp. G58, and the cowpea plant. PC3 showed the correlation for the nutrient-translocation process in the cowpea plant (Table 2). Pearson's correlation coefficient for highly weighted variables with high factor loading under principal component was used (Table 2).
Table 2 Results of the principal-components analysis of the variables in plant on greenhouse and aggrupation the components by multivariate statistics. PC = principal component. In bold letter factor loadings are considered highly weighted when within 10 % of variation of the absolute values of the highest factor loading in each PC

The treatment with low viscosity alginate showed better symbiosis and nutrient- transport processes in the cowpea plant. This result is shown in Figure 1 (A, B and C) for the different components; a positive response is displayed along the axis of the plane in the upper right quadrant of each graph. These positive responses may be explained by a greater root development of the plant, possibly stimulated by the controlled release of the microorganism in the solid-formulation (data not shown), which permitted the extraction of more nutrients that could be translocated to the plant for its metabolism. The effect of HPMC treatment on nutrient translocation is shown in Figure 1. HPMC is a synthetic polymer used by the pharmaceutical industry for the controlled release of drugs because of its hydrophilic matrix (Lee et al. 1999). This property may exert an indirect effect on the translocation of nutrients from the root to the aerial parts of the plant, based on the structure, additional components, or rheological properties of the polymer.

Fig. 1 Principal-components analysis of solid formulations of Rhi%obium sp. G58 in greenhouse. A. Scatterplot of the analysis components PC1 (Total biomass of the cowpea plant) and PC2 (Symbiotic relationship between the studied bacteria, Rhizobium sp. G58, and the cowpea plant). B. Scatterplot of the analysis components PC1 and PC3 (Nutrient-translocation process in the cowpea plant). C. Scatterplot of the analysis components PC2 and PC3. Treatments and symbols: Absolute control (■), liquid YEM medium control (•), solid formulation without polymer control (o), hydroxypropyl methylcellulose-HPMC (▲), Low viscosity sodium alginate (A), High viscosity sodium alginate (□). Each symbol represents the mean of four measurements.
In accordance with the results obtained and that were analyzed through the multivariate in greenhouse, it could be mentioned that the controlled release of the bacteria through the employment of a solid- formulation and the coating with polymers, offers an appropriate method to protect a Rhizobium sp. G58 against different conditions of stress in the soil (Figure 1). Hence, this method of using a solid-formulation could afford an opportunity to confront the conditions of the soil of Guajira (Rivera et al. 2014).
Furthermore, in reference to the realization of the greenhouse assay, it is important to consider that the Rhizobium sp. G58 could not be caught in the solid-formulation for a long period of time. Therefore, previous assays of degradation of microparticles and controlled release of the bacteria were evaluated (data not shown).
The results of these assays were conform and correlative with the emergence and the establishment of the legume seedlings. This liberation of the Rhizobium sp. G58 of the microparticles was essential for the process of symbiosis in this study (Figure 1). The release of the bacteria in the soil by covering of the polymers in the solid-formulation does not exceed more than five days (data not shown). This could be the point of time in which the seedling continuously will be in contact with the bacteria (continuous degradation of the microparticle). Thus, this can ensure that Rhizobium sp. G58 continuously will be available in the soil and that simultaneously the process of growth of the seedling is occurring (Figure 1 and Table 1).
SEM and TEM were performed on the low viscosity sodium alginate formulation -LVCR-, which yielded better biological-activity results for the Rh/'zobzum-plant interaction (Figure 2 A-D). The results showed the persistence of the bacteria on the surface of the beads, indicating the incorporation of polymers by the fluidized-bed coating technique (Figures 2 A and B). This demonstrated the capacity of bacterial resistance to temperature stress and the bacterial establishment. Related studies have been reported by Boza et al. (2004) who showed the viability of Beijerinckia sp. and its persistence following spray drying because of the adherence of the microorganism to microparticle surfaces. Similar results have been obtained by Young et al. (2006) who demonstrated the immobilization of B. subtilis on alginate microspheres indicating that the microorganism integrates itself into the polymer matrices through ionic interchanges.
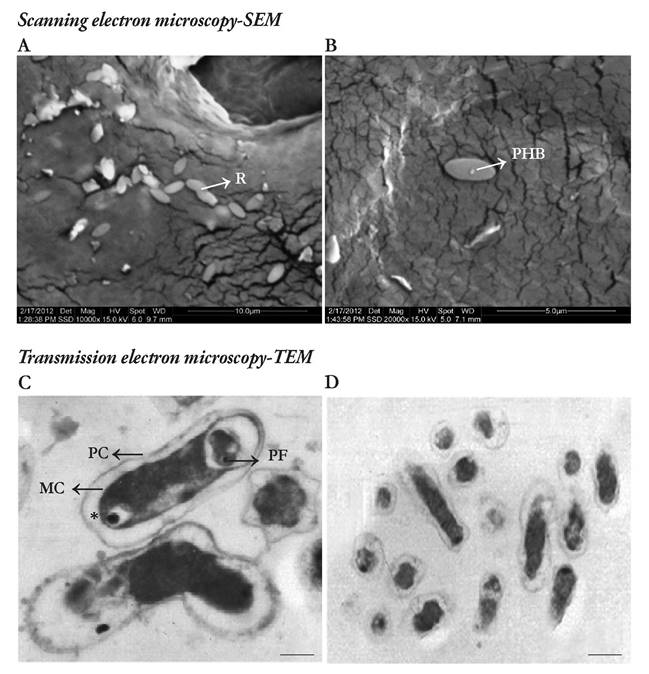
Fig. 2 A and B. Scanning electron micrographs of (R) Rhizobium sp. G58 in the low viscosity sodium alginate formulation - LVCR; PHB, Inclusion body of poly-/i-hydroxybutyrate. C and D. Transmission electron micrographs of Rhi%obium sp. G58 in the low viscosity sodium alginate formulation -LVCR. Abbreviations: PC: Cell wall, MC: Cell membrane, PF: polyphosphate granules, *Acidocalcisomes structures to be confirmed. C. 20.000 X magnification; scale bar represents 0.5 pm. D. 10.000 X magnification; scale bar represents 1.2 pm.
Likewise, the results obtained by TEM confirms the presence of specialized structures in the interior of the cell of Rhizobium sp. G58 (Figures 2 C and D). This observation is typical in Rhizobium, given that the bacterium induces the formation of inclusion bodies corresponding to poly-/?-hydroxybutyrate (PHB) granules. Furthermore, metachromatic polyphosphate granules were observed (Figure 2 C). These linear polymers act as storage deposits of phosphorus and energy for adenosine triphosphate (ATP) formation and confer a competitive advantage over other microorganisms that are unable to accumulate internal reserves (Serafim et al. 2002). In addition, electro-dense acidocalcisome organelles rich in calcium and phosphate were observed by TEM (Figure 2 C). These are implicated in the storage of phosphorus and various metal ions, polyphosphate metabolism, maintenance of intracellular pH, and osmoregulation (Docampo et al. 2005).
Conclusion
When the solid-formulations were applied to the legume rhizosphere, there was a greater effect on plant development and the establishment of symbiosis, as indicated by the formation of nodules in better condition. This observation indicates that the application of a solid-formulation results in a better release of the bacteria in a prolonged manner over time, allowing the nodulation process to progress in an effective way. In summary, these results lead to suggest that formulation of Rhizobium sp. G58 employing polymers as vehicles are among the most promising option for the encapsulation of beneficial microbes.














