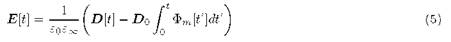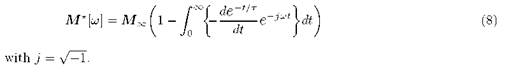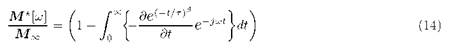Introduction
The high diffusion of ions in the so-called superionic or fast-ion conductor materials can be seen in the superionic phase for temperatures T > Tt, where Tt is the transition temperature from low-conducting to high-conducting phases. The influence of mobile ions such as Na+, Li+, Ag+ [1,2], among others, has been studied in these systems. The phase transition is characterized by an abrupt increase in ionic conductivity, a low activation energy, a latent heat typical for a first-order transition, a crystalline structure with vacancies available for mobile ions, and, in general, a change in the symmetry of the lattice. For these materials the structural disorder, below the melting point, is important for the increase of the ionic diffusion rate when either it is heated or a voltage is applied.
One of the most used experimental techniques to characterize the dynamics of ionic transport is the so-called impedance spectroscopy. In order to study the dielectric properties of materials using the impedance spectroscopy technique, an impedance bridge is used. It provides conductance (G), capacitance (C), and phase angle (0) measurements as a function of the angular frequency w [3]. All the experimental information about electric relaxation at a given temperature is found in G[w] and C[w]. These physical quantities are transformed in the complex permittivity e*[w], complex conductivity <r*[w] = jwe*[w], complex resistivity p*[w] = 1/<r*[w], and complex electrical modulus M*[w] = 1/e*[w] [4].
Electric answer due to ion dynamics
By applying a Heaviside step function to the electric displacement vector D, we have that for t > 0 the internal electric field E decreases due to the ion conventional hopping mechanism. This condition is expressed mathematically introducing the cm[t] function [5,6], that defines the electric field change with time:
cm [t] represents the relaxation of E at the interior of the material when a step function is applied to D, E[0] is the value of E at the instant t > 0:
where e0 is the electrical permittivity of vacuum and eTO is the electrical permittivity at the high-frequency limit.
The time derivative of (1) is:
By denominating
as the relaxation function and integrating the Eq. (3)
In the Eq.(5), the notation E[t] and D[t] are defined over the whole interval -∞< t < ∞.
Fourier transform of (5) is:
where Tm[w] is the electrical susceptibility.
being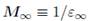 . For the case of an assembly of dipoles or ions non-correlated or Debye behavior (cm[t] ~ e-t/T), the electrical modulus would be:
. For the case of an assembly of dipoles or ions non-correlated or Debye behavior (cm[t] ~ e-t/T), the electrical modulus would be:
The solution of (8) gives the spectrum of M *[7]:
For the case of some compounds that do not follow the Debye type behavior, i.e., when the interactions between the ions and the structural disorder are taken into account, Havriliak-Negami proposed a susceptibility function of the form [8]:
where the exponents a, 7 and the characteristic time thn are chosen in such a way that they satisfy the experimental data which are asymmetric curves of slope different from one for the right branch, therefore expression (7) may be rewritten as:
where the new susceptibility must be equal to the susceptibility for the Debye case, multiplied by the time distribution function:
carrying out the inverse transform:
That is, a stretched relaxation function of the Kohlrausch-Williams-Watts (KWW) type [9,10]. Thus the expression (8) takes the form
This is the expression used to fitting experimental data.
Preparations of samples
Polycrystalline Agl powder were recrystallized using the solution technique with high-purity reagents [11] starting from 99.99% (Aldrich) high-purity compound. Pure single crystals of 0.5 cm in diameter were achieved. The obtained single crystals were subjected to thermal treatment at 413 K during 24 hours before the measurements to eliminate the 7-phase. The single crystals of chemical composition LiI-4AgI were prepared using the same method, mixing Agl and Lil Aldrich compounds of 99.99% in purity. During the recrystallization process, both Agl and Lil compounds do not react between them. The Agl and Lil compounds are combined forming a mix. Other phases in the formed crystals are not presents. The effect upon activation energy is produced by proximity among Lil and Agl compounds, by principle of conservation of mass all amount of the compounds of Agl and Lil used for the mix at the finish of process of recrystallization in the same initial.
The monocrystalline samples were painted with silver paint on their parallel faces. The impedance spectroscopy measurements were carried out in the LCR meters HP 4284A and HP 4284A in the range of frequencies from 20 Hz to 1 MHz for a temperature range from 200 K to 400 K. The measurements were carried out under N2 gas flow ensuring a controlled atmosphere.
Results and discussions
Figure 1 shows the dependence on the frequency of real part of ac conductivity σ[w] for LiI-4AgI crystals obtained by impedance spectroscopy measurements below the superionic transition in the range temperature from 258 K to 350 K. A potential dependence is observed at the highest frequencies and a cut frequency that separates the dc regime from the dispersive regime that increases with temperature. At low frequencies and high temperatures, ionic conductivity decreases due to the processes that happen in the frontier between the ionic conductor and the electrodes. The diminution effect of conductivity is greater in the Agl samples doped with Li, than in the pure Agl this is explainable due to a greater grain boundary effect. The values of dc conductivity are obtained from the plane regions of the isotherms of conductivity at the lowest frequencies. The solid lines are the best adjustments to experimental data.
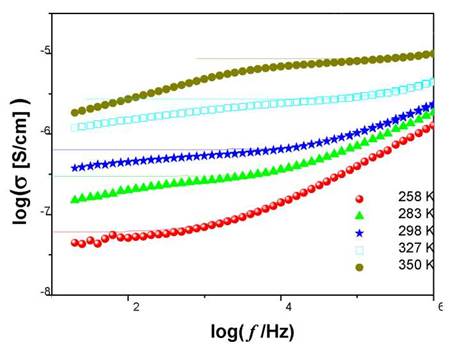
Fig. 1 Real part of the electrical conductivity as a function of frequency for LiI-4AgI at temperatures 258 K (red), 283 K (green), 298 K (blue), 327 K (cyan), and 350 K (dark yelow).
In Figure 2, the curves of the imaginary part of the electrical modulus are shown as a function of the frequency for various temperatures, of the LiI-4AgI sample. An asymmetric peak is observed, which was adjusted by expression (14), with a stretched relaxation function (13) KWW type. The solid lines in the figure represent better adjustment to the KWW relaxation function, from which the parameter was obtained.
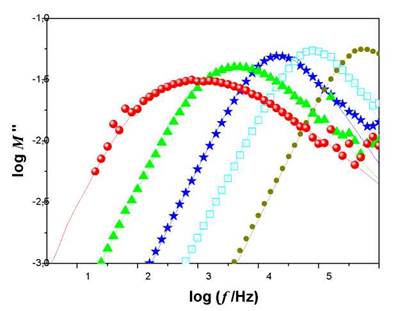
Fig. 2 Dependence of frequency of the real part of the electrical modulus for LiI-4AgI at temperatures 258 K (red), 283 K (green), 298 K (blue), 327 K (cyan), and 350 K (dark yelow).
In Figure 3, dependence of the [T] function is represented with temperature T for the Agl and LiI-4AgI systems. This function has been obtained starting from the slopes of the right branch of the curves of the imaginary part of the electrical modulus. The continuous line represents the behavior of the fl[T] function for the pure AgI. The changes observed in the behavior of fl[T] for the LiI-4AgI system, with respect to the AgI compound, are the consequences of the addition of the lithium phase to the pure AgI compound. The activation energies for the pure AgI and for the LiI-4AgI are similar; however the values of fl[T] are different. This fact suggests that the addition of the lithium phase would bring as a consequence the variation of microscopic energy and, consequently, a variation of the migration energy according with the coupling model [10].
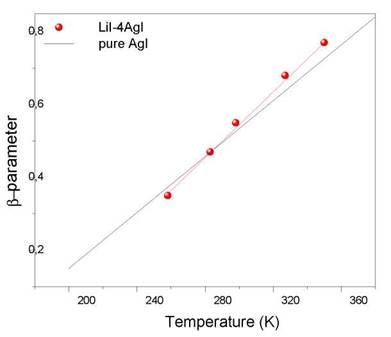
Fig. 3 Dependence of fl[T]-function with temperature T for both pure Agl and LiI-4AgI systems. The continues solid line representes the behavior of fl[T]-function for the pure Agl compound.
This is due to the presence of the non-conductor phase of LiI dispersed at nanometric level in the conductor phase of AgI which would favor the local ordering of Ag+ ions increasing slightly the microscopic energy. This observation confirms the hypothesis that the addition of the LiI phase would produce a decrease in the positional disorder of the sub-lattice of silver ions, with which the correlations at a given temperature would decrease with respect to the pure AgI [12].
Conclusions
In this work, we have researched the existence and importance of the ion-ion correlation effects on the relaxation of electrical conductivity of doped Agl crystals with Lithium Iodide at close temperatures and below the superionic transition.
It was found that correlations between mobile ions decrease when the concentration of the Lil doping phase increases. The experimental evidence suggests that the inclusion of the lithium phase in the Agl, favor the decoupling of interaction between the silver ions, and the sub-lattice of fixed iodide ions, remaining the constant activation energy for the silver ions, with a value of 0.50 ± 0.02 eV.
The decoupling of interaction between mobile ions can be due to the modification of relation among the microscopic energies.