Introduction
Temporary lakes contain water during periods as short as a few months and up to several years (Schwartz & Jenkins, 2000). These lakes are globally distributed, and their sizes range from a few square meters to hundreds of hectares (Williams, 1987; 2002; Schwartz & Jenkins, 2000). Many of these ecosystems are disappearing because of anthropic activity, including their transformation into agricultural fields (Belk, 1998; Williams, 2002; Jenkins etal. 2003; Eitam etal. 2004). The transformation and destruction of these ecosystems leads to species loss, thus significantly affecting local biodiversity (Waterkeyn et al. 2008).
Most water bodies in La Pampa province, a central semiarid region of Argentina, are shallow (maximum depth < 3 m), temporary lakes. These lakes occur in arheic basins, where surface runoff flows unpredictably due to lack of drainage patterns (Mann, 2002; World Meteorological Organization, 2012; Meybeck et al. 2013), and they are formed by phreatic input and, to a lesser extent, by rainfall (Dornes et al. 2016).
Most of the Pampean temporary lakes are saline; with ions such as Na+ and Cl- predominating in their composition (Echaniz, 2010), and concentrations of dissolved solids exceeding 3 g L-1 (Hammer, 1986). An important characteristic of saline ecosystems is that changes in water level cause considerable modifications of their physical and chemical parameters; thus, exerting major physiological constraints on the inhabiting biota (McCulloch et al. 2007). Further, salinity is an environmental variable with a strong influence on zooplankton. Salinity level is inversely associated with species richness and has been documented as an environmental stressor (Herbst, 2001). When salinity decreases, species richness increases (Wurtsbaµgh & Berry, 1990; Green, 1993; Williams, 1998; Hall & Burns, 2003; Ivanova & Kazantseva, 2006) and vice versa (Green, 1993; Hall & Burns, 2003; Kipriyanova et al. 2007). Zooplankton communities play an important ecological role in shallow temporary lakes because these organisms occupy different niches in aquatic food webs, and they contribute to element recycling and energy transfers (El-Shabrawy etal. 2015). Thus, changes in zooplankton community composition can alter characteristics of a lake, such as water transparency, phytoplankton composition, and chlorophyll-a concentration (Jenkins et al. 2003; Echaniz, 2010).
Several ecological aspects of temporary lakes have been studied in Australia (Williams etal. 1998; Bayly, 2001; Roshier etal. 2001), North America (Smith et al. 2003; Wallace et al. 2005), and Europe (Mura & Brecciaroli, 2003; Frisch et al. 2006). In Argentina, temporary lakes are common, yet their ecology has received little attention. Most of the studies on Argentine temporary lakes have been conducted in aquatic ecosystems, located in La Pampa Province, and many of them took place during annual cycles characterized by a certain hydrological stability. Therefore, many aspects of their ecology, under relatively limited salinity variation, are now documented (Echaniz et al. 2013a; b; 2015). Other studies have also compared the conditions recorded in different periods (annual cycles) for a given lake and showed differences in physical and chemical variables, as well as in zooplankton biological traits (Echaniz & Vignatti, 2011; Echaniz et al. 2006; 2012; 2013a; b; 2016; Vignatti et al. 2012a). Moreover, three studies conducted in La Pampa showed changes in limnological parameters and zooplankton traits during the filling or drying phases of temporary, subsaline and saline lakes, (Echaniz & Vignatti, 2010; Vignatti et al. 2012a; b). To the day, little is known about the ecological dynamics of species inhabiting a temporary water body as it experiences environmental transitions. Species replacement is one of such ecological dynamics, and it involves the emergence of organisms from sediments (resting egg bank) (Williams, 1987; Belk, 1998; Schwartz & Jenkins, 2000; Vignatti . This issue is of primary interest, because species assemblages recorded in the lakes of central Argentina differ from those in other continents. Particularly, many endemic species to the Neotropical region are represented (Adamowicz et al. 2004; Echaniz, 2010; Echaniz et al. 2006; 2012; Vignatti, 2011; Vignatti et al. 2012b; 2016), and ecological knowledge about them is relatively scarce.
The present study aimed to describe the variation in species composition, and in three biological parameters (i.e. individual density, biomass, and body size) of a zooplankton community inhabiting a saline, shallow, and temporary lake, that underwent drying in La Pampa Province. Further, the relationship among a set of environmental and biological parameters of the lake was investigated. Specifically, this study addressed two hypotheses: i) the increase in salinity produced by water evaporation has a negative effect on zooplankton richness, density, and biomass; and ii) because the lake lacks predatory fish, due to its saline and temporary nature, zooplankton is dominated by species with large body size.
Materials and methods
Study area
The Ojo de Agua Uriburu lake (36° 31' S, 63° 53' W) is located in the east of La Pampa Province (central Argentina), at the western boundary of the Pampa Plains phytogeographic region (Fig. 1). The lake was formed in an arheic basin and is sustained by phreatic inputs and rainfall. Water level fluctuations produce large movements of the coastline. Therefore, its outline is a beach with patches of halophytic vegetation.
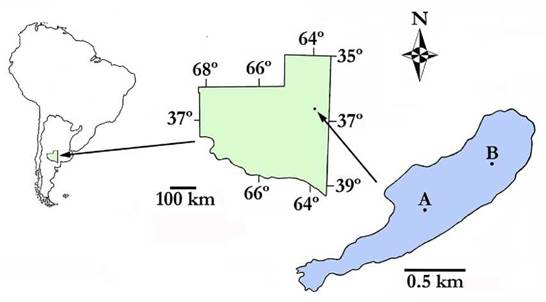
Figure 1 Geographic location of the Ojo de Agua Uriburu lake in the province of La Pampa (central region of Argentina). A and B indicate sampling sites. The outline denotes the morphometric dimensions recorded in December 2012.
The lake is surrounded by fields where cereals and oilseeds are cultivated, and, to a lesser extent, are used as pasture for cattle. This lake does not host any aquatic vegetation or fish.
At the beginning of the study period (December 2012), the maximum length of the lake was 2 351 m, its maximum width was 614 m, and its maximum area was 86.8 ha. These parameters gradually decreased until complete lake drying up.
Field and laboratory work
Monthly samples were taken from December 2012 to July 2013 (the lake dried up after the last sampling). Samples were taken at two sites along the lake’s major axis (Fig. 1). At each sampling spot, the following environmental variables were measured: water temperature, dissolved oxygen concentration (Lutron® OD 5510 oxygen meter), water transparency (Secchi disk, 22 cm in diameter), and pH (Cornning ® PS 15 pH sensor). Water samples were taken and kept refrigerated until laboratory analysis. Moreover, two 20-liter quantitative zooplankton samples were taken at each sampling site. Given the shallow depth of the lake, samples were taken with graduated pails, that integrated the water column, and were then filtered throµgh a net with a mesh size of 0.04 mm .In addition, at each sampling spot, a qualitative sample was also taken with a similar net. All samples were anesthetized with CO2 and kept refrigerated until fixation. Afterward, they were deposited in the collection of the Facultad de Ciencias Exactas y Naturales de la Universidad Nacional de La Pampa.
Water samples were taken for the following analyses: salinity determination, throµgh the gravimetric method with drying at 104 °C (APHA, 1992); chlorophyll-a concentration, determined by extraction with aqueous acetone and spectrophotometry (Arar, 1997); and concentration of suspended solids (total, organic, and inorganic), determined by weighing Microclar® FFG047WPH filters, drying at (103 °C - 105 °C) to a constant weight, and calcining at 550 °C (EPA, 1993). Water samples were taken twice during the study period (January and July), to determine ionic composition.
Macro and microzooplankton (Kalff, 2002) counts were made under stereoscopic and conventional optical microscopy in Bogorov and Sedgwick-Rafter chambers, respectively. 1-mL aliquots were prepared using a Russell sub-sampler. Samples were homogenized by shaking. Zooplankton density was obtained by counting the number of individuals (ind) per volume unit, expressed as ind L-1. Zooplankton biomass (expressed as u g L-1), was determined based on measurements of 30 specimens, of each species and from all stages, made with a micrometric Arcano® 10x eyepiece. For crustaceans, available formulae relating total length to dry weight were used (McCauley, 1984; Culver et al. 1985). For rotifers, the Ruttner-Kolisko’s method (1977) was employed. With this method, a specimen’s volume was estimated, assuming that the geometric formulae provide an accurate proxy (McCauley, 1984). Subsequently, the volume estimate allowed for fresh weight calculation (assuming a specific gravity of 1) and its subsequent conversion to dry weight (assuming a dry wet ratio of 0.05) (Ruttner-Kolisko, 1977; McCauley, 1984).
The obtained data followed neither normality nor it revealed variance homoscedasticity, thus non-parametric Spearman’s rank correlation coefficients were calculated (Zar, 1996). Software packages Past (Hammer et al. 2001) and Infostat (Di Rienzo et al. 2010) were employed for statistical analyses.
Results
Environmental variables
At the beginning of the study, in December 2012, the depth of the lake was around 0.7 m and its salinity barely exceeded 16 g L-1. However, in July 2013, its depth decreased to 0.06 m, and its salinity increased above 92 g L-1, before the lake dried up completely (Fig. 2). The correlation between lake depth and salinity was high (r s = -0.97; p = 0.0005).
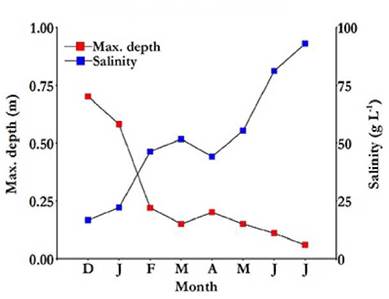
Figure 2 Maximum water depth and salinity variation in the Ojo de Agua Uriburu lake between December 2012 and July 2013.
Water chemical composition analyses revealed that Cl- and CO3 2- were the predominant anions, with concentrations exceeding 20 000 and 14 000 mg L-1, respectively, and that Na+ predominated among the cations, exceeding a concentration of 21 000 mg L-1. Divalent cations (Ca2+ and Mg2+) were present in low concentrations, and thus total hardness of the water was relatively low (Table 1). Furthermore, mean water pH was of 9.74 and fluctuated little along the study period (Table 1). Throµghout the study period, mean water temperature was of 19.74 °C (Table 1); water temperature showed an overall steady decline throµghout the sampling period. A maximum water temperature of 25.7 °C was recorded in January and a minimum of 10.9 °C in July. Mean dissolved oxygen concentration in water was high, around 8 mg L-1 (Table 1), and fluctuated from a minimum of 6.7 mg L-1 (February) to a maximum of 9.9 mg L-1 (July) (Fig. 3). A correlation was found between these two parameters (r s = -0.87; p = 0.0077).
Table 1 Mean and standard deviation of the water physicochemical variables measured in the Ojo de Agua Uriburu lake between December 2 012 and July 2013.
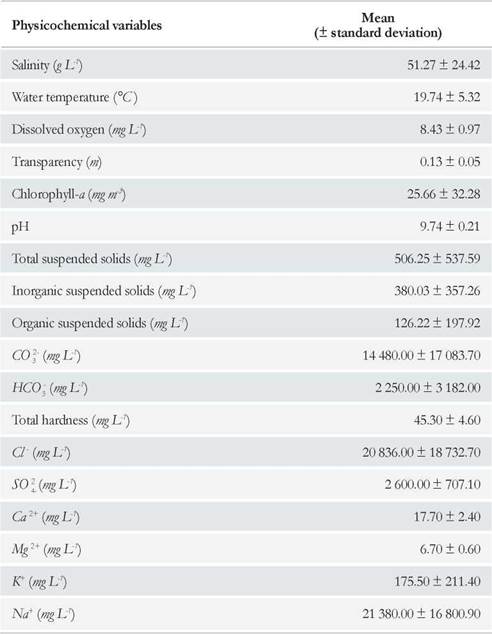
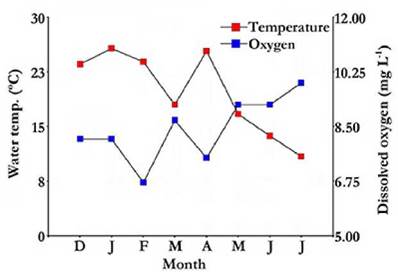
Figure 3 Water temperature and dissolved oxygen concentration variation in the Ojo de Agua Uriburu lake between December 2012 and July 2013.
Water transparency was always low (Table 1), with a maximum of 0.22 m, recorded in February (Fig. 4). Phytoplankton chlorophyll-a concentration was reduced at the beginning of the study but experienced a marked autumn peak, reaching 104.13 mg m-3 in April, followed by a sharp decline in March (Fig. 4). A significant correlation was found between water transparency and chlorophyll-a concentration (rs = -0.81; p = 0.0184).
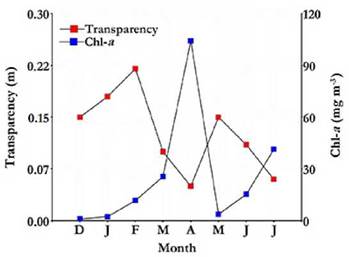
Figure 4 Water transparency and chlorophyll-a variation in the Ojo de Agua Uriburu lake between December 2012 and July 2013.
Throµghout the study period, the average concentration of total suspended solids was higher than 500 mg L-1 (Table 1).The correlation between water transparency and suspended solids concentration was high (rs = -0.84; p = 0.0096). Solids of inorganic origin were dominant and represented 75 % of the total solids, and organic solids accounted for the remaining 25 % (Table 1). Both solid types showed a strong peak in autumn, especially in April, exceeding 1 730 mg L-1 altogether.
Zooplankton community composition and parameters:
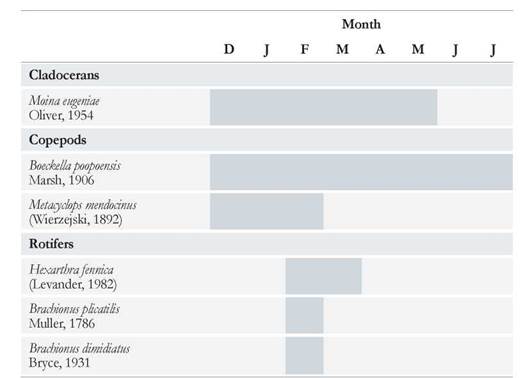
Six zooplankton species were recorded in the Ojo de Agua Uriburu lake throµghout the six-month study period. A maximum species number of five was observed in February, and it declined as lake drying proceeded, so that, only one species was recorded in July.
The copepod Boeckella poopoensis (Marsh, 1906) was found in each month, from December to July, even when salinity rose above 90 g L-1. Whereas the cladoceran Moina eµgeniae (Olivier, 1954) was registered between December and May (Table 2), disappearing from the lake when salinity exceeded 55 g L-1. Three rotifer species were first registered in the middle of summer (February), with Brachionus plicatilis (Müller, 1786) surviving the longest in the lake, almost until late autumn (Table 2).
Total average density and biomass values of the zooplankton community in Ojo de Agua Uriburu, were of 347.34 (±341.29) ind L-1 and 1 602.33 (± 1 485.55) µg L-1, respectively. Both parameters were correlated (rs = 0.86; p = 0.0072) and showed a strong peak at the beginning of autumn (April), when density was as high as 1 037.25 ind L-1, and recorded biomass was 4 912.3 µg L-1.From May on, both zooplankton density and biomass were severely reduced (Fig. 5 and 6). No correlations were found between zooplankton total density or biomass and the assessed environmental parameters.
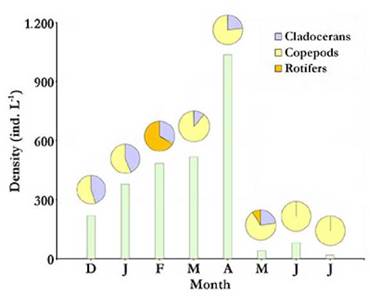
Figure 5 Total zooplankton density at each sampled month from December 2012 to July 2013 (bars) with relative contributions by taxonomic group (pie charts above bars) in the Ojo de Agua Uriburu lake.
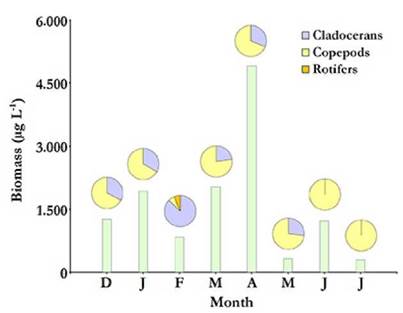
Figure 6 Total zooplankton biomass at each sampled month from December 2012 to July 2013 (bars) with relative contributions by taxonomic group (pie charts above bars) in the Ojo de Agua Uriburu lake.
For most of the study period, copepods accounted for the largest fraction of zooplankton density and biomass in Ojo de Agua Uriburu in five out of the six months that lasted the sampling (Fig. 5 and 6).Remarkably, rotifers accounted for the largest fraction of the zooplankton density in the lake in February (Fig. 5), as evidenced by an abundance peak of 305.8 ind L-1 for Hexarthra fennica (Levander, 1892), while, in the same month, the largest contribution to a biomass of 726.6 µg L-1 was made by the cladoceran M. eµgeniae (Fig. 6). During the first four months, parthenogenetic, ovigerous M. eµgeniae females were registered. These females accounted for 34 % and 6 % of the population density in January and April, respectively. From May on, no single M. eµgeniae individual bore eggs.
Among copepods, the species B. poopoensis dominated in the lake. Both B. poopoensis adults and copepodites were present throµghout the study with anaverage density of 114.8 ind L-1 (± 109.3) and a biomass of 1039.2 µg L-1 (± 954.6).
Adults and copepodites of this species, experienced a density and biomass peak in April,with parameter values of 318.5 ind L-1 and 3 029.1 µg L-1, respectively.
Further, B. poopoensis was the sole copepod species present in the lake in June and July, and its nauplii larvae reached an average density and biomass of 133.56 ind L-1 (± 202.53) and 97.16 µg L-1 (± 149.41), respectively; experiencing a density and biomass peak in April that exceeded 473 ind L-1 and 352 µg L-1, respectively. These larvae accounted for 4.15 % and 64.1 % of the population density in January and March, respectively. However, no nauplii larvae were recorded in the last two sampled months.
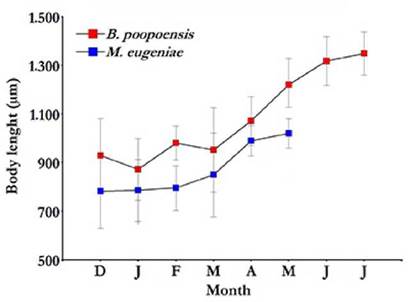
Average body sizes of the two most important crustaceans in the studied lake, M. eµgeniae and B. poopoensis, increased following a similar pattern (Fig. 7). Between December and March, mean body lengths of M. eµgeniae and B. poopoensis were 803.4 (± 27.4) µm and 933.0 (± 39.9) µm, respectively. In the subsequent months, larger average body sizes were observed. Specifically, M. eµgeniae individuals had an average size of 1 020 (± 84.2) µm in May, and B. poopoensis, individuals reached an average body size in July of 1 348.8 (± 89.0) µm. M. eµgeniae individuals were last found in May 2013 (Fig. 7).
Discussion
Most shallow lakes in the province of La Pampa are temporary, and their filling and drying depends on wet and dry climatic cycles (Viglizzo, 2010). These lakes are filled during rain cycles, associated with the El Niño southern oscillation phenomenon, with starting hydroperiods of variable duration. In a relatively prolonged hydroperiod (with greater-than-average precipitation) the amount of water stored in the soil increases and the piezometric level rises. Groundwater discharges in low-lying areas, where temporary lakes are located, constitute the main water source for these lakes, followed in importance by rain water or surface runoff (Dornes et al. 2016).
Ultimately, hydroperiod duration in these lakes is closely related to the amount of water stored in the ground and may extend for several years in some cases; water input from rain may not be constant, but phreatic water discharges keep the water influx into the lake (Echaniz et al. 2013a; b). The Ojo de Agua Uriburu lake remained filled for at least ten years, dried completely after July 2013, and was filled again by end of 2015 due to the beginning of a new period of abundant rainfall (Vignatti & Echaniz, pers. obs.).
Previous to its latest drying event, the Ojo de Agua Uriburu lake was rich in chloride - carbonate (sodic), and the hardness of its water was reduced given the low concentrations of bivalent cations. At the beginning of the present study, the lake was classified as hyposaline (Hammer, 1986). However, only three months into the study (in March 2013), the process of salt concentration, consequence of water evaporation, drove the lake above the hypersaline threshold (Hammer, 1986). The lake remained hypersaline until drying. Because of these salinity changes, any classification of such lakes into a specific category is of relative utility, and caution should be taken, as salinity depends on the ruling hydroperiod at the time of observation. Both chemical composition and salt concentration analyses confirmed that the lake chemistry was governed by evaporation - crystallization. This is the case for most lakes in the Central Chaco region of Argentina (Drago & Quiros, 1995). Water transparency was relatively low, likely due to the high amount of suspended solids, especially those of inorganic origin. This is a common situation for lakes in Central La Pampa. These lakes are located in flat landscapes, are of shallow depth, have relatively flat bottoms, are exposed to frequent winds, and commonly experience sediment resuspension from the bottom (Echaniz, 2010; Echaniz & Vignatti, 2013; Echaniz etal. 2013a; Vignatti etal. 2012a). Remarkably, the lowest water transparency was recorded in April, when the highest concentration of phytoplankton chlorophyll-a coincided with a very high amount of suspended inorganic solids.
The observed high concentration of dissolved oxygen in the water, even when chlorophyll concentration was relatively low, could have been a consequence of atmospheric dissolution, favored by wind-driven turbulence, rather than a contribution of phytoplankton autotrophic organisms. This phenomenon is common in the clear lakes of La Pampa, such as El Carancho, Utracan, and La Amarga, where, despite the fact that chlorophyll-a concentrations are below 1.5 mg m-3, dissolved oxygen concentrations can be found above 9.5 mg L-1 (Vignatti etal. 2012b).
A particular characteristic of Ojo de Agua Uriburu is that winter water temperature was not as low as that in other shallow lakes in the region, where values below 6 °C have been recorded (Vignatti et al. 2012b; Vignatti & Echaniz, unpublished data). This situation could be due to the large amount of inorganic, suspended solids rendering waters of an intense dark brown color and, in turn, favor heating by solar radiation. Similar conclusions have been drawn from the study of the Aime and Estancia Pey-Ma Lakes. These two lakes are similar to the Ojo de Agua Uriburu in their content of inorganic solids, color appearance, and recorded winter temperatures (above 9 °C) (Vignatti et al. 2012a; Echaniz et al. 2013a).
Zooplankton richness in Ojo de Agua Uriburu was low. However, this is common among fishless, saline lakes in Central Argentina. These lakes are inhabited by the crustaceans B. poopoensis and M. eµgeniae and by the rotifers Hexarthra fennica and Brachionusplicatilis (Vignatti, 2011; Vignatti et al. 2012a; 2012b; 2016; Echaniz, 2010; Echaniz et al. 2006; 2013a; 2013b; 2015; 2016). Whereas these crustacean species are endemic to Neotropical halophilic lakes, the rotifers are cosmopolitan and are broadly distributed given their wide tolerance to salinity (Fontaneto et al. 2006; Echaniz et al. 2016).
Moina eµgeniae has, thus far, been found in hypo-mesosaline ranges, specifically in the Pey-Ma Lake, with a salinity level of 48 g L-1 (Echaniz et al. 2013a). Therefore, the present study provides the first record for this species in the hypersaline range. However, the observation that only females without eggs were found in hypersaline conditions may sµggest that the reproduction of this species is affected by a high solute concentration, and that M. eµgeniae halotolerance is limited. Despite having been recorded as the most halotolerant Moinid in Argentina (Paggi, 1998), M. eµgeniae tolerance is lower than that of other cogeneric species from other latitudes, such as Moina salina (Daday, 1888), observed in hypersaline waters of Mongolia, at a salinity of 61 g L-1 (Alonso, 2010), and North Africa, at a salinity of 225 g L-1 (Amarouayache et al. 2012).
Althoµgh increases in salinity can negatively influence zooplankton richness, density, and biomass, other factors such as eutrophication, changes in biotic interactions, and food availability should also be considered (El-Shabrawy et al. 2015). Since, throµghout the study in Ojo de Agua Uriburu no predators were recorded and food availability (represented by phytoplankton chlorophyll-a) was always relatively high, the idea of a modulatory effect of salinity on the zooplankton community could be supported by the fact that richness was high while the lake remained in the mesosaline interval but declined once the lake surpassed the threshold of hypersalinity. Consequently, in the last two months of the sampling period, only the halotolerant B. poopoensis was found.
This decrease in richness is consistent with that recorded in the Great Salt Lake (USA), where four species were recorded when salinity was close to 50 g L-1 but only one species was found when salinity was close to 250 g L-1 (Wurtsbaµgh & Berry, 1990); and in the Siberian Lake Chany, where an increase in salinity from 0.8 g L-1 to 6.4 g L-1 caused a decrease in zooplankton richness from 61 to 16 species (Kipriyanova et al. 2007). However, caution should be taken before reaching any conclusion, since the absence of significant statistical correlations among the studied environmental variables (water temperature and salinity), and the zooplankton parameters (density and biomass) is by no means a confirmation of a negative effect of increasing salinity on these biological parameters.
The largest mean body sizes for both B. poopoensis and M. eµgeniae were recorded in the final three months of the study; this period was marked by low temperatures and hypersalinity. Whereas in ectotherm vertebrates, individuals of small size are likely to occur at lower environmental temperatures (Gillooly et al. 2002; Ohlberger, 2013), in zooplankton animals, such as cladocerans (Havens et al. 2015) and copepods (Anufriieva & Shadrin, 2014), larger body sizes have been registered at lower temperatures. In the present study, the observed increase in body size for B. poopoensis and M. eµgeniae is a likely consequence of the stress produced by increased salinity rather than that of low temperatures (Herbst, 2001). Such an effect could be obtained by negatively influencing reproduction, as evidenced by the lack of ovigerous, parthenogenetic females of M. eugeniae, in May, and the absence of nauplii B. poopoensis larvae in June and July. Moreover, low temperature could not affect the reproduction of these two species, since ovigerous M. eµgeniae females were found throµghout the winter months in two temporary saline lakes in 2006 (a period of relative hydrological stability) (Echaniz & Vignatti, 2011; Echaniz etal. 2013b). In addition, in three mesosaline lakes of LaPampa, with relatively stable salinity, the highest population densities of B. poopoensis were recorded during the winter months, when a high proportion of nauplii and different stages of copepodites were found (Vignatti et al. 2016).
A feature of many shallow lakes of the region is that the combination of their temporary nature and their high salinity hinders their colonization by fish (Menni, 2004; Rosso, 2006). This situation, verified in Ojo de Agua Uriburu, may also lead to relatively large-sized zooplankton crustaceans, such as B. poopoensis and M. eugeniae.
Althoµgh phytoplankton richness can also decrease because of increased salinity, thus lowering the concentration of chlorophyll-a, the presence of M. eµgeniae could also too help to explain the low concentrations of chlorophyll-a in this lake. Grazers are considered to play a key role in decreasing the amount of phytoplankton species of the genus Daphnia (Muylaert et al. 2006; Boveri & Quiros, 2007; Echaniz et al. 2010). It is likely that, as a relatively large size grazer, M. eµgeniae had been contributing to keeping low phytoplankton levels in the lake (Echaniz, 2010).
The environmental and biological changes that occur during lake drying have already been previously studied in two lakes of La Pampa. In comparison with the information obtained in the present study, variation in lake dynamics, due to differences in environmental conditions, can be highlighted. One of the previously studies lakes, El Guanaco, was subsaline, had a wide vegetation cover, showed a high biological richness (35 taxa), and hosted a species assemblage very different from that recorded in Ojo de Agua Uriburu, with rotifer predominance until complete drying (Echaniz & Vignatti, 2010). The other lake, Aime, was hypo-mesosaline, but shared some characteristics with Ojo de Agua Uriburu, namely, reduced water transparency caused by resuspended sediments and total lack of vegetation (Vignatti et al. 2012a). Furthermore, there are important ecological differences between these three ecosystems. For instance; their different species richness and contrasting zooplankton density dynamics during their drying phases. In Ojo de Agua Uriburu, species richness was low, and density and biomass decreased when salinity exceeded 50 g L-1; whereas in Aime Lake, species richness was higher (16 taxa), salinity barely exceeded 23 g L-1; and zooplankton density experienced a peak, triggered by rotifers, exceeding a density of 29 000 ind L-1.
This study is the first that documents the changes that occurred in an Argentine lake that reached hypersalinity. Comparison with existing data for other systems, confirms the remarkable variation in the ecology of saline lakes in the semiarid central region of Argentina. Therefore further studies are necessary to better understand the scarcely known ecological aspects of the filling and drying of a variety of water bodies, which may differ in their dynamics despite having similar characteristics.
Conclusions
The lakes in Central Argentina are temporary because they are strongly influenced by wet and dry climate cycles. Most of their ecological aspects have been studied during periods of relative stability; however, the dynamics that emerge as these lakes are being filled and dried constitute little studied scenarios of paramount interest.
The Ojo de Agua Uriburu lake, located in eastern La Pampa province, is in an arheic basin fed by phreatic inputs and rainfall. In December 2012, its depth was 0.7 m and its salinity was close to 16 g L-1. In July 2 013, its depth decreased to 0.06 m and its salinity increased to above 92 g L-1 before the lake dried up completely.
A total of six halotolerant species account for the zooplankton richness in the Ojo de Agua Uriburu Lake. Two of these six species, dominant in the lake, are the endemic, neotropical crustaceans Boeckella poopoensis and Moina eugeniae.
Currently, only two other lakes in the region have been studied as they dry. Their salinity is lower and their species richness is higher. In the light of the present study, it is safe to assume that there is high variation in the ecology of saline lakes in the central region of Argentina. Therefore, establishing generalizations about their dynamics is relatively difficult, and more studies should be conducted on this topic.