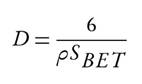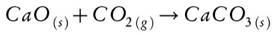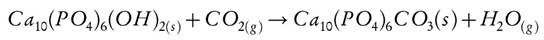Introduction
Millions of tons of fish scales are discarded and wasted around the world. In Colombia, fish scales are considered worthless, unusable, and are often eliminated as waste. Consequently, the local fish industry generates large amounts of fish waste per year, of which tilapia scales have a considerable share. Ideas have been proposed on how to create value for fish scales and how to use this resource[1-4]. The surface of fish scales is constituted by numerous high-value organic and inorganic components, such as hydroxyapatite (HAp) [5-7] and collagen[8,9], both of them are of interest to the food manufacturing, cosmetics, functional biomedical product, and catalytic material industries.
Whereas collagen is highly valued in the industry due to its applications in cosmetic production, interest in HAp has grown for its uses as biomaterial[10,11], adsorbent[12-14], and as catalytic reaction supporter[15-17]. HAp is commonly synthesized using wet methods or through solid state reactions to refine particle size or define a specific morphology. However, these methods can be costly and time-consuming, so their production from Ca-rich waste is of relevance from an industrial point of view. The HAp contained in fish scales is a crystallized form of calcium hydroxy-phosphate, and it can be easily extracted by thermal methods or acid treatment[18,19].
There is currently great concern about the negative effects of global warming on the planet’s climate. This climatic anomaly has its origin in the high accumulation of greenhouse gases such as CO2 and CH4, which are generated mainly by anthropogenic activities. In fact, the generation of energy from petroleum derivatives has significantly increased the concentrations of CO2 in the atmosphere. Because of the magnitude of the effects of this gas on the environment, the reduction of CO2 emissions is one of the great challenges of the century[20].
In order to reduce the harmful effects of CO2 on the environment numerous research efforts have been focused on developing methods of adsorption[21-23], capture and controlled release[24-26], and in boosting the catalytic transformation of CO2 into products of commercial interest[27-29]. Among these methods, that of CO2 capture on oxide or Ca-compounds deserves special mention because CaO can form bulk carbonates which, by thermal decomposition, easily release CO2. This type of solids can be obtained from Ca-rich biological waste, like fish scales, and can be directed to form crystalline phases with high thermal stability such as HAp.
Several authors have reported the use of CaO-based solids to capture CO2, and have provided evidence for the low thermal stability of CaO, because of the sintering of granules and destruction of the adsorbent porosity. Landi et al. [30], studied the formation of porous microgranules from a modified synthetic HAp, these solids capture CO2 at temperatures between 800 °C and 1000 °C. They suggested that HAp can capture CO2 through the formation of apatite carbonate (Ca10(PO4)6 CO3) inside the pores of HAp. Hap’s weight increase by CO2 capture was 2.5 % w/w, showing high thermal stability during several carbonation-release cycles, as compared to CaO which showed low thermal stability.
Regarding the thermal stability of CaO, Xu et al. [31] evaluated the performance of Ni/CaO-Al2O3 as a bifunctional catalyst in capturing and releasing CO2 , which they explained as a function of the decomposition of CaCO3 into CaO at 800 °C and the carbonation of CaO into CaCO3 at 650 °C. The CO2 capture capacity of catalysts Ni/CaO, Ni/Al2O3 and Ni/CaO-Al2O3 was evaluated by varying the CaO/Al2O3 mass ratio. The Ni/CaO catalyst showed a higher CO2 -capture capacity during the first cycles (0.5 g CO2 /g), but after 10 cycles its capacity drastically dropped until reaching 0.25 g CO2 /g, after 50 cycles, revealing its poor thermal stability for CaO. In contrast, the thermal stability of CaO-Al2O3 solids was significantly better, with a minimal loss of CO2 capture capacity after 10 cycles. This stability was attributed to the formation of Ca5Al6O14 species uniformly distributed in the catalyst, delaying the destruction of the CaO and CaCO3 granules at high temperatures[32].
In addition, Cesario et al. [33] studied CaO-mayenite systems (Ca12Al14O33), varying the CaO/Ca12Al14O33 ratio and the synthesis method. Their results showed a CO2 capture capacity of 0.43 g CO2 /g, and that the presence of another phase different than CaO (i.e. Ca12Al14O33) improved CO2 capture. Martavaltzi et al. [34] evaluated CaO synthesized from thermal decomposition of Ca(OH)2 and Ca (CH3COO)2. CaO from calcium acetate and obtained the best results with a weight increase of 0.5 g CO2 /g in the first cycle, followed by a drastic dropping when the number of cycles increase. Ca12Al14O33 was incorporated to improve thermal stability, providing a CO2 capture capacity of 0.325 g CO2 /g after 45 cycles. Furthermore, Castilho et al. [35] prepared various adsorbents for CO2 -capture from biogenesis waste treated at 950 °C to decompose the different materials into CaO. Their results showed that samples with greater CO2 capture capacity (0.25 g CO2 /g) were those with higher surface area and porosity, smaller particle size, and lower micro-element traces associated with the starting materials.
In the present paper, we report the results of a physicochemical and morphological characterization of HAps obtained from red tilapia scales found in Colombian fish markets. Tilapia-derived HAps were synthesized following direct calcination and acid-base treatment methods. Thermogravimetric analysis, elemental analysis by X-ray fluorescence, X-ray diffraction, scanning electron microscopy, surface area, infrared spectroscopy, and basicity measurements by CO2 -pulse titration at 298 K were used to carry out a complete description of the characteristics of the tilapia-derived HAps. Since tilapia scales are a biological Ca source, the present investigation also explores the CO2 capture and release capacities below 923 K for tilapia scale-derived HAps to study their potential use as low-cost adsorbents in mitigating atmospheric CO2 loads.
Materials and methods
Hydroxyapatite (HAp) extraction
Red tilapia (Oreochromis sp.) scales used as raw material were collected in common commercialization sites from the Department of Huila (2.9345 °N, 75.2809° W) in Colombia (Fig. 1) Scales were washed with distilled water, dried at room temperature and triturated before use. HAp was extracted from the raw material by two methods as reported in the literature[18,19]. According to method one, scales were directly calcined in static air at 973 K for 5 h with a ramp of 10 K min-1.
As to method two, known as the acid-base treatment, the scales were deproteinized with 0.1 M HCl and washed with distilled water; subsequently, they were treated with 5 % w/v NaOH at 343 K for 5 h. After this time, the fine white precipitated powder was rinsed with distilled water and finally treated with 50% w/v NaOH under reflux for 1 h. The final solid was washed, dried at 333 K, and calcined in static air at 973 K for 5 h with a ramp of 10 K min-1.
Characterization
Thermal decomposition of the raw material was examined by Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC) in air atmosphere of 80 % v/v N2, 20 % v/v O2. A TA-SDT-Q-600 apparatus (TA Instruments Corporate Headquarters, New Castle, UK) equipped with a TGA-DSC module was employed under a heating ramp of 10 Kmin-1. An Al2O3 crucible was used as reference for the tests.
Elemental analysis was performed by X-ray fluorescence (XRF) on a PHILIPS PANALYTICAL MAGIXPRO PW-2440 (Nottingham, UK) spectrometer equipped with an Rh-tube. The sensitivity of this equipment is 0.02 % w/w. X-ray powder diffraction (XRD) profiles were taken on a PANALYTICAL XPERT PRO MPD (Almelo, The Netherlands) with 2θ geometry, using a Cu-anode (λ= 0.154056 nm) and step/time ratio of 0.02 °2θ s-1.
The morphology of the solids was observed by Scanning Electron Microscopy (SEM) using a TESCAN VEGA 3SB microscope (Brno, Czech Republic) that operates with a W-filament. For this analysis, samples were deposited on carbon tapes and coated with a gold layer prior to visualization.
The surface area of a studied sample was assessed by the Brunauer-Emmett Teller (B.E.T.) method (0.05 < P/P0 < 0.35) entailing N2-adsorption at 76 K using a MICROMERITICS ASAP 2020 equipment (Norcross, USA), with prior degassing under vacuum at 573 K for 4 h. The average particle diameter (D) was determined by equation 1 referenced as in2:
Where D is the average particle size in um, p is the density of pure HAp (3.16 gcm-3), and Sbet is the B.E.T. surface area (m2g-1).
Fourier transform infrared spectroscopy (FTIR) analyses were conducted with a SHIMADZU IRTRACER-100 spectrometer (Kyoto, Japan). Samples were prepared as tablets by dilution with KBr.
Basicity measurements were carried out by CO2 -chemisorption by pulses using a CHEMBET 3000 QUANTACHROME equipment (Boynton Beach, USA) following a method reported elsewhere[36].
Solids were previously degassed under He flow at 773 K for 1 h and the sample’s CO2 uptake was quantified using a thermal conductivity detector through injection pulses of 50 µL of CO2 at 298 K36.
CO 2 capture capacity measurements
In order to evaluate CO2 capture capacity, a modified protocol was followed[32,35], and it was conducted in a TGA equipment, model TA SDT Q 600 (TA Instruments Corporate Headquarters, New Castle, UK). A sample of 10 mg of HAp’s with particle sizes less than 200 /um was placed in the microbalance of the TGA device and then heated to 1 073 K under N2 atmosphere to ensure total decarbonation. Subsequently, the sample was subjected to 15 carbonation-decarbonation cycles, where a cycle consists of 15 min of carbonation at 923 K with pure CO2 flow (50 mL min-1) and 10 min of calcination at 1 073 K in N2 (50 mL min-1) with a heating and cooling rate of 10 K min-1. The sample’s CO2 capture capacity, in mg CO2/g of adsorbent, was calculated according to equation 2:
Where, wt corresponds to the sample’s weight at time t, and w0 corresponds to its initial weight.
CO2 capture capacity for bulk CaO was performed to establish a reference to which sample results could be compared. CaO was synthesized from Ca(NO3)2.6H2O calcined at 973 K for 5 h with a ramp of 10 K min-1.
Results and Discussion
Fig. 2. illustrates the TGA and DSC profiles in air atmosphere of the raw material, namely tilapia scales thermal behavior. As revealed by its mass loss profile, at a temperature range of 300 to 500 K, there is an endothermic loss of 10.8 %, corresponding to a release of water and physisorbed gases. Subsequently there are two exothermic mass losses of 30.2 % between 500 and 600 K, and of 17.34 % loss between 600 and 800 K. The first exothermic event corresponds to thermal decomposition of organic macromolecules, while the second one corresponds to combustion of thermally stable molecular fragments and organic residues. The inorganic residue (ashes) obtained above 880 K, presumably HAp, constitutes 41.7 % of the initial mass.
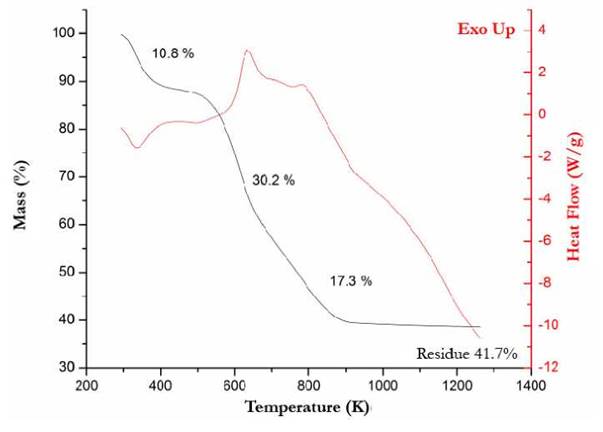
Figure 2 Thermogravimetric Analysis and Differential Calorimetry Scanning in air atmosphere for the raw material.
The absence of heat transfer or mass loss between 880 K and 1200 K suggests that the residue is thermally stable.
Since HAp synthesis of in this work was carried out by calcination at 973 K, a correct removal of organic compounds in the product after heat treatment should be assured. Additional TGA experiments performed on HAp extracted by both methods (not shown), revealed an endothermic mass loss of less than 3 % at temperatures below 200 K indicating an absence of organic residues.
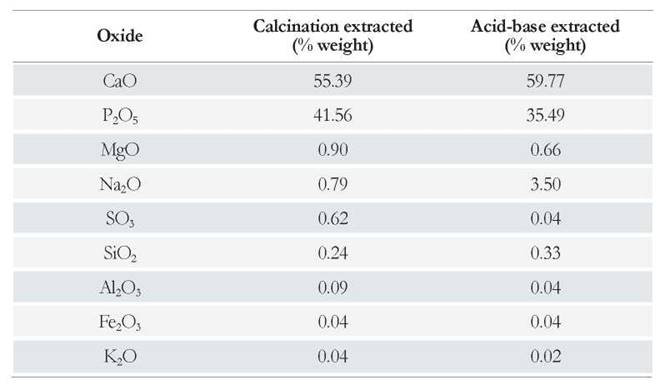
Results of the elemental analysis performed on the HAp samples, reported as % w/w of the more stable oxides of the elements, are presented in Table 1. This analysis revealed that the most abundant elements are Ca and P, in a Ca/P molar ratio of 1.69 for HAp obtained by the calcination method; and that it is very close to the stoichiometric value of 1.67 of Ca10(PO4)6(OH)2. The acid-base treatment yielded HAp with a Ca/P molar ratio of 2.13, which is above the stoichiometric value. Such an observation agrees with P removal during the acid-base treatment in the form of soluble phosphates, coupled with the decrease of S solubilized in the form of sulfates. These results can also be attributed to the exchange of phosphate ions by carbonate ions in the crystalline structure of HAp, as previously described in the literature[18,35].
The analysis results in Table 1 also revealed the presence of elements such as Mg, Na, Si, Al, Fe, and K; these elements are typically found in biological material. Traces of Cu, Zn, and Sr, among others, were detected in HAp obtained by calcination and their presence was evidenced by the blue coloration of the sample. Such blue color was not observed in the Hap extracted by the acid-base treatment; because the elements responsible for this coloration are removed from the sample in the form of soluble salts. Consequently, the acid-base treatment lead to an increase in the Na concentration, with respect to the calcination method, due to the exchange of metal ions by Na+ ions during the NaOH washes.
The XRD profiles for both the raw material (fish scales) and the obtained HAps are depicted in Fig. 3. The XRD for the scales (Fig. 3a) reveals the typical profile of a sample composed of both, crystalline and amorphous phases. Wide and low intensity signals in cps (counts per second) corresponding to the HAp phase, proteins and low crystalline polymeric carbohydrates, such as chitin and chitosan, belong to amorphous phases.
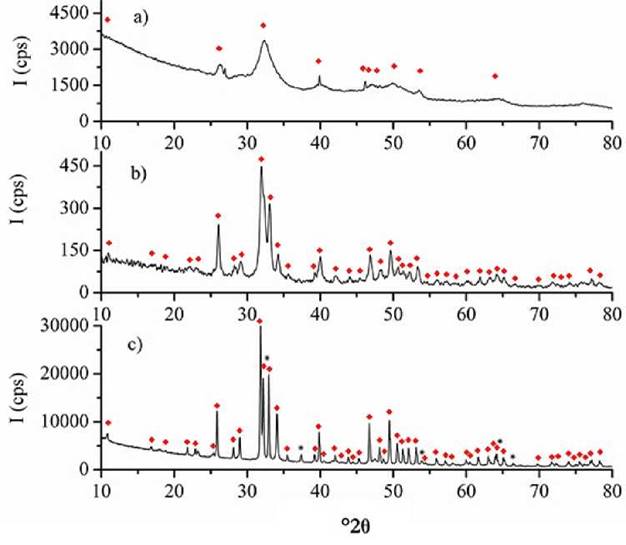
Figure 3 X-Ray Diffraction profiles for a) Raw material, b) HAp extracted by calcination and c) HAp extracted by acid-base treatment. ♦ HAp according to JCPDS 00-009-0432, * CaO according to JCPDS 00-082-1691.
The samples extracted by both treatments exhibited the typical XRD of an HAp phase, this was supported by the analysis results of the reference standard JCPDS 00-009-0432. Furthermore, these results are in accordance with the elemental analysis outcomes. However, the differences between the HAp obtained by the two methods are worth noting. The HAp that resulted from calcination (Fig. 3b), revealed a lower crystallinity than that of the HAp obtained by acid-base treatment (Fig. 3c), as more intense (in cps) and defined signals were obtained for the latter sample. In addition, HAp from the acid-base treatment, exhibited signals at 37.3, 53.9 and 66.5 °2θ corresponding to the CaO phase according to the results on the reference standard JCPDS 00-082-1691). The reason for this is the formation of CaO by solubilization and segregation of Ca from the HAp phase after treatment with strong acids and bases.
The low crystallinity of the HAp obtained by calcination suggests that heat treatment on the raw material hindered the formation of large HAp crystals due to the rapid decomposition of organic matter. In contrast, a gradual solubilization of proteins and carbohydrates, at places where small surface HAp crystals are embedded, occurred during the acid-base treatment. Thus, this method resulted in a gradual nucleation and growth of HAp.
To observe the surface morphology of the raw material and derived HAps, SEM micrographs were produced (Fig. 4). The raw material (Fig. 4a) has a typical morphology of a biogenesis material, characterized by a heterogeneous, irregular surface with a series of intermixed fibers corresponding to the organic matrix of the hard tissues. The HAp obtained by calcination (Fig. 4b) has a heterogeneous morphology composed of intermixed granules with a flat surface, rough edges and different sizes; whereas, the HAp yielded by acid-base treatment (Fig. 4c) shows a fine flower-like morphology caused by a slow crystal growth; this is correlated with the high crystallinity evidenced in XRD for this sample.

Figure 4 Scanning Electron Microscopy for raw material a), HAp extracted by calcination b) and HAp extracted by acid-base treatment c).
The FTIR spectra for the samples are summarized in Fig. 5. The FTIR for the raw material (Fig. 5a) evidences a wide peak at 3 420 cm-1 corresponding to -O-H stretching vibrations of polymeric carbohydrates and overlapped stretching bands of -N-H groups in the collagen; and a wide and intense peak at 2 960 cm-1, corresponding to -C-H stretches belonging to carbon skeletons. Furthermore, the raw material also exhibits peaks at 1 654 and 1 545 cm-1, assigned to type-I and -II amide (typical of samples containing collagen); peaks at 1440 and 871 cm-1,assigned to asymmetrical and out-of-plane stretching CO3 2-; and a peak at 1 026 cm-1, corresponding to P-O stretches in PO4 3-.
The FTIR spectra for HAp obtained by direct calcination (Fig. 5b) and acid-base treatment (Fig. 5c) lacked the signals found in the raw material; consequence of the elimination of organic compounds, such as collagen. The peaks at 3 566, 3 580, 3 466, and 3 418 cm-1 correspond to -O-H groups stretching vibrations. The shape and intensity of these signals are typical of an HAp phase 19.
The peak at 1470 cm-1 in (Fig. 5b) corresponds to a symmetric stretching of CO3 2-, this signal is more pronounced in the HAp prepared by acid-base treatment (precisely at 1 475 cm-1, (Fig. 5c). According to XRD results, the HAp obtained by acid-base treatment contains CaO, (see Fig. 3); CaO can be easily carbonated to give CaCO3. The intense and wide peaks, ranging between 1 000 cm-1 and 1100 cm-1, as well as the peaks around 595 cm-1 relate to the different types of stretches of the P-O bond in PO4 2- (Fig. 5b and 5c). The FTIR spectra of these materials are very similar to those reported for HAp from other types of fish scales, confirming the effective extraction of both methods [2,6].
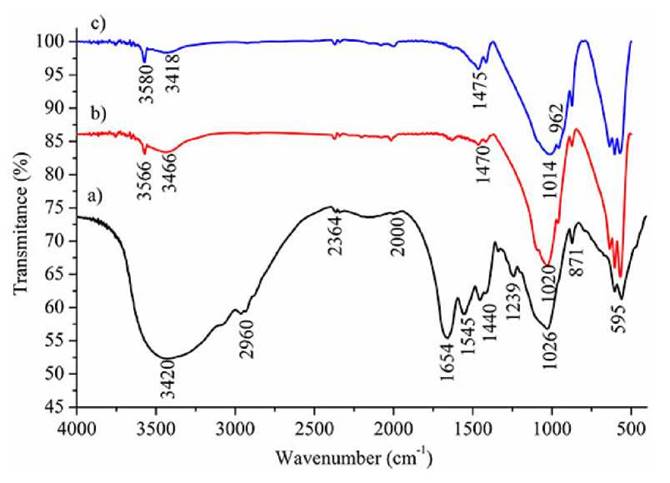
Figure 5 FTIR spectra for a) Raw material, b) HAp extracted by calcination and c) HAp extracted by acid-base treatment.
The B.E.T. area values registered for the obtained HAp samples are given in Table 2. The surface areas are particularly low (17.5 and 2.5 m2g-1), compared to the reported value from commercial HAp of 56.8 m2g-1 2. This HAp ha a low porosity in consonance with the type of morphology observed in the SEM micrographs in Fig. 4, showing compact and poorly porous granules. Contrastingly, the HAp obtained by calcination has a higher surface area because of its lower crystallinity and particle size. The average calculated particle sizes were 0.108 µm for HAp (calcination) and 0.759 µm for HAp (acid-base treatment).
The basicity analysis results of the obtained HAps are presented in Table 2. These analyses were based on CO2 -pulse titration at 298 K, and their results expressed as µmol/g and µmol/m2 of CO2 uptake Compared with other types of solids, such as MgO 37, CaO 38, or Mg-Al mixed oxides 39, which have basicities as high as 180 µmol/g, the basicity of the studied HAps is considerably low, indicating that these solids have a moderate surface basicity, and that they mainly have surface sites where physisorption mechanisms occur through formation of surface carbonates 36. The present analysis results are correlated with those of B.E.T areas, crystal size and crystallinity, all leading to the conclusion that basicity increases with increasing area and decreasing crystal size.
To establish whether HAp from tilapia scales retain any CO2 capture capacity at high temperature, thermogravimetric experiments were carried out through 15 carbonation-decarbonation cycles (Fig. 6). The CO2 capture mechanism on Ca-containing solids has been reported in the literature 40. In the first stage, the pores of the adsorbent are rapidly filled by CO2 forming a layer of surface CaCO3, subsequently CO2 molecules diffuse from the surface layer into granules in a stage governed by a diffusional regime. This mechanism is desired because it allows for the adsorbent to be easily regenerated after saturation. Then, in the calcination step, or second stage, the adsorbent partially or totally releases CO2 from the pores through a diffusive process, which happens at high temperature because of the strong interaction. There is evidence that total CO2 release happens between 700 °C and 750 °C 35.
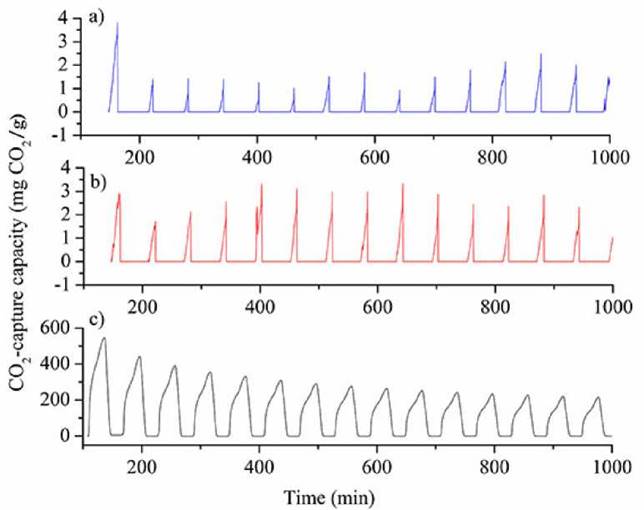
Figure 6 CO2 capture capacity measurements for: a) HAp extracted by acid-base treatment, b) HAp extracted by calcination and c) bulk CaO.
As can be seen in Fig. 6a, HAp obtained by acid - base treatment displays an initial CO2 -capture capacity of 3.9 mg CO2 /g, which decreases in the following cycles revealing a range of thermal stability from cycle two onwards with CO2 -capture capacities fluctuating between1.2 and 2.5 mg CO2 /g.
Fig. 6b reveals that HAp obtained via calcination has a CO2 capture capacity ranging between 2.5 mg and 3.2 mg CO2 /g. CO2 capture for this latter solid shows greater stability in all cycles without sudden losses and its CO2 capture capacity and thermal stability increased when surface area and basicity were also higher.
The CO2 capture capacity for bulk CaO is presented in Fig. 6c. CaO is expected to be a good adsorbent for CO2 because it can form CaCO3 stoichiometrically in a 1:1 ratio (Equation 3). As shown in Fig. 6c an initial capture capacity of 550 mg CO2 /g was achieved, and it progressively decreased through the cycles down to a capture capacity of 200 mg CO2 /g in cycle 15. The observed progressive decline is attributed to CaO-sintering and to the saturation of CO2 forming surface CaCO3 blocking the external pores of the solid.
CO2 capture capacities of tilapia-scale-derived HAps are markedly lower than those for (synthetic) bulk CaO. Nevertheless, the results are promising because the observed CO2 capture capacity values are of constant magnitudes, as it has been reported for synthetic HAp[30], and for CaO/CaCO3-based solids extracted from Ca-rich food wastes[35]. Unlike the results for bulk CaO, the CO2 capture capacity of the HAp obtained by calcination was not altered as cycles progressed, indicating that this solid is thermally stable. The calcination method is simple, inexpensive and does not involve the use of Al compounds to increase thermal stability[32-34].
The low CO2 capture capacities for HAp, may be explained by the fact that the HAp phase cannot easily form bulk carbonates due to its structural crystallinity and thermal stability. In the HAp structure, the possible carbonate (apatite carbonate) route of formation is presented in Equation 4[30].
In this reaction, a dehydroxylation of the structure must occur to allow carbonate formation. However, TGA in Fig. 2 showed that the dehydroxylation of extracted HAp does not occur before 1273 K, this suggests that the CO2 capture capacity registered for HAp are mainly the product of the formation of surface CaCO3 (not bulk). CO2 diffusion towards the internal pores of the material is limited because of crystallinity of the HAp phase.
A total reversibility in CO2 capture-release cycles for the obtained Haps was observed (Fig. 6a and 6b). This opens the possibility for these inexpensive solids to be employed in the reuse of CO2 captured from the atmosphere, through a controlled thermal release from the solid.
Conclusions
Tilapia scales are a Ca-rich biogenesis waste with an inorganic residue of 41.68 % w/w. Hydroxyapatites were synthesized from tilapia scales by two methods: calcination and acid-base treatment. The HAps obtained by the two methods displayed different characteristics in their crystallinity, morphology, composition, surface area and basicity. HAp derived from direct calcination had lower crystallinity, lower grain size, and higher surface area than the HAp provided by acid base treatment, which correlated with a higher surface basicity. CO2 capture capacities of the HAps were consequence of the formation of surface carbonates, finding values between 2.5 mg and 3.2 mg CO2 /g for the HAp obtained by calcination, and 1.2 mg and 2.5 mg CO2 /g for the HAp obtained by acid-base treatment that correlated with the basicity of solids. CO2 capture capacity ranges reported here are promising for CO2 capture solids at high temperature from biological sources, because their high thermal stability and reversibility in the capture/release detected.