Introduction
The lace bug, Corythucha gossypii (Fabricius), can be found across most of the American continent, ranging from southern United States and Mexico to Ecuador, as well as in the West Indies (Varón et al. 2010). The lace bug has the widest plan host range among foliage-eating insects, having been reported in sour soup or guanábana (Annona muricata), passion fruit (Passi lora edulis), and granadilla (Passi lora ligularis). In addition, certain perennial crops, such as papaya (Carica papaya), and annual crops like yucca (Manihot esculenta), sweet potato (Ipomoea batata), aubergine (Solanum melongena), and hot pepper (Capsicum baccatum) are also affected by this insect (Guidoti et al. 2015; Varón et al. 2010).
Lace bugs feed on the underside of leaves and their damage is characterized by premature leaf senescence, followed by focalized yellowing on the leaf blade, which can lead to complete leaf bleaching. These events can reduce plant vigor, thus decreasing fruit production or preventing its formation (Varón et al. 2010). Lace bug-directed insecticides of the neonicotinoid and the methyl-carbamate types, with long residual effect, are commonly used (Nair & Braman, 2012).
The use of chemical insecticides in pest control programs has triμgered secondary pest outbreaks and has been lethal to non-target organisms. Furthermore, increasing insecticide doses, rises the chance of collateral, negative effects on human health, due to the long-term persistence of insecticide residues and their inherent toxicity. In addition, contamination has been reported in water, in the atmosphere, and in the environment as a whole (Ratnadass et al. 2012; Savary et al. 2012). As an alternative to insecticide use, integrated pest management (IPM) strategies are being implemented, and they include, alternate crops with repellent plants, crop rotation, intercalated crops, and biopesticide use (Ratnadass et al. 2012).
Plant-derived extracts have been extensively studied to develop alternatives to conventional insecticides. To this end, species of the Meliaceae, Rutaceae, Asteraceae, Labiateae, Piperaceae, and Annonaceae families have been studied for their bioactivity (De Cássia Seffrin et al. 2010).
The Annonaceae family includes trees and shrubs exclusively found in the tropical and subtropical regions of America, Africa, and Asia, and it comprises 130 genera and 2 300 species (Castillo et al. 2010; Santos Lima et al. 2010). Numerous bioactive substances of diverse chemical nature are present in leaves, roots, fruits, and seeds of Annonaceae plant species. In addition, acetogenin and alkaloid bioactivities are associated with insecticide and pesticide effects (Galvis et al. 2010; Krinski et al. 2014; Solís et al. 2010). The Colombian territory harbors a large number of Annonaceae species, distributed in the following regions: Amazon (54%), Pacific (27.5%), and Andean (27%) (Murillo, 2001).
Rollinia mucosa, commonly called Amazon custard apple (anón), is a wild fruit native of the Amazon (Fonseca et al. 2012; Rodrigues et al. 2010). Previous research done on this fruit showed that its aporphine alkaloids exhibit antimicrobial and fungicidal activities (Caetano & Dadoun, 1987).
Furthermore, the acetogenins present in this plant have a cytotoxic effect against solid tumors (Chavez et al. 1998; Shi et al. 1997). To the best of our knowledge, the insecticidal activity of R. mucosa seed extracts has not yet been studied and reported. Based on previous studies of the Annonaceae family, we set out to evaluate R. mucosa seed ethanol extract to determine its potential use as a bio-pesticide against the lace bug and to identify the main active metabolites responsible for this activity.
Materials and methods
Chemical reagents: TEGO 51, Chloroform, and DMSO were purchased from Merck (Darmstadt, Germany). Ethanol (EtOH), Methanol (MeOH), and Formic Acid were purchased from Sigma-Aldrich (St. Louis Missouri, USA). Acetonitrile (HPLC grade), n-Hexane and Dichloromethane were purchased from J.T. Baker Chemicals (Center Valley PA, USA). Sea salt was purchased from Proquimel (Santa Rosa del Cabal, Colombia). Bifenthrin was purchased from Bayer Crop Science (Monheim am Rhein, Germany). Brine shrimp (Artemia salina) was gotten from San Francisco Bay Brand (Newark CA, USA). Finally, Corythucha gossypii at III, IV, and V nymphal stages were obtained from Hacienda Calamar, Pereira, Colombia.
Standards: Stock solutions of Bullatacin (1) (C37H66O7; molecular weight 622.928 g/mol), and Papaverine (2) (C20H21NO4; molecular weight 339.39 g/mol) were prepared in acetonitrile to a concentration of 100 μg/mL.
R. mucosa seed preparation
Plant material: R. mucosa specimens were collected in the municipality of Santo Domingo, department of Caqueta, southern Colombia. Species status was confirmed at the Herbarium of the Universidad de la Amazonia (HUAZ) with voucher number 1525.
Pre-treatment: Seeds from the collected R. mucosa fruits were washed with TEGO 51 and dried at 37 °C for 72 h. Dry material was then ground and degreased by means of the Soxhlet technique using hexane (1:4 w/v) for 24 h (Castro etal. 2010).
Chemical extraction from R. mucosa seeds and analysis
Obtaining the seed extract: Dry, ground seed material was subjected to passive maceration using ethanol as solvent, at a 1:4 sample-solvent ratio (Castro et al. 2010; Chen et al. 2012; McLaughlin, 2008). Maceration took place at room temperature for one week, with gentle stirring of the macerating material once a day. The Extract was then filtered and concentrated in a rotary flash evaporator and kept at -4 °C.
Chemical analysis: The chemical characterization of the R. mucosa active metabolites was carried out with a liquid-liquid extraction. Starting from a raw ethanolic extract, two fractions were obtained and designated as F1 and F2. F1 corresponds to the acetogenin fraction, obtained from an amount of the raw extract, which is diluted in an aqueous solution of dichloromethane. The organic phase of the resulting F1 solution was then diluted using a methanol-hexane solution. Only the phase obtained with methanol was retained (Castro etal. 2010). The F2 fraction, corresponding to the alkaloid fraction, was obtained from a volume of the raw extract diluted with an aqueous solution of 50% ethanol in a 1:10 ratio. Subsequently, the necessary volume of a hydrochloric acid solution was added until the solution’s pH was 4. Then, the necessary volume of an ammonium hydroxide solution was added until reaching a pH of 8. Afterwards some chloroform is added to the remaining phase which splits; fraction F2 goes to the chloroform phase (Rakotondramasy et al. 2008).
Profiling by HPLC: HPLC was performed on a Jasco 2000 Plus chromatography system (Jasco International Co Ltd, Tokyo, Japan), consisting of a quaternary gradient pump (PU-2089 Plus), a smart auto sampler (AS-2059 Plus), a column oven (CO-2065 Plus), and a UV-Vis detector with diode array (MD-2015 Plus). For the acetogenin fraction, an isocratic system was employed with a mobile phase consisting of water (A) and an acetonitrile (B) (8:92) at a flow rate of 0.4 mL/min for 10 minutes, and detection set at 220 nm. Profiling was based on a UV-VIS spectrum obtained by HPLC from a Bullatacin standard (Castro etal. 2010). For the isoquinoline alkaloid separation, an elution by binary gradient system was conducted with a mobile phase consisting of 0.1% Formic Acid pH 3.5 (A) and Acetonitrile (B). The analysis was initiated using 70% of A and progressed until reaching 60% of B for 35 minutes with at a flow rate of 0.5 mL/min and a detection wavelength set at 270 nm (Ghanavi etal. 2013). Profiling was based on a UV-VIS spectrum obtained by HPLC from a Papeverine standard.
Bioassays
Toxic activity: A toxicity assay of the raw ethanol extract, acetogenin fraction (F1), and alkaloid fraction (F2) were conducted on brine shrimp, A. salina following a modified McLaughlin method (McLaughlin, 2008). Stock solutions of the raw ethanol extract and the F1 and F2 fractions were prepared to a concentration of 100 μg/mL, and a mortality test was conducted in triplicate exposing A. salina to increasing concentrations of seed extract, ranging from 0.05 to 1 μg/mL (i.e. 0.05, 0.1, 0.2, 0.4, 0.6, 0.8, and 1.0 μg/mL). Artificial seawater was used as negative control, and anhydrous ethanol was used as positive control.
Mortality was assessed after 24 h and reported in terms of the Mean Lethal Concentration (LC50), that is the required concentration (μg/mL) to kill half of the experimental subjects in a given time frame. The data were subjected to Probit analysis.
Insecticidal activity against the lace bug: An in vitro assay was implemented with C. gossypii at III, IV, and V nymphal stages; on average in a lapse of three days individuals move from one nymphal stage to the next. Each experimental unit consisted of a plastic box with a lid and a ventilation system, one leaf of A. muricata, and five C. gossypii individuals of the three nymphal stages. The nymphs and the A. muricata leaves were collected at Hacienda Calamar, Pereira, Colombia.
Seed extract solutions of concentrations 5,10,25, 50, and 100 μg/mL, prepared from a stock solution at 1000 μg/mL, were sprayed over the content of the boxes; making sure that the underside of the A. muricata leaves was impregnated previous to placing five nymphs in each experimental unit.
Nymph mortality was assessed 24 h, 48 h, and 72 h after exposure to seed extracts. The negative control consisted of nymphs without any extract exposure, and nymphs exposed to Bifenthrin were used as positive control. In addition, a group of nymphs were exposed to an aqueous ethanol solution to rule out any effect of the ethanol (contained in the extracts) on the insects.
Results and discussion
The average yield of the degreased R. mucosa seed ethanol extract was 1.78%, and those of the enriched acetogenin (F1) and alkaloid (F2) fractions were of 1.19% and 0.38%, respectively.
Chemical analysis: The Chemical characterization results of the R. mucosa seed extract made by HPLC is presented in Fig. 1A, Fig. 1B, and Fig. 1C. The chromatographic profile of the raw ethanol extract revealed six peaks (2-5, 7, and 9 in Fig. 1A). Likely corresponding to acetogenin compounds. This was ascertained thanks to their UV-VIS spectrum, detecting peaks of maximum absorption in the 200-220 nm range (Avelar Lage, 2011).
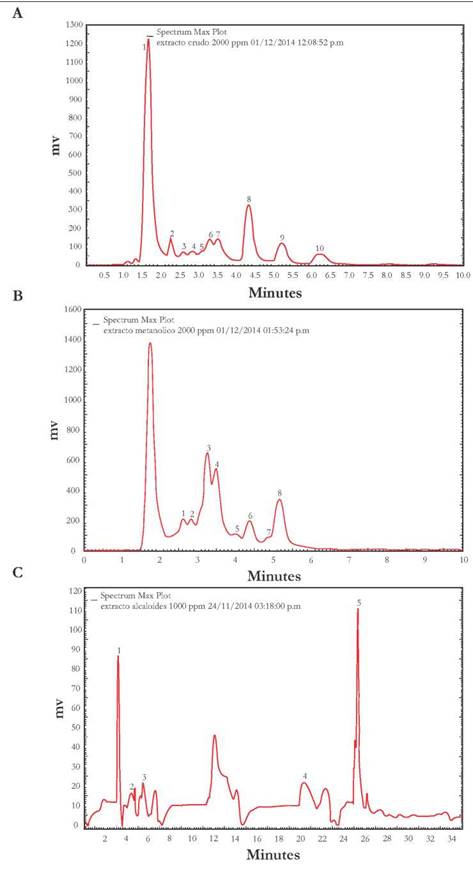
Figure 1 Rollinia mucosa degreased seed extract chromatograms. A. Ethanol extract, B. Acetogenin (F1) fraction, and C. Alkaloid (F2) fraction.
The other peaks shown in Fig. 1A. likely correspond to phenolic compounds, which have maximum absorption peaks at 270 nm (Ferreres et al. 2017) and lignans, which have maximum absorption peaks in the 220-225 and 278-281 nm regions (Fuentealba et al. 2015).
Fig. 1B. shows the chromatographic profile of the acetogenin (F1) fraction. A total of eight peaks were observed, and with the help of their UV-VIS spectra, it was possible to determine that these correspond to acetogenin compounds.
The alkaloid (F2) fraction’s chromatographic profile revealed five peaks (Fig. 1C). With the help of UV-VIS spectra, these peaks were identified as corresponding to isoquinoline alkaloids. According to their chemical nature, these alkaloids were of the aporphynic type, which have maximum absorption peaks at 215, 265, 280, and 294 nm; and of the phenanthrene type, which have maximum absorption peaks at 250 and 279 nm (Hu et al. 2010).
Two of the detected metabolite groups in the of the R. mucosa seed ethanol extract, namely acetogenin and alkaloids, were expected to have insecticidal activity.
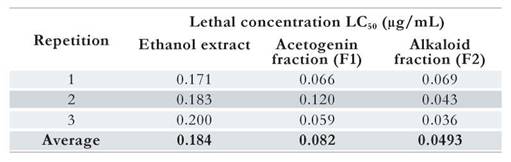
Cytotoxic activity: Median lethal concentrations (LC50) of the raw ethanol extract, the acetogenin fraction (F1), and the alkaloid fraction (F2) on A. salina are shown in Table 1.
Table 1 Toxicity screening of raw Rollina mucosa seed ethanol extract, its acetogenin (F1) fraction, and its alkaloid (F2) fraction on the brine shrimp, Artemia salina.
Insecticidal activity: Increasing concentrations of R. mucosa seed extract did not have an overall negative effect on lace bug mortality (Fig. 2). The highest level of mortality, 86.67%, was observed after 72 h of treatment with R. mucosa seed extract at a concentration of 5 ¡ug/mL. As shown in Fig. 2, the obtained data reveals that a longer the exposure time, leads to increased mortality rates of the lace bug for the five concentrations studied.
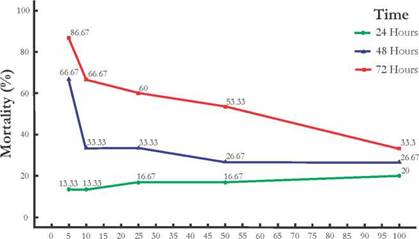
Figure 2 Effect of the concentration of Rollinia mucosa seed extract and exposure time on lace bug mortality (lace bug at III, IV, and V nymphal stages were exposed for 24, 48, and 72 hours).
The LC50 concentration of R. mucosa seed ethanol extract was determined as 74 ug/mL for an exposure time of 72 h. The LC50 concentrations for the other exposure times could not be determined. According to the observed insecticidal effect of the tested concentrations, the most promising concentration range was that between 5 ug/mL and 50 ug/mL; whereas, for a concentration of 100 ug/mL there were no significant rises in insect mortality.
The observation that the highest concentrations of seed extract led to lower mortality rates as exposure time increased, prompted us to hypothesize that at low concentrations insects do not detect the extract and easily feed on the impregnated leaves. Thus, mortality increased in the long term. Contrastingly, at high concentrations the extract itself becomes phagorepellent, impeding insect nourishment, and leading to death by starvation.
Interestingly, during the whole experiment female insects were laying eμgs; this behavior could be related to the effect produced by the compounds present in the extract (Biddinger et al. 2009).
Our results agree with previous reports, in that acetogenins and alkaloids of the Annonaceae family have the same mechanisms of action. It has been reported that the three types of Annonaceae acetogenins of R. mucosa, bis-tetrahydrofuran (THF), mono-tetrahydrofuran (THF), and epoxides have cytotoxic potential, as well as pesticidal, herbicidal, and antifeedant activities (Kuo et al. 2001). The bioactive potential of these compounds is elicited by means of the reduction of ATP levels. This reduction affects the process of electron chain transport in the mitochondria, leading to cell death (Castillo et al. 2010).
There is evidence that isoquinoline alkaloids of to the Annonaceae family have multiple biological activities, including antifungal, cytotoxic, deterrent, repellent, antifeedant, and insecticidal (Torres et al. 2007). These compounds inhibit the enzyme acetylcholinesterase, thus impeding the degradation of the neurotransmitter acetylcholine. As the concentration of acetylcholine increases, hyperexcitation of the Central Nervous System (CNS) causes insect death (W02013043031 A1, 2013).
Conclusions
The active compounds of R. mucosa extract have great potential as bio controllers of the lace bug (Corythucha gossypii), due to their insecticidal and deterrent actions. The results of the present work further support the case for the potential utilization of plant species native to the Colombian Amazon, particularly that of R. mucosa, as biocontrol agents. In addition, this study has provided fundamental information about the biological activity reported for the Annonaceae family species. All the above constitutes a starting point for new research proposals.














