Introduction
Cellulose is an important structural component of the plant cell wall and therefore, one of the most abundant biological materials on Earth. It is a polysaccharide consisting of a linear chain of several hundreds to many thousands of (1 → 4) linked D-glucose units [1]. Cellulose, hemicellulose, and lignin are the main components of the lignocellulosic biomass, such as seed husks, bagasse, woodchips, straw, dry leaves, and sawdust. This lignocellulosic biomass represents an economical, plentiful, renewable energy source because it is generally waste material [2, 3].
These waste materials can be used to produce biofuels, the use of which can help reduce carbon dioxide emission as well as dependence on fossil fuels. For this process, polymers in the lignocellulosic biomass must be broken down into fermentable sugars. The enzymatic hydrolysis of these compounds is an environment-friendly process catalyzed by both types of cellulases (endo-1,4-b-D-glucanase, EC 3.2.1.4; exo-b-1,4-glucan cellobiohydrolase, EC 3.2.1.91; and b-glucosidase, EC 3.2.1.21) and hemicellulases (exo-1,4-b-xylosidase, EC 3.2.1.37 and endo-1,4-b-xylanase, EC 3.2.1.8) [4, 5]. Also, this hydrolysis can be performed under neutral pH and low temperature and with low by-product formation, thus being highly efficient [6].
Nevertheless, high concentrations of enzymes are required to scale-up cellulose hydrolysis to industrial levels. Therefore, the study of biotechnology-based approaches is important for their use in production of cellulase-producing microorganisms, which are of interest also in the textile, paper, pharmaceutical, food, and detergent industries [6, 7].
This study focussed on Trichoderma, one of the most studied cellulase- producing genera of fungi [4]. T. reesei is the most studied species for cellulolytic enzyme production at an industrial level. It is a common soil fungus found in the rhizospheres of crop plants, decaying wood, and other decomposing materials. It is characterized by rapid growth, mostly bright green conidia, and a repetitively branched conidiophore structure [8]. Although it is believed that T. reesei is the only and indispensable choice for enzymatic cellulose saccharification and mutant strains with high cellulolytic activity, such as T. reesei Rut C30 have been developed, recent publications have increasingly demonstrated that fungi other than T. reesei are used for cellulolytic enzymes production and it is necessary to optimize the culture conditions for these fungi as well as the technology required for efficient cellulase production in bioreactors [9-12].
Several strains of Trichoderma have been used in submerged fermentations to study cellulase production using substrates, such as cellulose [5], pulp mill lime mud [13], flower stems [14, 15], and crop residues [16]. Likewise, researchers have also used corncob [6], mushroom compost [17], oat straw [18], wheat bran [18, 19], rice husk and bran [20], rice straw [19, 21], cauliflower and legumes residues [21], and sugarcane bagasse [19, 22] as substrates in solid state fermentations (SSF). Additionally, enzymatic hydrolysis of corn stover, rice straw, sawdust, and paper has been performed with cellulases produced by Trichoderma to obtain fermentable sugars [2, 5, 6, 23]. However, these substrates must undergo pretreatment for lignin removal, because lignin constitutes a barrier to cellulose breakdown by microorganisms [6, 24].
Waste paper can be used as a substrate in SSF to produce cellulases with fungi of the genus Trichoderma [3]. This type of fermentation, in comparison to others, enables the use of low-cost substrates, recovery of enzymes with higher concentrations, and faster growth of aerobic microorganisms, such as the filamentous fungi. SSF also uses less energy and has lower sterility requirements than those of submerged fermentations [25]. Paper has low lignin and high cellulose contents and does not require any chemical pretreatment for its use in cellulase production. Hence, besides being environment-friendly, waste paper is an ideal substrate for fungal cellulase production.
The objective of this work was to evaluate cellulolytic enzyme production in SSF with a native isolate of T. asperellum using paper and sawdust as substrates and to ascertain the effect of lignin on enzyme production. A strain of T. reesei was used as reference organism.
Materials and methods
Microorganisms
T. reesei T114 was donated by the Environmental and Agricultural Biotechnology Unit of the Biological Research Corporation (CIB). T. asperellum and other fungi were isolated from coffee pulp after 60 days of ensilage by serial dilutions in saline solution and culturing on potato dextrose agar (PDA) supplemented with gentamicin. The colonies were screened for the characteristics reported for Trichoderma and subcultured to obtain the isolates.
To obtain monosporic cultures, conidia suspensions from each colony were serially diluted and inoculated onto agar-agar plates. Next, cultures were made from the plates containing conidia separated enough to transfer them individually to a new plate [26], which was then incubated at 25 °C for 6 days and further stored at 4 ◦C.
Morphological and molecular characterization
Isolateswereobservedafter6daysofincubationonPDAplatesat25°C.Fungi were identified according to their macroscopic (color, texture, and appearance) and microscopic (appearance of hyphae, conidia, and conidiophores) features. Genomic DNA of native T. asperellum was extracted from the pure culture and the internal transcribed spacer (ITS4 and ITS5) regions of the ribosomal DNA were amplified by PCR and subsequently sequenced. Sequences were aligned and compared against available sequences in the databases GenBank, EMBL (European Molecular Biology Laboratory) and UNITE (https://unite.ut.ee) using the BLAST of NCBI (National Centre for Biotechnology Information, (http://www.ncbi.nlm.nih.gov/).
Validation of fungal cellulolytic activity
To establish their cellulolytic activity, isolates were cultured in a medium containing the following components: carboxymethyl cellulose (CMC), 10 g/L; (NH4)2SO4, 0.5 g/L; CaCl2, 0.5 g/L; KH2PO4, 0.1 g/L; K2HPO4, 0.1 g/L; and agar-agar, 15 g/L. Additionally, three modifications of this culture medium were used to further evaluate fungal growth: i) use of sawdust with an average particle size of 0.15 mm instead of CMC, ii) addition of 2.5 g/L of yeast extract and peptone to the original composition, and iii) use of CMC without adding salts. Hydrolysis of cellulosic substrates was confirmed after incubation for 5 days at 30 ◦ C.
Solid state fermentation
Sawdust and bond paper were used as substrates. Paper was cut into pieces with sizes of approximately 5 x 5 mm2. Sawdust was ground to an average particle size of 2 mm. Both the substrates were sterilized in an autoclave at 121 ◦ C for 15 min, followed by drying at 70 ◦ C for 24 h.
SSF was performed in 250-mL Erlenmeyer flasks sealed using cotton balls, with each flask containing 10 g of dried substrate. The substrates were moistened with a sterile solution (yeast extract, 2.5 g/L; peptone, 2.5 g/L; (NH4)2SO4, 0.5 g/L; CaCl2, 0.5 g/L; KH2PO4, 0.1 g/L, K2HPO4, 0.1 g/L; pH, 6.0) to obtain an initial humidity content of 80 %. Conidia from PDA cultures were suspended in a solution of Tween 80 (0.1 % v/v) and inoculated into the above mentioned solution to obtain a final concentration of 1 x 107 conidia/mL. Erlenmeyer flasks were incubated at 28 ◦ C with 80 % relative humidity for 12 days.
Enzyme assays
Crude enzymatic extracts were obtained by washing the cultures with 50 mM citrate buffer solution (1:2.5, w/v, pH 4.8) for 30 min. Solids were separated by centrifugation at 16 000 x g and 4 ◦ C for 15 min. Supernatants were stored at -20 ◦C.
Total cellulolytic activity (FPase) was measured using the filter paper assay (FPA) according to Ghose, 1987 [27]. Whatman filter paper N1 was soaked in 1 mL of the enzyme extract that was diluted in 1 mL of 50 mM citrate buffer (pH 4.8). The reaction mixtures were incubated at 50 ◦C for 30 min. To measure endoglucanase activity (CMCase), 1 mL of the enzyme extract was added to 1 mL of CMC (2 % w/v) prepared in citrate buffer. The mixture was incubated at 50 ◦ C for 30 min.
The concentration of reducing sugars released was measured using the dinitrosalicylic acid (DNS) method [2,8]. For this method, 0.5 mL of DNS solution (NaOH, 1.6 g; sodium and potassium tartrate, 30 g; 3,5-dinitrosalicylic acid, 1 g; in 100 mL of distilled water) was added to 0.5 mL of each sample and incubated in boiling water for 5 min. After the samples were cooled to room temperature, the absorbance at 540 nm was measured with a Nanocolor® spectrophotometer. A glucose solution (4 g/L) was used to plot the calibration curve. The extract obtained from uninoculated substrate was used as negative control.
One enzyme unit was defined as the amount of enzyme required to release 1 μmol of reducing sugars in 1 hour at 50 °C. The results were calculated using Equation 1 [6].
where E A is the enzyme activity (U/g), RS is the concentration of reducing sugars released (mg/mL), u e is the extract volume (mL), E is the mass of fermented substrate (g), and t is the reaction time (h).
Experimental design and statistical analysis
The solid state fermentation was performed through a randomized design and a factorial arrangement with three replicates for each treatment was followed. A two-way analysis of variance (ANOVA) for enzymatic activities with substrate and fungal isolate as factors was conducted with a significance level of 0.05. Significant differences were analyzed using Tukey's multiple comparison test. All tests were performed using R®.
Results and discussion
Fungi isolation and characterization
Besides Trichoderma, 12 other fungi were isolated from coffee pulp. After 2 months of ensilage, the coffee pulp from Coffea arabica contained ashes (14.68 %), lipids (1.49 %), proteins (19.91 %), fibers (29.47%), soluble carbohydrates (34.47 %), nitrogen (3.19 %), phosphorus (0.23 %), potassium (6.55%), calcium (0.75%), and magnesium (0.18%) [29]; these micro- and macronutrients are required for the growth of microorganisms. Hence, many microorganisms can use coffee pulp as a substrate for growth.
Microorganisms of the genera Aspergillus, Candida, Enterobacter, Penicillium, Streptomyces, Fusarium, Geotrichum, Escherichia, and Pseudomonas have been isolated from coffee pulp ensilaged for 2 months [29]; coffee pulp is an ideal medium for the growth of fungi and bacteria due to its high humidity content [30]. Likewise, some native fungi of the genus Aspergillus isolated from coffee husk can degrade caffeine and tannins [31]. In this study, we confirmed the presence of some already reported fungi in coffee pulp. In addition, a strain of Trichoderma, which can use cellulosic polymers as an energy source, was found.
One isolate was identified as specie belonging to the genus Trichoderma by observing the morphological features of his colony and his microscopic structures. This isolate presented the following features: hyaline tabicated microhyphae, regularly branched conidiophores, 3-5 hyaline bottle-shaped phialides on the conidiophore's edge, green ovoid unicellular conidia, fast-growing colonies with colorless, reverse, nonaerial mycelium at early stages, and tufty aerial mycelium at later stages. The native isolate was white-spotted green, whereas T. reesei exhibited white and green concentric rings [8, 32]. The results of ITS sequencing of the native Trichoderma indicated 99 % identity with T. asperellum.
Fungi growth on solid media with cellulosic substrates
After 5 days of incubation, native T. asperellum and T. reesei grew on solid culture media prepared with CMC and sawdust as substrates. The colonies were larger on media supplemented with yeast extract and peptone.
Culture medium composition modified T. reesei morphology as well as its cellulase production. Strains growing on media supplemented with yeast extract, peptone, and glucose showed denser and more highly branched mycelia, and thus, larger surface area, which enhanced their enzyme production due to higher enzyme-substrate interaction [32].
Microbial growth was limited in culture media that lacked salts, suggesting that salts are necessary for the production of cellulolytic enzymes; these results validated previous studies, which used pulp mill lime mud as a substrate for the growth of T. asperellum [13].
Enzyme production by SSF
After 12 days of incubation, fungal growth was higher on paper than on sawdust. FPase and CMCase activities were exhibited by both the isolates (Fig. 1 and 2), but these activities were significantly higher in case of T. reesei grown on paper. The concentrations of reducing sugars released in FPA with extracts produced by T. reesei growing on paper and sawdust were 2.82 and 0.96 g/L, respectively, while those of CMCase were 5.24 and 1.84 g/L, respectively.
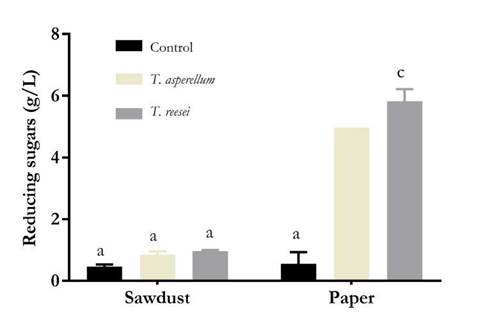
Figure 1 FPase activity of extracts obtained by SSF. Letters indicate significant differences (p < 0.05) between concentrations of released reducing sugars. Source: Authors.
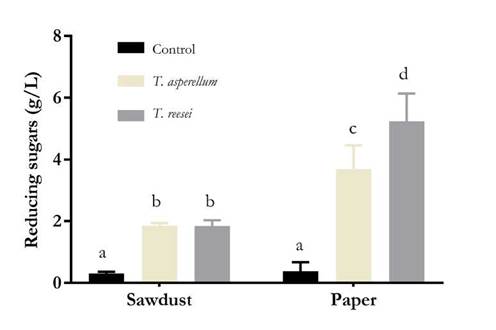
Figure 2 CMCase activity of extracts obtained by SSF. Letters indicate significant differences (p < 0.05) between concentrations of released reducing sugars. Source: Authors.
CMCase activity was also observed for isolates grown on sawdust, although the level was significantly lower than that observed for isolates grown on paper. Similar to other lignocellulosic materials, sawdust is composed of cellulose, hemicellulose, and pectin, forming a complex crystalline lattice with lignin, which makes the material refractory and blocks the interaction between cellulose and Trichoderma endo- and exoglucanases [10]. Other scholars have reported similar results. A considerably low enzyme activity using wood sawdust as a substrate for the production of cellulases with T. harzianum on SSF was found [23]. Similarly, more than 0.6 mg/mL of reducing sugars released using cellulolytic extracts from SSF of lignocellulosic materials with T reesei could not be recovered [19].
In other studies, in contrast with this work, substrates were delignified before SSF to enhance cellulose production [6]. High cellulolytic activity in extracts from mixed and pure cultures of T. reesei and A. niger using wheat bran, rice straw or soybean hulls as substrates, which are much less refractory than sawdust, was found [21, 33]. Given that lignin is the main obstacle for enzyme production using sawdust as a substrate, further studies concerning delignification of sawdust before SSF with Trichoderma should be conducted [34, 35].
The ANOVA calculations (Table 1) showed that both the variables, i.e., the type of substrate and isolate, affected FPase and CMCase activities. Furthermore, interaction between these variables also affected enzyme production, suggesting that enzyme production is markedly different for each isolate depending on the substrate used.
Table 1 Two-factor ANOVA performed on the data of total cellulolytic (FPase) and endoglucanase (CMCase) activities (p < 0.05).
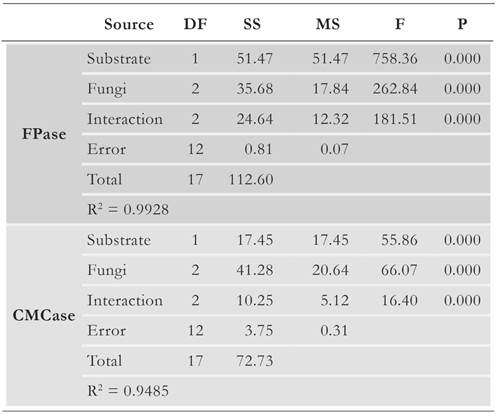
Table 2 shows FPase and CMCase activities measured in enzyme units per gram of dried substrate (U/g). T. asperellum and T. reesei exhibited FPase activity that was higher than that of the negative control when using paper as the substrate. In contrast, no significant differences were found when using sawdust as the substrate, probably due to the barrier effect of lignin. FPase activity comprises the activity of endo- and exoglucanases, which can be produced by Trichoderma in the presence of an inducer, such as sophorose or cellulose, the latter being highly accessible in the fibers of paper pulp but not in sawdust [36].
Table 2 FPase and CMCase activity of extracts obtained by SSF reported in enzyme units per gram of dried substrate. For each enzyme activity measure, letters indicate significant differences (p < 0.05).
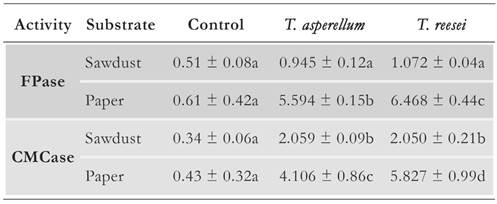
Furthermore, significant differences were found in the CMCase activities between the two isolates and the negative control, which were higher on paper than on sawdust as the substrate (Table 2). Trichoderma endoglucanases cleave glycosidic bonds in a random manner, preferentially in portions of low molecular weight [23]. These enzymes may be induced by molecules present in both paper and sawdust.
CMCase activity was higher than FPase activity when sawdust was used as the substrate. Other studies using isolates, such as those consistent with Trichoderma, SSF, and lignocellulosic substrates, such as wheat bran and straw, rice husk and straw, and sawdust, have reported similar results [18, 20, 21, 23]. This probably occurs because endoglucanases are produced in high quantities to reduce the size of cellulose chains, thus increasing the availability of energy sources to fulfill the energy demand in phases of higher biomass production [20].
On the contrary, FPase activity was higher than CMCase activity when paper was used as the substrate. Although this phenomenon is not common for Trichoderma cultures grown on lignocellulosic substrates, it has been observed in other studies a higher FPase activity with low concentration of nitrogen source and inoculum proportion in the cultures [37].
It should be considered that FPase activity was measured using filter paper in the enzymatic reaction; hence, it is possible that Trichoderma enzymes specific for this type of substrate were synthesized. As reported in other studies, T. reesei exhibited the highest cellulolytic activity. However, the native isolate of T. asperellum also produced cellulolytic enzymes that catalyzed the release of fermentable sugars using paper as the substrate. It will be noteworthy to investigate the ability of both the fungi to utilize different kinds of waste papers as an alternative source of reducing sugars and cellulolytic enzymes, which can hydrolyze other lignocellulosic materials.
Reliable comparisons with other studies cannot be made because there are only a few reports regarding the production of fungal enzymes by SSF using paper as a substrate, and those experiments were not conducted using similar temperature, incubation time, and buffer concentration as used in the current experiment.
Conclusions
This study confirmed that it is possible to isolate Trichoderma, widely reported as cellulolytic enzyme producers, from decomposing lignocellulosic materials. T. reesei isolate showed a higher production of endo- and exoglucanases than that by native T. asperellum. FPase and CMCase activities were very low when sawdust was used as a substrate in SSF with both the fungal isolates, probably because of the high lignin content, which acts as a barrier for fungi-cellulose interactions.
T. asperellum has been used for the biological control of some phytopathogenic fungi and although the cellulolytic activity of native T. asperellum in this study was lower than that by T. reesei, it was demonstrated that paper can be useful as a substrate for biomass and conidia production of T. asperellum.















