Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Ciencia y Tecnología Agropecuaria
versão impressa ISSN 0122-8706versão On-line ISSN 2500-5308
Cienc. Tecnol. Agropecuaria vol.20 no.1 Mosquera jan./abr. 2019
https://doi.org/10.21930/rcta.vol20_num1_art:1248
Salud animal
Babesia bigemina in cattle from the municipality of Los Palmitos (Sucre, Colombia)
1Investigadora, Universidad de Sucre, Grupo de Investigaciones Biomédicas. Sincelejo, Colombia.
2Docente-Investigador, Universidad de Sucre, Facultad de Educación y Ciencias, Departamento de Biología, Grupo Investigaciones Biomédicas. Sincelejo, Colombia.
3Docente-Investigador, Universidad de Sucre, Facultad de Educación y Ciencias, Departamento de Biología, Grupo Investigaciones Biomédicas. Sincelejo, Colombia.
Bovine babesiosis is a disease that usually causes losses in the livestock sector, so it is necessary to diagnose early the haemoparasite in cattle. The aim of this study was the detection, by duplex PCR, of Babesia species that are present in cattle of the municipality of Los Palmitos in the department of Sucre, Colombia. For this, a sample of 218 individuals distributed in 12 farms, between the ages of three months and nine years was studied, through blood samples that were taken and analyzed by microscopic identification in blood smears with Giemsa stain, and by polymerase chain reaction (PCR) with specific primers for the species Babesia bovis and B. bigemina. Three positive samples were obtained for Babesia bigemina by blood smear, corresponding to 1.4 % of the total samples; by PCR, five infected cattle were identified corresponding to 2.33 % of the total, detecting the haemoparasite B. bigemina, which corresponds to the samples identified by optical microscopy. Bovines positive for B. bigemina are asymptomatic; four of them males under two years of age, and only one female of three years of age. In Los Palmitos, B. bigemina is being actively carried by bovines, which remain as asymptomatic carriers of the infection.
Keywords Babesia bigemina; babesiosis; PCR; Sucre (Colombia); tickborne diseases
La babesiosis bovina es una enfermedad que suele causar perdidas en el sector pecuario, por lo que es necesario el diagnóstico temprano del hemoparásito que la transmite a los bovinos. El objetivo de esta investigación fue la detección mediante la reacción en cadena de la polimerasa (pcr) dúplex de especies de Babesia que estén presentes en bovinos, en el municipio Los Palmitos, en el departamento de Sucre, Colombia. Para ello, se estudió una muestra de 218 individuos distribuidos en 12 predios, en edades comprendidas entre tres meses y nueve años, a los que se les tomaron muestras de sangre que se analizaron mediante identificación microscópica en frotis sanguíneo con coloración de Giemsa y por PCR, con cebadores específicos para las especies Babesia bovis y B. bigemina. Se obtuvieron tres muestras positivas para Babesia bigemina por frotis sanguíneo, correspondientes al 1,4 % del total de las muestras; por PCR, se identificaron cinco bovinos infectados, correspondientes al 2,33 % del total, cuyo hemoparásito detectado fue B. bigemina, que obedece a las muestras identificadas por microscopia óptica. Los bovinos positivos para B. bigemina son asintomáticos: cuatro de ellos machos, menores de dos años de edad, y solo un individuo hembra, de tres años de edad. En Los Palmitos, B. bigemina está siendo portada de manera activa por los bovinos, que se mantienen como portadores asintomáticos de la infección.
Palabras clave Babesia bigemina; babesiosis bovina; frotis sanguíneo; hemoparásitos; PCR
Introduction
Babesiosis is a disease transmitted by ticks whose causative agent are intracellular haemoparasites of the Apicomplexa phylum, Piroplasmorida order, Babesiidae family and Babesia genus (Montes- Farah, De la Vega-del Risco, Bello-Espinosa, & Fortich-Salvador, 2012). Within this genus the most important species associated with cattle infections are Babesia bovis and B. bigemina (Mosqueda, Olvera-Ramirez, Aguilar-Tipacamu, & Canto, 2012), and their transmission is carried out naturally by the bite of the tick species Rhipicephalus (Boophilus) microplus (Canestrini, 1888) (Ixodida: Ixodidae). This species has a development related to the climatic and ecological conditions characteristic of the tropical and subtropical zones (Mosqueda et al., 2012), where they are abundant and parasitize domestic animals. Therefore, the presence of the parasite and its vector, makes it difficult to eradicate the disease (Polanco & Rios, 2016).
Babesiosis is considered an emerging zoonosis, which affects domestic and wild animals (Becker, 2011). When outbreaks of the disease occur in cattle, it causes haemoglobinuria, fever, jaundice, anemia, weight loss, dehydration, muscle tremor and weakness (Benavides & Sacco, 2007; Rios, Zapata, Reyes, Mejia, & Baena, 2010). Furthermore, there is also a decrease in the production of milk and meat, a reduction in the reproductive rate due to alterations in the quality of the semen in males, abortions in females, and finally, the death of the individual (Rios et al., 2010). This in turn causes an increase in handling costs of bovine exploitation system due to pharmacological treatments, veterinary attention and control over the haemoparasitic vector (Polanco & Rios, 2016).
In Colombia, the livestock industry is the main cattle production modality with great importance in the national economy. This economic resource is affected by haemoparasitic infections such as bovine babesiosis, which has an incidence of two cases per 100 cattle heads in the country according to the Animal Health Bulletin [Boletín de Sanidad Animal] of Instituto Colombiano Agropecuario (ica) for 2014 (Diaz-Martinez et al., 2017). Its transmission is determined by the vector-parasitehost relationship, which is conditioned by the biotic and abiotic factors of the environment (Rios et al., 2010). In Colombia, the first report of the disease was described by Lleras in 1908, who cataloged the etiological agent as Piroplasma bigeminum (currently known as Babesia bigemina) by microscopic observation of erythrocytes from infected cattle (Mosqueda et al., 2012), and later was recognized as a widely distributed disease (Lleras, 1908). Colombia has the right geoclimatic characteristics for babesiosis to manifest in cattle, by massive infestation of ticks distributed in most of the geographical areas of the country, recorded initially below 2,100 m.a.s.l. Nonetheless, although these have been reported at altitudes above 2,800 m.a.s.l., it has yet not been proven whether the vector reproduces under these conditions (Benavides, Polanco, Vizcaino, & Betancur, 2012).
Current records published in the country indicate the presence of the species Babesia bovis and B. bigemina. In several regions of Bajo Cauca (Antioquia) and Alto San Jorge (Cordoba), the prevalence of the Babesia sp. parasite is 3.1 % (Herrera et al., 2008). In Puerto Berrio (Antioquia) the seroreactivity before specific IgG antibodies against B. bovis and B. bigemina was 65 % in cattle between three and nine months of age (Rios et al., 2010); in the municipality of Gomez Plata (Antioquia) the seroprevalence found in cattle was 89.8 % for at least one species of Babesia spp. (Zapata et al., 2011). Moreover, in Cordoba the seroprevalence of Babesia spp. in purebred Gyr cattle was 3.05 % (Blanco-Martinez, CardonaAlvarez, & Vargas-Viloria, 2015).
However, the data is mostly restricted to the departments of Antioquia and Cordoba, whereas, the department of Sucre is an area without registration, control or published studies of haemoparasites of the genus Babesia that affects cattle. However, in 2014 a study carried out in Ovejas (Sucre) found an infection frequency of 17.34 % for B. bigemina in bovines (Barboza, 2018), however, this disease is not found within the zoonoses monitored by ica (Osorio et al., 2013). From the above, and taking into account that the northern coast of Colombia meets the conditions that contributes to the preservation of the biological cycle of many diseases transmitted by arthropods (Buelvas-Alvis, Buelvas, Miranda, & Mattar, 2008) as Babesiosis, the aim of this study was to detect Babesia spp. that are present in bovines in the municipality of Los Palmitos (Sucre) employing duplex PCR.
Materials and methods
Study área
The municipality of Los Palmitos is located at 185 m.a.s.l. at 09°22´52" latitude N, and 75°16´17" longitude W, with a geographical extension of 211 km2. This municipality has an average annual temperature of 25.5 °C and an average annual precipitation of 1,319 mm, according to Instituto Geografico Agustin Codazzi (IGAC, 1996). The bovine inventory of Los Palmitos, according to Instituto Colombiano Agropecuario (ica, 2016) was 18,041 individuals, from which a sample of 218 bovines was evaluated. The population size was estimated using the Epi-Info program version 7.2.0.1., with a confidence level of 95 %, an error of 0.05 %, and a prevalence of 17.34 %. This last value was obtained from a study conducted on bovine babesiosis in the municipality of Ovejas, in the same department (Barboza, 2018).
Ethical considerations
For the current study, owners or managers of the properties signed an informed consent before the bovines of all ages were sampled. This format explains the purposes, benefits, objectives of the study and potential risks according to what is established in the Resolution 8430 of 1993 of Ministerio de Salud of Colombia (1993).
Sample collection and storage
Each blood sample was extracted by puncture in the jugular vein, using a 5 mL syringe and collected in a Vacutainer* tube with ethylenediamine tetraacetic acid (edta) anticoagulant. The sample was then mixed by inversion with the anticoagulant and sent to the Biomedical Research Laboratory of Universidad de Sucre where it was stored under cold conditions until its analysis (Benavides et al., 2012).
Microscopic identification
The observation of the haemoparasites was performed through blood smears; for this, a drop of blood was deposited on a slide, and with another slide, blood was extended forming an angle of 45 °, and allowed to dry for 3 minutes at room temperature. Subsequently, methanol was added to fix the smear allowing it to dry for 4 minutes.
Then, the Giemsa dye was added and allowed to dry for another 10 minutes. Afterwards, the slide was washed to remove the excess dye, and dried at room temperature. Finally, the parasites were observed under a microscope in a 100X objective with immersion oil, as proposed by Herrera et al. (2008) and Mosqueda et al. (2012).
DNA extraction
DNA extraction was performed following the protocol of high concentrations of salts (Miller, Dykes, & Polesky, 1988) with the following modifications. Initially, 200 μL of blood was transferred to an Eppendorf R tube, then centrifuged at 12,000 rpm for 5 minutes. The supernatant was discarded, and 500 μL of tes lysis solution (Tris- HCl 10 mM pH 8.0, edta 1 mM pH 8.0, sds 0.1 %) was stirred vigorously, and 3 μL of proteinase K was added for digestion at 55 °C for 1 hour. The proteinase was inactivated at 95 °C for 1 minute, and again, it was centrifuged at 12,000 rpm. The supernatant was transferred to a new vial to which 300 μL of potassium acetate (5 M) was added. The precipitate obtained was washed with 400 μL of 70 % ethanol, and finally, the DNA was resuspended in 30 μL of TE buffer (10 mM Tris-HCl and 0.1 mM edta).
Duplex PCR
The reaction mixture was made in a volume per sample of 25 μL which included buffer Taq (1X), MgCl2 (1.5 mM), dNTP Mix (0.2 mM), primers (10 pmol) (table 1) and DNA Taq Polymerase (1 U/ μL). The thermal amplification profile started with a denaturation at 90 °C for 3 minutes, followed by 35 cycles of denaturation at 95 °C for 30 seconds, alignment at 60 °C for 30 seconds, and extension at 72 °C for 30 seconds. In addition, a final extensión was made at 72 °C for 5 min. Finally, the products were kept at 4 °C, and modifications were carried out as proposed by Smeenk et al. (2000).
Visualization of the PCR products
From the products obtained, an aliquot of 5 μL was taken and a 1.5 % agarose gel electrophoresis was performed in which the DNA fragments were visualized in a uv light trans-illuminator (Smeenk et al., 2000).
Purification and sequencing of PCR products
The amplimers that were positive in the PCR were selected; the amount of DNA was quantified and purified with the PureLink Gel Quick Extraction Kit, following the manufacturer's instructions.
Subsequently, the products were transferred into 1.5 mL vials and sent to be sequenced. Nucleotide sequence determination of the amplified products was carried out in an automatic capillary electrophoresis sequencer (abi 3730xl DNA analyzer), based on the chain termination method with dideoxynucleotides marked with different fluorescent groups (Sanger, Nicklen, & Coulson, 1977). In this process, the primers described in Table 1 were used (Service provided by the company Macrogen).
Phylogenetic analysis
The resulting electrophoregrams were edited with the program mega* version 6.0 (Tamura, Stecher, Peterson, Filipski, & Kumar, 2013). Then, the sequences acquired were compared with the sequences registered in the GenBank database using Basic Local Alignment Search Tool (Blast)2 available online to corroborate the species of the genus Babesia that parasitizes infected cattle.
Statistical análisis
A univariate and bivariate analysis of the data recorded in the survey was made, including origin, race, age and sex. These were contrasted with the results of microscopic observation and the PCR. For this, an exact Fisher's test was used (table 2) in order to establish the relationship between the variables and analyzes carried out. The statistical test was performed using the software R version 2.9.1.
Results
From the sample of 218 bovines, 215 individuals were analyzed, of which 69.77 % were females (150), and 30.23 % were males (65), distributed according to age in ranges, as follows. Between 0 and 2 years of age (161 animals; 74.88 %); between 3 and 4 years old (23 animals; 10.70 %), between 5 and 6 years old (23 animals; 10.70 %), and between 7 and 9 years of age (8 animals; 3.72 %) (figure 1). In relation to the breed, all the bovines studied belong to the Cebu breed. The positive samples obtained (5/215) for Babesia bigemina were found in the same field of 12 respondents during the field phase, which are also asymptomatic animals carrying the parasite.
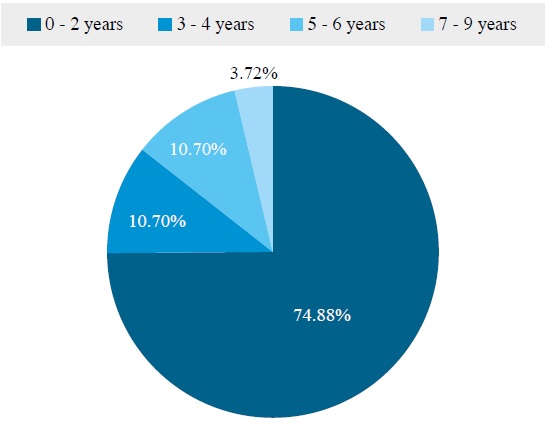
Source: Elaborated by the authors
Figure 1. Percentage distribution of different cattle age groups involved in the study.
Under direct observation, the samples that allowed the evaluation of intracellular parasitic forms morphologically compatible with Babesia spp., were considered positive. The visualization of the smears was carried out in the form of a labyrinth, observing between 100 and 200 fields of the blood smears, whose result indicated that 1.4 % (3/215) of the bovines contained parasites in their red blood cells, which also corresponded to male individuals younger than two years old (figure 2).

Fuente: Elaboracion propia
Figura 2. Observacion microscopica de Babesia bigemina en frotis sanguineo de bovinos (La flecha indica el merozoito de B. bigemina observado a traves de microscopia optica con objetivo 100x).
When using the duplex PCR technique for the molecular determination of Babesia in cattle, five samples were positive for the species B. bigemina, which corresponds to the 278 bp amplified fragment (figure 3), corresponding to 2.32 % of the total number of animals studied. These samples also coincide with the three positive results found in blood smear. In no animal, DNA amplification for B. bovis was found.

Source: Elaborated by the authors
Figure 3. PCR of the positive samples for Babesia bigemina (Numbers 11b, 12b, 13b, 14b and 42b correspond to the positive samples found by PCR, C+b is the positive control, C-b the negative control, and mpm is the molecular weight marker).
The statistical F-test reflected in table 2 indicates that there is a significant difference between the sexes (gender) of the bovines that were detected as positive by PCR. The bovines of the age group between 0 and 2 years old (2.48 % of the 161 individuals sampled with this age range), are the most susceptible to infection with the protozoan B. bigemina, being these four individuals males.
Only one bovine of the age group between three and four years (4.34 % of a 23 individuals sampled) was positive by using the PCR technique for the species B. bigemina, being the only female animal infected.
In addition, these results agree with the three positive ones found by direct search in blood smear.
Discussion
In the 12 livestock farms assessed, babesiosis was found to be caused by the haemoparasite B. bigemina, identified by duplex PCR in five infected animals, which were located in the same field.
In these, tick treatments and manual removal of arthropods are carried out. However, ticks were not found in infected cattle. In the area, tick-vector control measures are the application of tickicide or other substances such as poison or natural extracts every four months, in addition, also the manual removal of the arthropods during the milking process in the mornings. This interrupts the transmission cycle of Babesia spp. (Mtshali & Mtshali, 2013), since female ticks can become infected at the end of the wet phase of the infection, when there is a manifestation of a parasitaemia in the bovine host. However, this parasitaemia is rare and the infestation time is limited. Therefore, according to Rios et al. (2010), only a small number of arthropods contract the parasite. Moreover, these authors state that the high frequency of tick treatments generates a lower degree of infection of R. microplus, and the low frequency of treatment results in a higher degree of infection by ticks.
The microscopic identification of Babesia bigemina in blood smears resulted in three bovines infected with the parasite, however, this method requires trained personnel to identify the Babesia species that infect the erythrocytes (Benavides et al., 2012; Homer, Aguilar-Delfin, Telford, Krause, & Persing, 2000), since, its morphological difference lies in their size, i.e. B. bigemina is larger than B. bovis (Benavides et al., 2012; Smeenk et al., 2000). Although microscopic detection is the reference technique in the identification of babesiosis (Homer et al., 2000), particularly during the acute stage of the disease, the number of intraerythrocytic parasites increases in such a way that they can be detected microscopically (Mosqueda et al., 2012). This technique is not very useful in chronically infected animals in which the disease occurs subclinically or asymptomatically. For this reason, it is necessary to resort to molecular methods such as pcr to detect nucleic acids, due to their high sensitivity and specificity to identify Babesia species in hosts and vectors (Mosqueda et al., 2012; Mtshali & Mtshali, 2013). Employing this technique, results showed that B. bigemina is the haemoparasite that can cause babesiosis in Los Palmitos, finding it in five of the 215 animals assessed in this study. By amplifying a 278 bp fragment (figure 3) corresponding to the gp45 gene specific for B. bigemina (merozoite surface glycoprotein with 45 kD) (Figueroa et al., 1993; Mtshali & Mtshali, 2013), it was established that the species B. bigemina coincides with this species registered in the GenBank database (figure 4). Moreover, it also corresponds to the species found in a study carried out in the municipality of Ovejas (Sucre). Furthermore, it also corresponds to the recent publications that register the presence of the species Babesia spp., B. bovis and B. bigemina as the etiological agents of bovine babesiosis in the region of Bajo Cauca and Alto San Jorge (Herrera et al., 2008), in Puerto Berrio (Antioquia) (Rios et al., 2010), in the municipality of Gomez Plata de Antioquia (Zapata et al., 2011); in Magdalena Medio (Lopez, Florez, Munera, Rios, & Rios, 2012) and in Cordoba (Blanco et al., 2015).
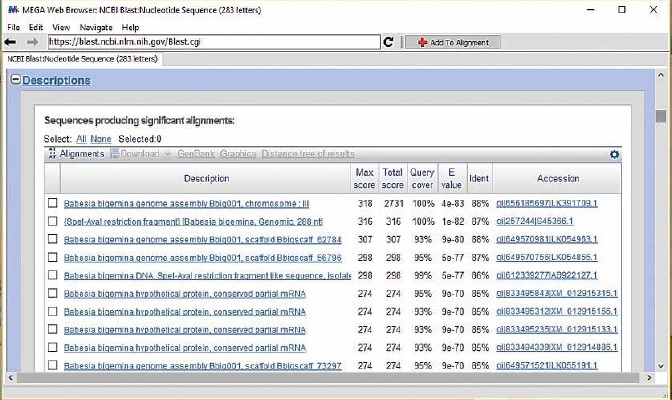
Source: Elaborated by the authors
Figure 4. Comparison of the amplified nucleotide sequences with those registered in the GenBank database employing the BLAST tool.
In the municipality of Los Palmitos (Sucre) the frequency of infection found was 2.32 % (5/215), and is considered low in relation to a study carried out in sheep from the same department (17.34 %) and in Cordoba, where the Babesia spp. frequency was 3.05 % in pure Gyr cattle (Blanco et al., 2015).
The animals studied were asymptomatic, which indicates that, even though they are positive for Babesia, the globular damage by the protozoan is minimal. This shows that in these animals there are no signs of anemia (Blanco et al., 2015), due to the use of tickicides that interrupts the Babesia spp. transmission cycle, and thus, decreases the number of infected cattle in the area.
In addition, the haemoparasite occurs mostly in cattle between zero and two years of age (4/5), which are less susceptible to the clinical effects of the disease than adult cattle (1/5) (Benavides et al., 2012; Benavides & Sacco, 2007). Individuals that become infected with the parasite at an early age acquire passive immunity provided by the mother through the colostrum that lasts until the age of 9 months (Blanco et al., 2015; Rios et al., 2010), although it may persist for four more years (Bock, Jackson, De Vos, & Jorgensen, 2004), making them asymptomatic.
From the above, the individual animal develops an acquired immunity that is conditioned by the inoculation of parasites by the vector, which allows the immunity acquired in the hosts to be maintained, and therefore, the absence of clinical signs or symptoms that causes the disease (Rios et al., 2010). Similarly, in an endemic region for the vector and the haemoparasite such as the northern coast of Colombia, the infection can remain unnoticed when it occurs at an early age, a situation known as enzootic stability (Benavides et al., 2012; Rios et al., 2010). Together, these mechanisms contribute to the natural control of babesiosis.
Conclusions
In the municipality of Los Palmitos (Sucre, Colombia), bovines under two years old are carriers of Babesia bigemina, detected by duplex PCR. The absence of clinical symptoms in infected cattle may be because they act as reservoirs of microorganisms, which awaits the optimal physiological conditions to cause the pathology or the transmission by the vector to other cattle.
Acknowledgements
The authors want to thank God, their families and Grupo de Investigaciones Biomedicas [Biomedical Research Group] of Universidad de Sucre, for their help and technical support during all the research process.
REFERENCES
Barboza, W. (2018). Detección parasitológica y molecular de Babesia spp. en ganado bovino del municipio de Ovejas, Sucre-Colombia (tesis de pregrado). Universidad de Sucre, Sincelejo, Colombia. [ Links ]
Becker, K. (Ed.). (2011). Apicomplexan parasites: Molecular approaches toward targeted drug development. Weinheim, Germany: Wiley-VCH. [ Links ]
Benavides, E., Polanco, N., Vizcaino, O., & Betancur, O. (2012). Criterios y protocolos para el diagnóstico de hemoparásitos en bovinos. Revista de Ciencia Animal, 5, 31-49. [ Links ]
Benavides, M. V., & Sacco, A. M. S. (2007). Differential Bos taurus cattle response to Babesia bovis infection. Veterinary Parasitology, 150(1-2), 54-64. doi:10.1016/j.vetpar.2007.08.022 [ Links ]
Blanco-Martínez, R., Cardona-Álvarez, J., & Vargas-Viloria, M. (2015). Prevalencia de parásitos hematropicos endoglobulares en bovinos gyr puros en Córdoba (Colombia). Revista Medicina Veterinaria, 31, 67-74. doi:10.19052/mv.3710 [ Links ]
Bock, R., Jackson, L., De Vos, A., & Jorgensen, W. (2004). Babesiosis of cattle. Parasitology, 129(7), S247-S269. doi:10.1017/s0031182004005190 [ Links ]
Buelvas, F., Alvis, N., Buelvas, I., Miranda, J., & Mattar, S. (2008). Alta Prevalencia de Anticuerpos contra Bartonella y Babesia microti en Poblaciones Rurales y Urbanas en dos Provincias de Córdoba, Colombia. Revista Salud Pública, 10(1), 168-177. [ Links ]
Diaz-Martínez, O. L., Mendoza-Nino, E., Linares-Chaparro, C., Gasca-Cardenas, H. H., Jaramillo-Ramírez, D. C., Baron-Moya, J. P., … Gonzalez-Garibello, P. M. (2017). Colombia, sanidad animal 2014. Bogota, Colombia: Instituto Colombiano Agropecuario (ica). Recuperado de https://www.ica.gov.co/getattachment/986dd783-8f37-4ab3-bc33-39995bd8c065/2014.aspx [ Links ]
Figueroa, J. V., Chieves, L. P., Johnson, G. S., & Buening, G. M. (1993). Multiplex polymerase chain reaction based assay for the detection of Babesia bigemina, Babesia bovis and Anaplasma marginale DNA in bovine blood. Veterinary Parasitology, 50(1-2), 69-81. [ Links ]
Herrera, M., Soto, A., Urrego, V., Rivera, G., Zapata, M., & Ríos, L. (2008). Frecuencia de hemoparásitos en bovinos del bajo Cauca y alto San jorge, 2000-2005. Revista MVZ Córdoba, 13(3), 1486-1494. [ Links ]
Homer, M. J., Aguilar-Delfín, I., Telford, S. R., Krause, P. J., & Persing, D. H. (2000). Babesiosis. Clinical Microbiology Reviews, 13(3), 451-469. doi:10.1128/CMR.13.3.451 [ Links ]
Instituto Geográfico Agustín Codazzi (igac). (1996). Diccionario Geográfico de Colombia. Bogota: Universidad de los Andes. [ Links ]
Instituto Colombiano Agropecuario (ica) (2016). Censo Pecuario Nacional-2016. Censo bovino en Colombia. Recuperado de http://www.ica.gov.co/getdoc/8232c0e5-be97-42bd-b07b-9cdbfb07fcac/Censos-2008.aspx [ Links ]
López, L., Florez, S., Munera, A., Ríos, S., & Ríos, L. (2012). Evaluacion de la infección por Babesia spp. en garrapatas Rhipicephalus (Boophilus) microplus y la infestación en bovinos de 3 a 9 meses de edad en 9 hatos ganaderos del Magdalena medio Colombiano. Hechos Microbiologicos, 3(2), 37-44. [ Links ]
Lleras, F. (1908). Ranilla o Malaria Bovina en la Sabana de Bogotá. Diagnóstico diferencial con la fiebre carbonclosa. Bogota, Colombia: Imprenta Nacional. [ Links ]
Miller, S. A., Dykes, D. D., & Polesky, H. F. (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research, 16(3), 1215. doi:10.1093/nar/16.3.1215 [ Links ]
Ministerio de Salud de Colombia. (1993). Resolución número 8430 de 4 de octubre, por la cual se establecen las normas científicas, técnicas y administrativas para la investigación en salud. Bogota, Colombia: Ministerio de Salud. [ Links ]
Montes-Farah, J., De la Vega-del Risco, F., Bello-Espinosa, A., & Fortich-Salvador, A. S. (2012). Coinfeccion de Babesiosis y Ehrlichiosis: un caso en Cartagena de Indias, Colombia. Revista Ciencias Biomédicas, 3(2), 339-345. [ Links ]
Mosqueda, J., Olvera-Ramírez, A., Aguilar-Tipacamu, G., & Canto, G. J. (2012). Current Advences in Detection and Treatment of Babesiosis. Current Medicinal Chemistry, 19(10), 1504-1518. doi:10.2174/092986712799828355 [ Links ]
Mtshali, M. S., & Mtshali, P. S. (2013). Molecular diagnosis and phylogenetic analysis of Babesia bigemina and Babesia bovis hemoparasites from cattle in South Africa. BioMed Central Veterinary Research, 9(1), 154. doi:10.1186/1746-6148-9-154 [ Links ]
Osorio, M., F. J., Patino, A., A., Linares, C., C., Romero, G., L. A., Ortiz, C., J., Reina, B., J. F., & Gonzalez, G., P. M. (2013). Colombia, Sanidad Animal 2012. Bogota, Colombia: Instituto Colombiano Agropecuario (ICA). Recuperado de https://www.ica.gov.co/getattachment/bce28fb3-c2c7-4f46-99fc-6bae850353fc/2012.aspx [ Links ]
Polanco, D., & Ríos, L. (2016). Aspectos biologicos y ecologicos de las garrapatas duras. Corpoica - Ciencia y Tecnología Agropecuaria, 17(1), 81-95. doi:10.21930/rcta.vol17_num1_art:463 [ Links ]
Ríos, L., Zapata, R., Reyes, J., Mejía, J., & Baena, A. (2010). Estabilidad enzootica de babesiosis bovina en la región de Puerto Berrio, Colombia. Revista Científica, 20(5), 485-492. [ Links ]
Sanger, F., Nicklen, S., & Coulson, A. R. (1977). DNA sequencing with chain-terminating inhibitors. Proceedings of the National Academy of Sciences of the United States of America, 74(12), 5463-5467. [ Links ]
Smeenk, I., Kelly, P. J., Wray, K., Musuka, G., Trees, A. J., & Jongejan, F. (2000). Babesia bovis and B. bigemina DNA detected in cattle and ticks from Zimbabwe by polymerase chain reaction. Journal of the South African Veterinary Association, 71(1), 21-24. [ Links ]
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Molecular Biology Evolution, 30(12), 2725-2729. doi:10.1093/molbev/mst197 [ Links ]
Zapata, R., Lara, N., Baena, A., Reyes, J., & Ríos, L. (2011). Seroprevalencia de babesiosis bovina en la hacienda Vegas de la Clara, Gómez Plata (Antioquia), 2008. Revista Medicina Veterinaria, 21, 63-71. doi:10.19052/mv.577 [ Links ]
2Available online http://blast.ncbi.nlm.nih.gov/Blast.cgi.
Received: January 19, 2018; Accepted: October 10, 2018











 texto em
texto em 





