Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Ciencia y Tecnología Agropecuaria
versão impressa ISSN 0122-8706versão On-line ISSN 2500-5308
Cienc. Tecnol. Agropecuaria vol.20 no.1 Mosquera jan./abr. 2019
https://doi.org/10.21930/rcta.vol20_num1_art:1249
Salud animal
Evaluation of essential coriander (Coriandrum sativum L.) oil against the red poultry mite (Dermanyssus gallinae) (De Geer, 1778) (Acari: Dermanyssidae) under laboratory conditions
1Docente auxiliar, Fundación Universitaria Juan de Castellano (FUJC), Facultad de Ciencias Agrarias y Ambientales, Investigacion en producción animal y tecnologias agroalimentarias (Inpanta). Tunja, Colombia.
2Docente, Universidad Pedagógica y Tecnológica de Colombia Facultad de Ciencias, Escuela de Ciencias Biológicas, Grupo Biología para la Conservación. Tunja, Colombia.
The red mite Dermanyssus gallinae affects poultry production mainly of laying hens by feeding on its blood. Its control is carried out using chemical acaricides, toward which its resistance increases. The aim of this study was to evaluate the acaricidal activity of essential oil (eo) of coriander (Coriandrum sativum) at concentrations of 1, 2 and 3 mg/cm2 through contact bioassays on adults of D. gallinae, compared to a chemical acaricide (Neguvon*). The mites were collected from backyard hens (Gallus gallus L.). Six replicates were performed per treatment. Statistically, mortality percentages were established and an anova was carried out. The lethal activity percentage was established. Additionally, the median lethal concentration (LC50) and the median lethal time (TL50) were estimated. Coriander eo caused 100 % mortality on D. gallinae population 25 min after contact with a concentration of 2 and 3 mg/cm2 and after 30 min with a lower concentration of 1 mg/cm2. The LC50 of the coriander eo was 1.3 mg/cm2, with a 13 min TL50 on the D. gallinae population. The eo of coriander is suggested as an alternative to be used instead of the chemical acaricides for the control of the red poultry mite D. gallinae.
Keywords acaricides; Apiaceae; aviculture; haematophagy; pest control; repellents
El acaro rojo Dermanyssus gallinae afecta las producciones avicolas, principalmente de ponedoras, al alimentarse de su sangre. Su control se realiza a través de acaricidas químicos, hacia los cuales aumenta su resistencia. Se evaluo la actividad acaricida de aceite esencial (ae) de cilantro (Coriandrum sativum) a concentraciones de 1, 2 y 3 mg/cm2 a través de bioensayos por contacto sobre adultos de D. gallinae y se comparo con un acaricida químico (Neguvon*). Los ácaros fueron colectados de aves (Gallus gallus L.) de traspatio. Se realizaron seis repeticiones por tratamiento. Estadísticamente, se determinaron los porcentajes de mortalidad y se calculó el anova. Se estableció el porcentaje de la actividad letal. Se estimo la CL50 y el TL50. El ae de cilantro causo el 100 % de mortalidad sobre la población de D. gallinae a los 25 min después del contacto con una concentración de 2 y 3 mg/cm2 y a los 30 min con una concentración de 1 mg/cm2. La CL50 del ae de cilantro fue de 1,3 mg /cm2, con un TL50 de 13 min sobre la población de D. gallinae. Se sugiere el aceite esencial de cilantro C. sativum como una alternativa a los acaricidas químicos para el control del acaro rojo de aves D. gallinae.
Palabras clave acaricidas; Apiaceae; avicultura; control de plagas; hematofagia
Introduction
The red poultry mite Dermanyssus gallinae (De Geer, 1778) belongs to the Arachnida (De Geer, 1778) class and to the Dermanyssidae family.
Further, it is the most abundant species within this group (Walter & Proctor, 2013). It is an hematophagous ectoparasite that causes important damage in poultry production systems (Rodriguez- Vivas & Cob-Galera, 2005), especially in breeding operations of laying hens, in which egg production can fall up to 25 %, decreasing its quality and causing weight loss, anemia and mortality in birds (Green, Sparling, & Sperling, 2007). Moreover, this pest is commonly found worldwide (Kim, Na, Yi, Kim, & Ahn, 2007).
Synthetic acaricides are used for its control but there are populations that have developed resistance to pesticides such as carbamates and pyrethroids (George et al., 2015), increasing the problem (George, Callaghan, Guy, & Sparagano, 2008). Alternative substances have been assessed for its control, including essential oils of coriander (Coriandrum sativum), lavender (Lavandula angustifolia), thyme (Thymus vulgaris), manuka (Leptospermum scoparium), pennyroyal (Mentha pulegium), sweet basil (Ocimum basilicum), mint (Mentha spicata), garden savory (Satureja hortensis), cinnamon (Cinnamomum zeylanicum) and eucalyptus (Eucalyptus globulus), with toxic results on D. gallinae through contact trials (Kim, Yi, Tak, & Ahn, 2004; Kim et al. al., 2007; George, Smith, Shiel, Sparagano, & Guy, 2009; Magdaş, Cernea, Baciu, & Şuteu, 2010; Nechita, Poirel, Cozma, & Zenner, 2015). Likewise, entomopathogenic fungi such as Beauveria bassiana and Metharhizium anisopliae with high mortality rates have been studied (Steenber & Kilpinen, 2003; Tavassoli, Ownag, Porseyed, & Mardani, 2008). However, other alternatives must be evaluated, because the final control solution has not been determined.
Pesticides based on natural products have been used as an alternative to mitigate the attack of mites, both hematophagous as well as phytophagous. Such is the case of essential oils (eo) whose use is viable thanks to their low toxicity for mammals and due to their biodegradability (Toloza, Zygadlo, Biurrun, Rotman, & Picollo, 2010). EOs with insecticidal properties have been successfully marketed in specific agricultural applications and meet minimum risk criteria as pesticides (United States Environmental Protection Agency [us epa], 2004). According to Toloza (2010), natural insecticides such as eo are a viable option and should be taken into account for the development of new environmentally friendly products.
On the other hand, coriander (Coriandrum sativum L.) is an annual herbaceous plant, which belongs to the family Apiaceae (Mahendra & Bisht, 2011) and contains eo in leaves, stems, flowers, fruits, seeds, roots and bark (Mandal & Mandal, 2015). eo yield of C. sativum and its chemical composition undergo changes during ontogenesis (Bhuiyan, Begum, & Sultana, 2009), but its major constituent is linalool; in addition, it also contains limonene, α-pinene, camphene, geranyl acetate, linalyl acetate, geraniol, terpin-4-ol, α-terpineol, c-terpenepinene, myrcene and camphor (Zeb, 2016). These compounds have a broad spectrum of biological activities, including their insecticidal (Pavela & Vrchotova, 2013) and acaricide (Song, Yang, Suh, & Lee, 2011) functions. The activity of EOs depends on their composition, which is affected by factors such as their genetic structure, climate and agricultural practices (Telci, Toncer, & Sahbaz, 2006).
According to the aforementioned, the aim of this study was to evaluate the acaricidal activity of the coriander (C. sativum) eo against the red poultry mite (D. gallinae), under controlled laboratory conditions.
Materials and methods
Dermanyssus gallinae breeding
Colonies of D. gallinae mites were collected under the feathers and chest of Gallus gallus hens (L., 1758) (Birds: Galliformes: Phasianidae), and from slits in the poultry houses in a backyard production located in Finca Samaria, in Duitama (department of Boyaca, Colombia). The specimens of D. gallinae were immediately transferred to polyethylene containers (4.8 cm × 8.4 cm) and covered. The mites were transported to the Plant Health Laboratory of Fundacion Universitaria Juan de Castellanos in the city of Tunja (Department of Boyaca, Colombia).
In the laboratory, the mites were placed in a Petri dish on filter paper and a piece of wet cotton and sealed with ParafilmR; they were maintained at a temperature between 20+} 9 °C and a relative humidity of 60-90 %, with cycles of 16: 8 light-dark hours. Tests were carried out within 2 days after the mites were collected. These specimens were not fed because they can survive up to eight months away from poultry without feeding and they also resist desiccation (Chauve, 1998).
Bioassays
For this type of test, parameters of established and standardized methodologies were followed with some modifications due to laboratory conditions, such as those published by Kim et al. (2004) and George et al. (2009).
Coriander eo was purchased at Bioryz Biovegetal (Bogota, Colombia). In order to evaluate their acaricidal activity, contact bioassays were carried out with filter paper on D. gallinae adults. To do this, concentrations of 1.0, 2.0 and 3.0 mg/cm2 of C. sativum eo were applied on filter paper (Whatman No. 2, and 4.25 cm of diameter) in 50 μl of ethanol 70 %. The control treatment was carried out with 50 μl of ethanol and using the commercial acaricide (Neguvon*) as a standard, whose active ingredient is trichlorfon, in order to compare the toxicity tests.
The filter paper was dried in an extractor hood for 2 minutes; later, the filter paper was placed in the lower part of a Petri dish (4.8 cm in diameter x 1.2 cm in height). Ten adult mites were placed in each box together with a piece of cotton (5 mm x 5 mm) impregnated with 100 μl of distilled water. Then, each Petri dish was covered with a lid and sealed with ParafilmR. Each treatment was repeated six times.
Finally, mortality was determined every 5 min after exposure to treatments, for which a Motic* SMZ-168 stereomicroscope was used. Adults were considered dead if their appendages did not move when they were punctured with a fine pin. Twentyfour hours later, residuality tests were carried out.
Statistical análisis
Mortality percentage was established with the equation of Henderson and Tilton (1995), meanwhile, the lethal activity was classified according to Kim et al. (2007) as follows. Strong mortality is > 80 %; moderate i 80-61 %; weak is 60-40 %; and little or no activity is < 40 %. The analysis of variance (anova) was carried out and a nonparametric analysis was performed, using the Shapiro-Wilk test (n < 30). In addition, significant differences were analyzed through the Kruskal- Wallis test, and with the Tukey test, significant differences were evaluated between the treatments and the exposure time. The median lethal concentration (LC50) was estimated with the Probit analysis using the BioStat program (2009).
Likewise, the median lethal time (TL50) for each concentration was determined with the slope of the line containing 50 % of the mortality value and the X and Y values in the equation (Y = A + BX) were replaced to find A for each treatment.
Results and discussion
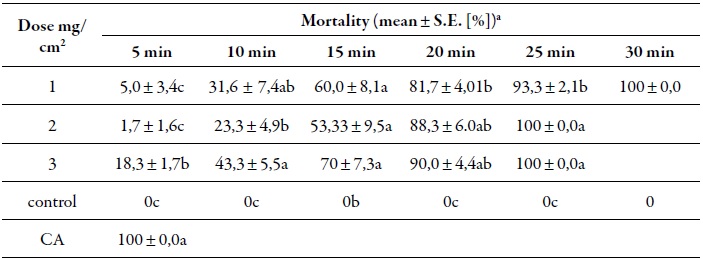
The acaricidal activity of coriander (C. sativum) on adult red poultry mites (D. gallinae) through contact bioassays, according to different doses and exposure times is presented in table 1.
Table 1. Acaricide activity of coriander (C. sativum) EO against adult red poultry mites (D. gallinae) through contact bioassays
Source: Elaborated by tje authors
a data with the same letter in each column expresses that there are no significant differences at p = 0.05.ca: Commercial acaricide Neguvon*, chemical control treatment.
According to the data reported in table 1, it can be established that the dose of 3 mg/cm2 is the one that offers the highest mortality at any time (5, 10, 15, 25 and 30 minutes). The efficacy of the evaluated doses of coriander eo (1, 2 and 3 mg/cm2) showed strong mortality (100 % mortality) after 30 min of contact. In the control group, all individuals survived, meanwhile the chemical control group, from the first moment there was contact with the mites the mortality was 100 %. The mean and standard error is reported with the untransformed data. The times and treatments are compared with the Kruskal-Wallis test and there were significant differences p < 0.05 according to the dose applied and the exposure time.
Kim et al. (2004) reported 100 % mortality in D. gallinae after 24 hours of contact with coriander eo at 0.07 mg/cm2. In the current study, coriander eo caused 100 % mortality on the population of D. gallinae 25 min after contact with a concentration of 2 and 3 mg/cm2, and after 30 min with a lower concentration of 1 mg/cm2. Magdaş et al. (2010) reported 100 % mortality after 24, 48 and 72 hours of contact of D. gallinae with coriander eo at 0.4 and 0.6 mg/cm2 in in vitro tests, using the direct contact method. When comparing previous works with the current study, the effect of coriander eo on D. gallinae is faster, reaching 100 % mortality with the three concentrations evaluated after 30 minutes of exposure.
The doses of coriander eo in ethanol evaluated (1.0, 2.0 and 3.0 mg/cm2) on D. gallinae, are relatively high compared to the results published by other authors (Kim et al., 2004; Magdaş et al., 2010; Nechita et al., 2015), which demonstrates a faster way to eliminate the target pest with a low residual toxicity, as proposed by George, Olatunji, Guy and Sparagano (2010). In this case, no residual activity of coriander eo was recorded on D. gallinae 24 hours after application and 100 % mortality was reached after 30 min of exposure.
According to Isman (2000), the lack of residual toxicity of volatile compounds could be considered beneficial, because the environmental persistence of bioactive compounds will be low. On the other hand, according to George et al. (2008), essential oils are very volatile in nature, so the effect is of short duration, and if they are used as acaricides, they act in the vapor phase. In addition, according to the results of Kim et al. (2004, 2007), coriander eo was more effective on D. gallinae in closed containers, indicating that the route of action of essential oils is largely in the vapor phase through the respiratory system; although the authors do not know its mode of action.
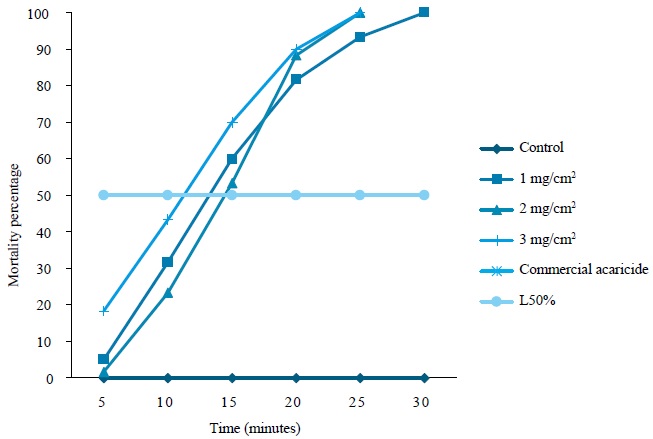
The LC50 of the coriander eo was 1.301 mg/cm2 and it was reached in a TL50 of 13 min, which reduced 50 % of the population of D. gallinae (figure 1).
For the control group, no values were recorded, because they were null (zero) from 5 min to 30 min after exposure; similarly, there are no values for the commercial acaricide, as mortality was 100 % after 5 min (figure 1).
Source: Elaborated by authors.
Figure 1. Lethal concentration time of AE of C. sativum on D. gallinae. It was determined from the slope of the straight with 50% mortality; values were replaced X and Y on ecuation (Y = A + BX).
It could be considered that the lethality of the coriander eo on D. gallinae is due to the presence of linalool. According to Khani and Rahdari (2012) and Chung et al. (2012), approximately 70 % of the chemical composition of this oil is linalool. Studies on the mode of action of EOs indicate that there is neurotoxic activity, including hyperactivity, convulsions, and tremors, followed by paralysis, symptoms similar to those produced by pyrethroid insecticides (Kostyukovsky, Rafaeli, Gileadi, Demchenko, & Shaaya 2002) and also by the behavior observed in D. gallinae in this study. On the other hand, Chung et al. (2012), with an LC50 value of 21.5 ppm of the evaluated coriander eo, observed that it has significant toxic effects against the larvae of Aedes aegypti (L., in Hasselquist, 1762) (Diptera: Culicidae), acting as an immunotoxin for the insect. It has been recognized that EOs are potent neurotoxins that affect, through the inhibition of the enzyme acetyl cholinesterase, the central nervous system (Keane & Ryan, 1999).
In the current study, the potential of coriander essential oil varied according to the concentration and time of exposure. The results of this work show that the eo of C. sativum can play a controlling role on D. gallinae and reduces risks associated with the use of synthetic insecticides. The mode of action of the eo of C. sativum is of special interest, so other investigations should focus on the way they penetrate the cuticle of insects and their effects on mammals fed with the treated material. In addition, it should be noted that the leafy coriander eo has low cytotoxicity in human cells, considering it safe for human consumption (Mandal & Mandal, 2015).
Conclusions
This study shows that the eo of C. sativum has acaricidal activity against D. gallinae through direct contact and has no residual activity. Therefore, it can be used as a natural acaricide for the management of adult individuals of D. gallinae and is considered as an interesting alternative to reduce the conventional use of chemical control.
Acknowledgements
The authors wish to thank Fundacion Universitaria Juan de Castellanos for their support facilitating the laboratories and the time spent in this research.
Moreover, also to the peer reviewers and the editors of this journal for their comments, which helped improve this text.
REFERENCES
Bhuiyan M. I., Begum, J., & Sultana, M. (2009). Chemical composition of leaf and seed essential oil of Coriandrum sativum L. from Bangladesh. Bangladesh European Journal of Pharmacology, 4,150-153. doi:10.3329/bjp.v4i2.2800. [ Links ]
Chauve, C. (1998). The poultry red mite Dermanyssus gallinae (De Geer, 1778): current situation and future prospects for control. Veterinary parasitology, 79(3), 239- 245. doi:10.1016/S0304-4017(98)00167-8. [ Links ]
Chung, I. M., Ahmad, A., Kim, E. H., Kim, S. H., Jung, W. S., Kim, J. H., … Nagella, P. (2012). Immunotoxicity activity from the essential oils of coriander (Coriandrum sativum) seeds. Immunopharmacology and immunotoxicology, 34(3), 499-503. doi:10.3109/08923973.2011.637500. [ Links ]
George, D. R., Finn, R. D., Graham, K. M., Mul, M. F., Maurer, V., Moro, C. V., & Sparagano, O. A. (2015). Should the poultry red mite Dermanyssus gallinae be of wider concern for veterinary and medical science? Parasites and vectors, 8(1), 178. doi:10.1186/s13071-015-0768-7. [ Links ]
George, D. R., Olatunji, G., Guy, J. H., & Sparagano, O. A. E. (2010). Effect of plant essential oils as acaricides against the poultry red mite, Dermanyssus gallinae, with special focus on exposure time. Veterinary parasitology, 169(1), 222-225. doi:10.1016/j.vetpar.2009.12.038. [ Links ]
George, D. R., Smith, T. J., Shiel, R. S., Sparagano, O. A. E., & Guy, J. H. (2009). Mode of action and variability in efficacy of plant essential oils showing toxicity against the poultry red mite, Dermanyssus gallinae. Veterinary parasitology, 161(3), 276-282. doi: 10.1016/j.vetpar.2009.01.010. [ Links ]
George, D. R., Callaghan, K., Guy, J. H., & Sparagano, O. A. E. (2008). Lack of prolonged activity of lavender essential oils as acaricides against the poultry red mite (Dermanyssus gallinae) under laboratory conditions. Research in veterinary science, 85(3), 540-542. doi:10.1016/j.rvsc.2008.02.001. [ Links ]
Green, B., Sparling, J., & Sperling, M. B. (2007). What's eating you? Pigeon mite (Dermanyssus gallinae). Cutis, 80, 461-462. Recuperado de https://www.mdedge.com/sites/default/files/Document/September-2017/080060461.pdf. [ Links ]
Henderson C. F., & Tilton, E. W. (1955). Acaricides tested against the brown wheat mite. Journal of Economic Entomology, 48, 157-161. [ Links ]
Isman, M. B. (2000). Plant essential oils for pest and disease management. Crop Protection, 19(8-9), 603-608. doi:10.1016/S0261-2194(00)00079-X. [ Links ]
Keane, S., & Ryan, M. F. (1999). Purification, characterisation, and inhibition by monoterpenes of acetylcholinesterase from the waxmoth, Galleria mellonella (L.). Insect biochemistry and molecular biology, 29(12), 1097-1104. doi:10.1016/S0965-1748(99)00088-0. [ Links ]
Khani, A., & Rahdari, T. (2012). Chemical composition and insecticidal activity of essential oil from Coriandrum sativum seeds against Tribolium confusum and Callosobruchus maculatus. ISRN Pharmaceutics, Article id 263517. doi:10.5402/2012/263517. [ Links ]
Kim, S. I., Na, Y. E., Yi, J. H., Kim, B. S., & Ahn, Y. J. (2007). Contact and fumigant toxicity of oriental medicinal plant extracts against Dermanyssus gallinae (Acari: Dermanyssidae). Veterinary Parasitology, 145(3), 377-382. doi:10.1016/j.vetpar.2006.12.021. [ Links ]
Kim, S. I., Yi, J. H., Tak, J. H., & Ahn, Y. J. (2004). Acaricidal activity of plant essential oils against Dermanyssus gallinae (Acari: Dermanyssidae). Veterinary parasitology, 120(4), 297-304. doi:10.1016/j.vetpar.2003.12.016. [ Links ]
Kostyukovsky, M., Rafaeli, A., Gileadi, C., Demchenko, N., & Shaaya, E. (2002). Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: possible mode of action against insect pests. Pest Management Science, 58(11), 1101-1106. doi:10.1002/ps.548. [ Links ]
Magdaş, C., Cernea, M., Baciu, H., & Şuteu, E. (2010). Acaricidal effect of eleven essential oils against the poultry red mite Dermanyssus gallinae (Acari: Dermanyssidae). Science of Parasitology, 11(2), 71-75. Recuperado de http://Users/Ultra%20Book/Downloads/Acaricidal_effect_of_eleven_essential_oils_against%20(1).pdf. [ Links ]
Mahendra, P., & Bisht, S. (2011). Coriandrum sativum: A daily use spice with great medicinal effect. Pharmacognosy Journal, 3(21), 84-88. doi:10.5530/pj.2011.21.16. [ Links ]
Mandal, S., & Mandal, M. (2015). Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pacific Journal of Tropical Biomedicine, 5(6), 421- 428. doi:10.1016/j.apjtb.2015.04.001. [ Links ]
Nechita, I. S., Poirel, M. T., Cozma, V., & Zenner, L. (2015). The repellent and persistent toxic effects of essential oils against the poultry red mite, Dermanyssus gallinae. Veterinary parasitology, 214(3), 348-352. doi:10.1016/j.vetpar.2015.10.014. [ Links ]
Pavela, R., & Vrchotova, N. (2013). Insecticidal effect of furanocoumarins from fruits of Angelica archangelica L. against larvae Spodoptera littoralis Boisd. Industrial crops and products, 43, 33-39. doi:10.1016/j.indcrop.2012.06.044. [ Links ]
Rodríguez-Vivas R.I., & Cob-Galera L. A. (2005). Técnicas Diagnósticas en Parasitología Veterinaria (2a ed.). Merida, Mexico: Universidad Autonoma de Yucatan. [ Links ]
Song, H. Y., Yang, J. Y., Suh, J. W., & Lee, H. S. (2011). Acaricidal activities of apiol and its derivatives from Petroselinum sativum seeds against Dermatophagoides pteronyssinus, Dermatophagoides farinae, and Tyrophagus putrescentiae. Journal of agricultural and food chemistry, 59(14), 7759-7764. doi:10.1021/jf201945y. [ Links ]
Steenberg, T., & Kilpinen, O. (2003). Fungus infection of the chicken mite Dermanyssus gallinae. IOBC WPRS Bulletin, 26(1), 23-26. [ Links ]
Tavassoli, M., Ownag, A., Pourseyed, S. H., & Mardani, K. (2008). Laboratory evaluation of three strains of the entomopathogenic fungus Metarhizium anisopliae for controlling Dermanyssus gallinae. Avian Pathology, 37(3), 259-263. doi:10.1080/03079450802043718. [ Links ]
Telci, I., Toncer, O., & Sahbaz, N. (2006). Yield essential oil content and composition of Coriandrum sativum varieties (var. vulgare Alef and var. microcarpum DC.) grown in two different locations. Journal Essential Oil Research, 18, 189-193. doi:10.1080/10412905.2006.9699063. [ Links ]
Toloza, A. (2010). Bioactividad y toxicidad de components de aceites esenciales vegetales, en Pediculus humanus capitis (Phthiraptera: Pediculidae) resistentes a insecticidas piretroides (tesis doctoral). Universidad de Buenos Aires. Buenos Aires, Argentina. Recuperado de http://digital.bl.fcen.uba.ar/download/tesis/tesis_n4665_Toloza.pdf. [ Links ]
Toloza, A., Zygadlo, J., Biurrun, F., Rotman, A., & Picollo, M. (2010). Bioactivity of Argentinean essential oils against permethrin – resistant head lice, Pediculus humanus capitis. Journal of Insect Science, 10, 185. doi:10.1673/031.010.14145. [ Links ]
United States Environmental Protection Agency (us epa). (2004). Biopesticides—25b Minimum Risk Pesticides. Recuperado de http://www.epa.gov/oppbppd1/biopesticides/regtools/25b_list.htm. [ Links ]
Walter, D. E., & Proctor, H. C. (2013). Mites-ecology, evolution and behaviour: life at a microscale (2a ed.). Amsterdam, Paises Bajos: Springer. doi:10.1007/978-94-007-7164-2. [ Links ]
Zeb, A. (2016). Coriander (Coriandrum sativum) oils. En V. Preedy (Ed.), Essential Oils in Food Preservation, Flavor and Safety (pp. 359-364). Londres, Reino Unido: Elsevier. doi:10.1016/B978-0-12-416641-7.00040-7. [ Links ]
Received: November 11, 2017; Accepted: October 10, 2018











 texto em
texto em 



