Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Ciencia y Tecnología Agropecuaria
versão impressa ISSN 0122-8706versão On-line ISSN 2500-5308
Cienc. Tecnol. Agropecuaria vol.20 no.1 Mosquera jan./abr. 2019
https://doi.org/10.21930/rcta.vol20_num1_art:1251
Sanidad vegetal y protección de cultivos
Scale insects (Hemiptera: Coccomorpha) on coffee roots (Coffea arabica L.) in Colombia, with records of associated ants (Hymenoptera: Formicidae)
1Investigador, Universidad Nacional del Altiplano Puno, Universidad Nacional de Cañete, Instituto de Cultivos Tropicales, Departamento de Fitopatología. San Martin, Perú.
2Investigador y coordinador, Universidad Nacional Agraria La Molina, Universidad Nacional Autónoma del Alto Amazonas, Instituto de Cultivos Tropicales. San Martin, Perú.
3Estudiante de PhD, University of Aberdeen, School of Biological Sciences.
In the Atusparia sector, Tocache, of the San Martin region in Peru, two-year-old plants of Theobroma cacao L. (Malvaceae) of the CCN-51 clone showed for the first time in January 2010, symptoms of leaf yellowing, wilt and dieback, with an internal obstruction of the vascular system of the plant. To isolate the fungus, diseased tissues were cultured in Petri dishes containing potato dextrose agar oxytetracycline, and the identification was based on morphological and microscopic analyses and by the Sanger sequencing. Pathogenicity tests were carried out on three-months-old T. cacao ICS-1 clone plants and were inoculated with a conidia suspension of 1x107 cfu/mL employing two inoculation methods (Drench to the soil and stem puncture). Likewise, the antagonistic capacity (mycoparasitism and antibiosis) was established in vitro with endophytic Trichoderma isolates on the pathogen. With both inoculation methods symptoms of wilt of different degrees of severity were observed and the causal agent of sudden death in T. cacao was confirmed to be the fungus Verticillium dahliae Kleb. blast sequence analysis revealed that the isolate showed 100 % homology with V. dahliae sequences in GenBank. Trichoderma TE-91 was identified as the most aggressive mycoparasite because its metabolites inhibited completely the mycelial growth of the pathogen.
Keywords biological control; endophyte; wilt; Trichoderma
En el sector Atusparia, Tocache, de la Región de San Martin, Perú, plantas de Theobroma cacao L. (Malvaceae) clon CCN-51 de dos años de edad presentaron, por primera vez en enero de 2010, síntomas de amarillamiento en hojas, marchitez y muerte, e internamente obstrucción del sistema vascular. Para el aislamiento del agente causal, tejidos enfermos fueron sembrados en placas Petri que contenían medio papa dextrosa agar oxitetraciclina, cuya identificación fue con base en un análisis morfológico, microscópico y secuenciación de Sanger. Las pruebas de patogenicidad se hicieron en T. cacao clon ICS-1 con edad de tres meses, inoculadas con una suspensión de conidias de 1x107 ufc/mL por dos métodos de inoculación (Drench al suelo y punción en el tallo). La capacidad antagónica (micoparasitismo y antibiosis) se determinó in vitro con aislamientos de Trichoderma endofito sobre el patógeno. En ambos métodos de inoculación, se observaron síntomas de marchitez de diferentes grados de severidad y se confirmó que el agente causal de la muerte súbita del T. cacao fue el hongo Verticillium dahliae Kleb. El análisis de la secuencia blast revelo que el aislamiento tuvo un 100 % de homología con las secuencias de V. dahliae en GenBank. El Trichoderma TE-91 fue identificado como el micoparasito más agresivo, debido a que sus metabolitos inhibieron completamente el crecimiento micelial del patógeno.
Palabras clave control biológico; endofito; marchitez; Trichoderma
Introduction
Theobroma cacao L. (Malvaceae) is a plant species with its center of origin found in the humid forests of South America; its beans and derivatives are the basic input for chocolate, cosmetics and for the pharmaceutical industry (Zhang & Motilal, 2016). However, there are three major diseases that limit global cocoa production, including brown rot (caused by Phytophthora spp.), Moniliasis (Moniliophthora roreri) and witch's broom (Moniliophthora perniciosa) (Bailey & Meinhardt, 2016). These phytopathogens are able to limit significantly its commercial production and reduce yields by up to 20 %, e.g. the decline in cocoa production in 2012 was estimated at 1.3 million tonnes of dry beans (Bailey & Meinhardt, 2016). Moreover, another disease that severely limits production in Uganda, Brazil and Colombia is the sudden death of T. cacao plants caused by Verticillium dahliae Kleb (Resende, Flood, & Cooper, 1994).
The species V. dahliae and V. albo-atrum are highly pathogenic as they cause wilt in more than 200 plant species, including economically important crops (Pegg & Brady, 2002; Schnathorst, 1981). Solely V. dahliae results in yields losses of up to 80 % and its control is a challenge, because its pathogenicity is highly variable and it has a high survival capacity (Wang et al., 2016).
Currently, there is no biocontrol management or treatment for infected plants, and no effective fungicides are available to control wilt caused by Verticillium; basically, prevention is the only effective method to avoid losses (Yildiz & Benlioglu, 2010; Yuan et al., 2017). Furthermore, the available control and prevention measures are not completely successful when applied individually. Therefore, the only way to effectively control the disease in the field is through an integrated management aimed to reduce the disease to coexistence levels (Lopez- Escudero & Mercado-Blanco, 2011).
A promising ecological strategy within the integrated disease management is the use of endophytic fungi of the crop of interest, as they are taxonomically and biologically diverse, they colonize the internal tissues of plants without causing visible symptoms and they can improve plant growth and health.
Additionally, they can induce the production of compounds and can activate some genes involved in plant defense responses to biotic and abiotic factors (Bae et al., 2009; De Souza et al., 2008; Hanson & Howell, 2004). Among the main endophytes used as biological control agents are some species of the Trichoderma genus considered as natural antagonists of phytopathogens, that have widely been used in agriculture, and contribute to the improvement of plant growth and development (Guedez, Canizalez, Castillo, & Olivar, 2009, Harman, 2006, Junaid, Dar, Bhat, Bhat, & Bhat, 2013, Toghueo et al., 2016). In T. cacao, recent studies have shown that endophytic fungi can limit damage by Moniliophthora perniciosa, M. roreri and Phytophthora palmivora (Bailey et al., 2008; De Souza et al., 2008; Mejia et al., 2008).
In Peru, staff from Instituto de Cultivos Tropicales (ict) conducted an expedition to the upper Amazon region in 2008, in order to search for endophytes that have coevolved with pathogens, obtaining a diverse collection. These researchers collected 126 Trichoderma isolates from stems and leaves (Marquez et al., 2010). However, only a small number of Trichoderma spp. isolates of the collection have been studied to establish the endophytic and biocontrol capacities related to main cacao pathogens (Arevalo, Canto, Leon, & Meinhardt, 2010a; Arevalo, Canto, Leon, Meinhardt, & Cayotopa, 2010b; Leon et al. 2010a; Leon, Rojas, Rodriguez, Arevalo, & Marquez, 2010b).
Currently, V. dahliae has not been confirmed as being present in Peru. Therefore, the causal agent was isolated from two-year-old T. cacao clone CCN-51 plants collected in Atusparia, Tocache sector of the San Martin region of Peru, that showed for the first time in January 2010 symptoms of leaf yellowing, generalized wilt and dieback, as well as internal obstruction of the vascular system. According to the aforementioned, the aim of this study was to identify and characterize the causal agent of the sudden death of T. cacao plants and establish the in vitro antagonistic capacity of endophytic Trichoderma isolates on the causal agent.
Materials and methods
Location of the experiment
This research was carried out in the nursery and in the phytopathology laboratory of the experimental station Juan Bernito of ict, located in the district of La Banda de Shilcayo, province and region of San Martin, Peru, at 76°00´18" W and 06°00´28" S, at an altitude of 315 m.a.s.l. The temperature, relative humidity and precipitation were 25 ° C, 73 % and 1,188 mm, respectively.
Plant material
For the isolation and identification of the causal agent of the sudden death of T. cacao, samples of affected tissues with death symptoms and plants with "sudden death" typically affected by Verticillium were collected in January 2010 on a two-year-old CCN-51 cacao plantation of the Atusparia sector, in the district and province of Tocache in the San Martin region of Peru. In this plantation, 5 % of the plants exhibited symptoms of leaf yellowing, widespread wilting and plant death. When performing transverse cuts of the stem, internal vascular occlusion was observed as points, meanwhile in the longitudinal cuts, brown striae were observed in the vascular system. Samples were taken to the ict Phytopathology Laboratory for their isolation and identification.
Isolation and identification
Isolation of the causative agent was obtained from segmented tissues of the stem, root and neck of diseased plants. The segments were washed with water and disinfested with 1 % sodium hypochlorite for 10 minutes to remove surface microorganisms; in addition, they were washed with sterile distilled water and placed on paper towels for drying. From each segment, 1 cm portions were cut and planted in Petri dishes containing potato dextrose agar medium with oxytetracycline (pdao), and incubated for 7 days at 25 °C (Resende, Flood, & Cooper, 1995). The plates were subjected to artificial illumination at a room temperature of 28 °C ± 0.57 °C and a relative humidity of 70 % ±} 1.71 % for 10 days ±} 1 to favor fungus sporulation. The taxonomic identification of the fungus was made by observing the macroscopic morphological characteristics of the colony (appearance and coloration) as well as the microscopic morphological characteristics (type and size of vegetative and reproductive structures). The keys published by Barnett and Hunter (1998), Barron (1968) and Watanabe (2010) were used for identification of the pathogen.
Further, the identification of the isolates was performed using Sanger's sequencing. For this purpose, the isolates were cultivated in flasks containing 50 mL of potato dextrose broth (pdb, 24 g/L) at 25 °C until the mycelium colonized the surface of the broth. The mycelium was washed, filtered and stored at -20 °C until dna extraction was carried out. The mycelium was triturated in liquid nitrogen and the dna was extracted using the DNeasy PowerPlant Pro kit (Qiagen, Germany), following the manufacturer's instructions. Partitions ITS1/ITS4 (ITS1: 5'-tccgtaggtgaacctgcgg-3'; ITS4: 5'-tcctccgcttattgatatgc-3') (White, Bruns, Lee, & Taylor, 1990) were used to amplify dna.
The pcr mixture was prepared using Master Mix 2x pcr (Promega, U.S.A.), in a final volume of 25 μL containing Milli-Q water, 1x MasterMix pcr, 0.5 μM of each 5' to 3' partitions (forward or direct), 3' to 5' (reverse) partitions and 2 μL of cDNA. The pcr was performed on a Techne TC-412 thermal thermocycler (Techne). The conditions employed were 94 °C for 2 minutes, followed by 35 cycles of 94 °C for 10 seconds, 54 °C for 30 seconds and 72 °C for 1 minute, with a final elongation phase of 72 °C for 10 minutes. Amplicons were monitored by agarose gel electrophoresis at 1 % prior to purification, using the QIAquick pcr purification kit (Qiagen, Germany). Amplicons were sequenced in both directions using Sanger’s sequencing (GATC Biotech, Konstanz, Germany).
Sequences were trimmed and aligned with the BioEdit Sequence Alignment Editor (Hall, 1999) and compared with the accessions of V. dahliae in GenBank using the Nucleotide blast tool.
Pathogenicity test
To carry out the pathogenicity test, V. dahliae inoculum was grown from a young culture in pdao medium. Three discs of fungus colonized medium (6 mm of diameter) were placed in 100 mL of potato broth with 2 % dextrose, and incubated under agitation at 110 rpm at room temperature (27 °C) for one week, to favor the development of the fungus. To remove the mycelia formed and to obtain the spore suspension used as inoculum, the broth was filtered through four layers of sterile gauze. The inoculum obtained was adjusted to a concentration of 107 cfu/mL (Resende et al., 1995). For the inoculation of the pathogen, 40 plants from three-month-old ICS-1 T. cacao seeds (Resende et al., 1994, 1995) grown on sterile soil contained in black polyethylene bags were used.
The soil was autoclaved at 121 °C and 15 lb/in2 for two consecutive days, for 45 and 30 min (Ormeno- Orrillo & Davila, 1999). Two inoculation methods were evaluated: a) via drench to the soil with 50 mL of the conidia suspension (107 cfu/mL) around the root of 20 plants (Resende et al., 1995); b) puncture in the stem of 20 plants employing a sterile needle.
Wounds were made in four points on the stem up to a depth of 2 mm (two on the bud of the cotyledonal leaves and two on the bud of the first true leaves), inoculating 5 μL of conidia suspension into each localized wounds (Trapero, Diez, Rallo, Barranco, & Lopez-Escudero, 2013). Plants were maintained in the greenhouse of the ict Phytopathology Laboratory at a temperature of 28 °C ±} 0.57 °C, at a relative humidity of 70 % ±} 5.71 % and soil conditions at field capacity (Resende et al., 1995).
On the other hand, the severity of the disease was evaluated with a scale of 0 to 6 (Villarroel- Zeballos, Feng, Iglesias, du Toit, & Correll, 2012), where 0 = no symptoms; 1 = between 0 and 10 % of chlorosis or necrosis in the plant; 2 = between 10 and 25 % of chlorosis or necrosis; 3 = between 25 and 50 % chlorosis or necrosis; 4 = between 50 and 75 % of chlorosis or necrosis; 5 = between 75 and 100 % chlorosis or necrosis, and 6 = dead plant. Pathogen reevaluation was performed from sections of plant tissue sections (root and stem) in which the symptoms of the disease were observed, following the same isolation methodology described previously.
Endophytic Trichoderma (ET)
Endophytic Trichoderma (ET) isolates used in this study were obtained from the ict collection. Ten et isolates (table 1) with endophytic (Arevalo et al., 2010b) and antagonistic capacities against Moniliophthora perniciosa, M. roreri and Phytophthora palmivora (Arevalo et al., 2010a; Leon et al., 2010a, 2010b), were selected to establish the antagonistic capacity against V. dahliae.
Antibiosis
Soluble inhibitory metabolites were produced in three culture broths and the antibiosis test was adapted to the method described by Bailey et al. (2008). Trichoderma isolates were cultured in Petri dishes of 90 cm in diameter, containing malt extract agar (mea) culture medium and incubated at 25 °C +} 0 °C for five days. From these cultures a suspension of conidia was obtained by addition of sterile distilled water (sdw) to each plate. For this test, three culture broths were used: 1) based on minimum salts (min), 2) malt extract (me), and 3) potato sucrose (ps). For each broth, 1 mL of conidia suspension (106 cfu/mL) was added per 100 mL; then, it was incubated at 25 °C under agitation at 110 rpm for one week. Mycelia were filtered through a layer of sterile cotton and then centrifuged for 20 minutes at 2,500 rpm. The supernatant was stored at -20 °C until it was used.
Before being incorporated into the medium, the centrifugated filtrate was placed in a water bath at 90 °C +} 0 °C for two hours to inactivate the enzymes; then, an equal volume of psa medium with solidified agar (3 %) was added to the Petri dishes. A 4-mm-diameter broth slice of medium with the developed pathogen from a five-day-old colony was placed in the center of the Petri dish. Inhibition of the mycelial growth of the pathogen was recorded when the control covered the entire box (Osorio Hernandez et al., 2016).
Mycoparasitism
For this test the precolonized plaque method described by Bailey et al. (2008) was used. A 2.5 × 0.5 cm piece of inoculum was extracted from a young culture of each Trichoderma isolate and located at one end of the plates containing pdao medium precolonized by V. dahliae. Three plates were used as replicates. The Petri dishes were incubated at 25 °C under darkness conditions.
Two weeks later the fungi were re-established. A total of eight samples (medium disks) of each box or replica were removed with a 5 mm diameter punch, from the point where the pathogen was planted. These samples were located in Petri dishes with pdao medium and incubated at 25 °C under darkness conditions; after 14 days the growth of Trichoderma and of the pathogen was observed, and the colonization percentage of Trichoderma biocontrol on medium disks with the pathogen was established.
Statistical análisis
Antibiosis and mycoparasitism tests were established under a completely randomized experimental design. Ten treatments were evaluated and corresponded to selected endophytic Trichoderma isolates. Three replicates per treatment were performed. The parameters evaluated were inhibition percentage of mycelial growth and colonization by Trichoderma of mycelium precolonized by V. dahliae. The percentage data was transformed angularly (arcsine √X) to confirm normality and homogeneity of variances as recommended by Montgomery (2008). An analysis of variance (andeva) and the Duncan contrast test with a level of significance of 0.05 % were carried out in the statistical software InfoStat version 2008 (Di Rienzo et al., 2008).
Results and discussion
In situ symptoms
Figure 1 shows the different symptoms observed in sudden death in a two-year-old CCN-51 T. cacao plantation located in the Atusparia sector, district and province of Tocache, Peru. Initially, flaccidity, epinasty, chlorosis, and necrosis of upper leaves are observed, and finally, wilting of the entire plant. When a cross-section of the upper limb was performed, necrotic points of the vascular system were identified and, in a longitudinal section, necrotic striae showing blackened infected vessels in the wood were observed. Similarly, necrotic rings were detected in the cross-section of the root of affected plants.

Figure 1. Symptoms of sudden death of cocoa in a plant of T. cacao CCN-51 clone located in the Atusparia sector, district and province of Tocache, Peru a. Yellowing, epinasty and wilting; b. Discoloration of the vascular system; c. Necrotic points throughout the vascular system; d. Necrotic striae in a longitudinal cut of the branch; and e. Necrotic rings in a cross section of the root.
Morphological and molecular identification of Verticillium
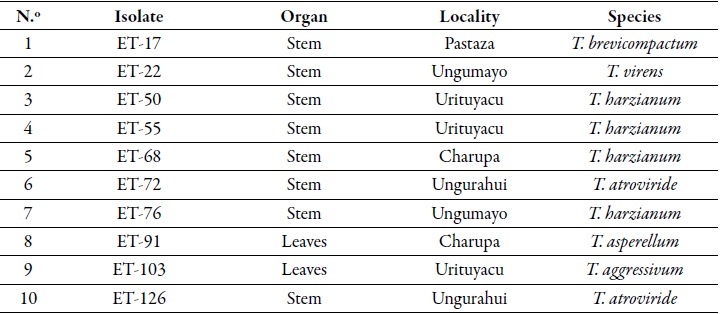
The coloration of the colony in pdao medium was grayish to blackish due to microsclerotia formation (figure 2a). In the mycelium immersed in the middle and spase, with the help of a stereoscope, conidia immersed in mucilage were clearly observed as small water droplets. These colony characteristics correspond to those observed in the genus Verticillium (Barnett & Hunter, 1998; Barron, 1968).
The conidiophores are hyaline, erect and carry a mass of conidia apically on each verticilated phialide in 2-3 fertile portions (figure 2b).
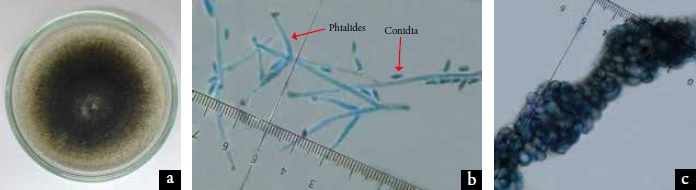
Figure 2. Macroscopic colony and microscopic morphology of vegetative and reproductive structures of Verticillium dahliae Kleb. a. The 15-days-old colony in potato dextrose agar (pda) medium; b. Conidia and verticilial phialides (40 X); c. Microsclerosis (40X).
Conidia are hyaline, smooth-walled, ellipsoidal, unicellular, non-septated, and measure on average 6.7 ±} 1.4 μm × 2.9 μm ±} 0.5 μm. In figures 2a and 2c, microesclerotes immersed in the pdao culture medium regularly distributed throughout the colony, are blackish brown in color, in chains and aggregated, rounded and with a thin cell wall. The microscopic morphology characteristics mentioned place the pathogen as belonging to the species V. dahliae (Inderbitzin et al., 2011; Watanabe, 2010).
Blast sequence analysis revealed that the Verticillium isolate showed 100 % homology to the V. dahliae sequences in GenBank (accession number NCBI: NR_126124.1 and HQ206718.1) and V. longisporum (HQ206832.1).
Pathogenicity
With the two methods of inoculation the Koch postulates were achieved and the causal agent of the sudden death of T. cacao was confirmed to be the species V. dahliae. Both the drench to the soil and the stem puncture inoculation methods using suspensions of V. dahliae conidia were able to reproduce wilting symptoms in 28 young inoculated cacao plants. However, the incubation period in the plants inoculated using the drench to the soil method was longer, and in which the first symptoms of leaf flaccidity followed by chlorosis and necrosis of the upper leaf apex were observed in ten plants (50 %) 30 days after inoculation (dai) with the pathogen (figure 3a); furthermore, ten days later the first symptoms of necrosis in the leaf area of the upper leaves (figure 3b) with a severity of 55 % ±} 1.70 were perceived. However, with the stem puncture method, the first symptoms were observed 10 dai (figure 3c) in 18 plants (90 % ±} 2.37), showing symptoms similar to those obtained with the drench to the soil method. The progression of the disease was severe; four days after observing the first symptoms, epithelia, wrinkling and necrosis of all leaves of the plant (figure 3d) occurred, and two days after that, some plants died showing a severity of 80 % ±} 2.38 % (table 2).
Table 2 Incidence and severity of sudden death of T. cacao by V. dahliae employing two inoculation methods at the Juan Bernito experimental station of ict, district of La Banda de Shilcayo, province and region of San Martin, Peru, in 2010
Source: Elaborated by the authors
Note: Average data of 20 plants for severity per inoculation method. Different letters indicate significant differences according to the Duncan test (p ≤ 0.05).

Figura 3. Symptoms of pathogenicity with V. dahliae in plants of T. cacao ICS-1 clone employing the drench to the soil inoculation method. a. Wilt; b. Necrosis caused by the stem puncture inoculation method; c. Chlorosis; d. Wilt; e. Healthy plant; f. Stem showing necrotic striae; g and h. Necrotic points in the vascular system; i. Pathogen observed in pdao medium (inside the yellow circles).
The current study comprises the first report that shows the presence of the sudden death of cacao plants in Peru, corroborating with the Koch´s postulates that V. dahliae is the causal agent of the disease. Of the 40 plants inoculated with the pathogen, 50 % and 90 % showed typical symptoms of the disease with the drench to the soil and the stem puncture inoculation methods, respectively. This disease caused by V. dahliae was reported in Uganda in 1965 (Emechebe, Leakey, & Banage, 1971; Leakey, 1965), and in Brazil it has became a serious problem in the states of Bahia and Espiritu Santo, where 85 % of the cocoa is produced (Resende et al., 1995). In addition, T. cacao in north-eastern Brazil was considered as a favorite host for Verticillium, because all Brazilian isolates of T. cacao were reported as pathogenic and destructive (Resende et al., 1994).
In this study, V. dahliae infections were studied in three-month-old cacao plants of the ICS-1 clone using the drench to the soil and the stem puncture methods, finding that the first symptoms were observed 30 days with the first method. On the contrary, Resende et al. (1994) reported the first symptoms at an earlier development stage, i.e. after only 20 days, and after 36 days, the disease rate was higher causing wilt in a small number of ICS-1 clone plants. Probably, this difference in days is due to the climatic conditions found in each location, as the temperature (28 °C ±} 0.57 °C) and the relative humidity (70 % ±} 5.71 %) were different from those reported by Resende et al. (1994) (temperature of 25 °C ±} 2 °C and relative humidity of 60 % ±} 10 %).
However, the progression of the disease was rapid and showed more severe symptoms in 90 % of the seedlings inoculated by stem puncture, which agrees with what Resende et al. (1995) reported.
These last authors used the same inoculation method, inoculum concentration and cacao plant genotypes (ICS-1 clone), observing the first symptoms after 10 days, which probably shows that the isolated pathogen of Peru has the same behavior as the one from Brazil. In addition, with the re-isolation of the pathogen, Koch's postulates were fulfilled and internal distribution within the plant was established.
Likewise, Trapero et al. (2013) indicated that inoculation through stem injection in young olive seedlings (40 days old) was the most effective inoculation method to begin the selection process of olive genotypes resistant to the wilting due to Verticillium, constituting an effective method that saves work, space and resources. This method was also used to isolate cacao V. dahliae in Abelmoschus esculentus Medik and Gossypium hirsutum L. (Malvaceae), which induced similar symptoms on artificially inoculated T. cacao seedlings (Emechebe et al., 1971).
Antagonistic capacity of endophytic Trichoderma against V. dahlia
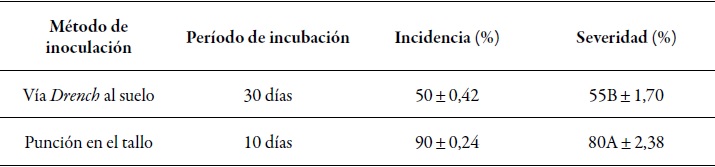
In the antibiosis test, the endophytic Trichoderma cacao isolates were different in their ability to prevent the mycelial growth of V. dahliae in psa medium with mixtures of inhibitory metabolites (table 3).
Table 3 Effect of inhibitory metabolites of endophytic Trichoderma isolates obtained in three culture broths on the percentage of mycelial inhibition of V. dahliae.
Source: Elaborated by the authors
Note: min: Broth based on mineral salts; ps: potato sucrose broth; me: Malt extract broth. Averages followed by different letters indicate significant differences between treatments according to the Duncan test (p ≤ 0.05). Values to the right of the mean indicate the standard error.
The metabolites obtained in ps medium had a higher effect on the mycelial inhibition (29.1 %) of V. dahliae, followed by min (22.2 %) compared to the metabolites obtained in me medium, which showed the lowest percentage (16.6 %). According to the mean culture media data, metabolites of the isolates TE-91, TE-17 and TE-126 prevented the growth of the pathogen with 66.5 %, 55.2 % and 51.9 %, respectively, compared to the other isolates that inhibited less than 17.2 %. Figure 4 shows that metabolites obtained from the TE-91 isolate in min broth prevented completely the mycelial growth of V. dahliae, followed by TE-17 with 77.2 % and 75.0 % in ps and me media, respectively; additionally, TE-126 with 72.8 % and 70.5 % in ps and me media, respectively, and TE-22 with 44.3 % in min. The other isolates had a low percentage of inhibition, i.e. less than 13.5 %.
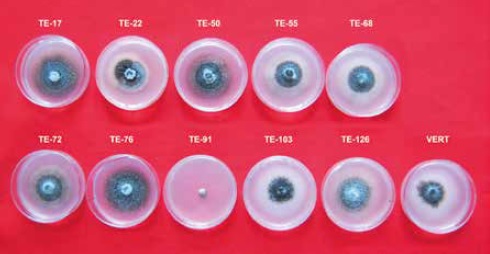
Figure 4. Inhibition of mycelial growth of V. dahliae with soluble inhibitory metabolites of endophytic Trichoderma.
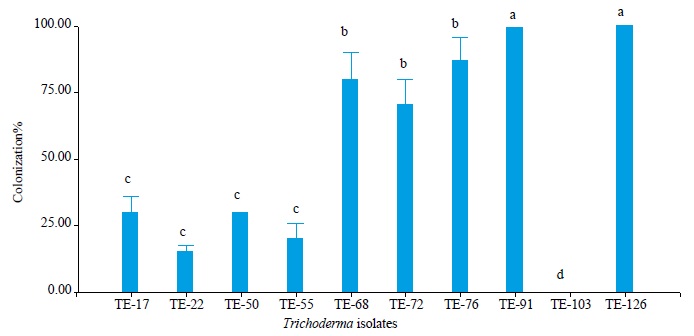
In the myoparasitic test, the anova showed significant differences between the endophytic Trichoderma isolates (p = 0.05) in the colonization of V. dahliae (figure 5). The isolates TE-91 and TE- 126 were the most aggressive mycoparasites of V. dahliae with 100 % colonization on the pathogen, followed by TE-68, TE-72 and TE-76 with 80 %, 70 % and 87 % colonization, respectively, compared to isolates TE-17, TE-22, TE-50 and TE-55, which colonized V. dahliae in percentages lower than 30 %.
Source: Elaborated by the authors
Figure 5. Percentage of endophytic Trichoderma colonization on V. dahliae. Different letters indicate significant differences according to the Duncan test (p ≤ 0.05).
Antibiosis, the production of antimicrobial compounds, mycoparasitism and the feeding of a fungus by another organism are some of the mechanisms of Trichoderma species that provide protection to plants against pathogens ( Junaid et al., 2013; Martinez, Infante , & Reyes, 2013). The colonization and production of soluble inhibitory metabolites of endophytic Trichoderma isolates obtained from different media varied in their ability to mycoparasite and inhibit the mycelial growth of V. dahliae in cccc medium, respectively.
This agrees with the results reported by Bailey et al. (2008), whose Trichoderma isolates varied greatly in their ability to produce inhibitory compounds of M. roreri and in their ability to parasitize these. In this study, Trichoderma asperellum (TE-91) proved to be the most aggressive mycoparasite that colonized and completely inhibited mycelial growth of the pathogen. This is probably due to a combined action between secondary metabolites such as terpenoids, which this species of Trichoderma releases into the pathogen, that inhibits germination and the synergistic interactions of hydrolytic enzymes such as chitinases, glucanases and proteases that degrade the cell wall of the pathogen until it causes its death (Lorito, Farkas, Rebuffat, Bodo, & Kubicek, 1996; Sivasithamparam & Ghisalberti, 1998; Zeilinger, Gruber, Bansal, & Mukherjee, 2016). In addition, isolates of Trichoderma species have been reported as biocontrollers of V. dahliae (Carrero-Carron et al., 2016; Gao, Jun, Pan, Wu, & Zhang, 2007; Jabnoun- Khiareddine, Daami-Remadi, Ayed, Mahjoub, 2009; Meng, Tang, Huang, Ye, & Kan, 2007) and Phytophthora megacarya (Mbarga et al., 2014).
Furthermore, culture broths have an influence on the production of inhibitory metabolites such as non-ribosomal peptides (peptabiotics, siderophores and diketopiperazines), such as gliotoxin and gliovirine, polyketides, terpenes, pyrones and isocyano metabolites, which vary with the species of fungi and the environmental conditions (Zeilinger et al., 2016). The metabolites of isolates TE-17, TE-91 and TE-126 obtained in ps culture were the ones that inhibited most of the mycelial growth of V. dahliae and belong to the species T. brevicompactum, T. asperellum and T. atroviride, respectively. At the same time, TE-126 also proved to be a very aggressive mycoparasite, to such an extent that the pathogen could not be recovered.
Trichoderma atroviride, reported as a mycoparasite, responds to a number of external stimuli, produces hydrolytic enzymes in the presence of a fungal host and wraps around the host hyphae (Harman, 2006). In the same way, Degenkolb, Grafenhan, Nirenberg, Gams and Bruckner (2006) analyzed the formation of polypeptide antibiotics from four isolates of T. brevicompactum selected for their potential antagonism against regressive death. Isolates TE-50, TE-55, TE-68 and TE-76 belonging to the T. harzianum species inhibited the mycelial growth of the pathogen in the antibiosis test. However, TE-68 and TE-76 turned out to be aggressive mycoparasites. Bailey et al. (2008) indicated that T. harzianum isolates tend to have moderate to low antibiosis activity, and moderate to high mycoparasitism activity. This is probably due to the parallel formation of hydrolytic enzymes, together with a group of membrane-activating polypeptides called peptabiotics, whose synergistic action induces an important role in mycoparasitism between T. harzianum and its fungal host (Lorito et al. 1996; Schirmbock et al., 1994).
The selection of Trichoderma strains with good endophilic capacity, in addition to other attributes such as mycoparisitism, antibiosis or induced resistance, can greatly improve the possibilities of developing strategies for biological control of cacao diseases (Bailey et al., 2008).
Conclusions
The presence of the species V. dahliae is reported for the first time in Peru, resulting in the unexpected or sudden death of cacao plants. Metabolites extracted from endophytic Trichoderma isolates in ps broth had a higher effect on V. dahliae mycelial inhibition. The isolation of TE-91 in min and ps culture media showed very good in vitro biocontrol capacity due to their ability to completely inhibit the mycelial growth of V. dahliae, followed by TE-17 and TE-126 obtained in PS and ME culture media, respectively. Also, isolates TE-91 and TE-126 were the most aggressive mycoparasites for the V. dahliae species. These results indicate that the use of Trichoderma could be a biological alternative to consider in the integrated management of the cacao sudden death disease.
Acknowledgements
The Authors wish to thank the The Agricultural Research Service of the United States Department of Agriculture (usda-ars), for their technical support. Moreover, also to the United States Embassy in Lima, Peru, for their support while carrying out this work. Further, to Instituto de Cultivos Tropicales (ict) for allowing us to work in their facilities and laboratories, and to all the cocoa producing organizations of Peru for enabling carrying out the sampling in their areas.
REFERENCES
Arévalo, E., Canto, M., León, B., & Meinhardt, L. (2010a). Biocontrol potencial de Moniliophthora roreri y Moniliophthora perniciosa con aislamientos de trichoderma endófito de cacao in vitro. Documento presentado en el xxi Congreso Peruano de Fitopatología, Tarapoto, Perú. [ Links ]
Arévalo, E., Canto, M., León, B., Meinhardt, L., & Cayotopa, J. (2010b). Colonización de plántulas de Theobroma cacao por aislamientos de Trichoderma endófitos con potencial de control biológico. Documento presentado en el xxi Congreso Peruano de Fitopatología, Tarapoto, Perú. [ Links ]
Bae, H., Sicher, R. C., Kim, M. S., Kim, S.-H., Strem, M. D., Melnick, R. L., & Bailey, B. A. (2009). The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. Journal of Experimental Botany, 60(11), 3279-3295. doi:10.1093/jxb/erp165. [ Links ]
Bailey, B. A., Bae, H., Strem, M. D., Crozier, J., Thomas, S. E., Samuels, G. J., ... Holmes, K. A. (2008). Antibiosis, mycoparasitism, and colonization success for endophytic Trichoderma isolates with biological control potential in Theobroma cacao. Biological Control, 46(1), 24-35. doi:10.1016/j.biocontrol.2008.01.003. [ Links ]
Bailey, B. A., & Meinhardt, L. W. (2016). Cacao diseases: A history of old enemies and new encounters. Nueva York, EE. UU.: Springer. [ Links ]
Barnett, H. L., & Hunter, B. B. (1998). Illustrated genera of imperfect fungi, (3.a ed.). Minnesota, EE. UU.: Macmillan. [ Links ]
Barrón, G. L. (1968). The genera of Hyphomycetes from soil. Baltimore, Canada: The Williams and Wilkins Co. [ Links ]
Carrero-Carrón, I., Trapero-Casas, J. L., Olivares-Garcia, C., Monte, E., Hermosa, R., & Jimenez-Diaz, R. M. (2016). Trichoderma asperellum is effective for biocontrol of Verticillium wilt in olive caused by the defoliating pathotype of Verticillium dahliae. Crop Protection, 88, 45-52. doi:10.1016/j.cropro.2016.05.009. [ Links ]
De Souza, J. T., Bailey, B. A., Pomella, A. W. V., Erbe, E. F., Murphy, C. A., Bae, H., & Hebbar, P. K. (2008). Colonization of cacao seedlings by Trichoderma stromaticum, a mycoparasite of the witches’ broom pathogen, and its influence on plant growth and resistance. Biological Control, 46(1), 36-45. doi:10.1016/j.biocontrol.2008.01.010. [ Links ]
Degenkolb, T., Grafenhan, T., Nirenberg, H. I., Gams, W., & Bruckner, H. (2006). Trichoderma brevicompactum complex: rich source of novel and recurrent plantprotective polypeptide antibiotics (peptaibiotics). Journal of agricultural and food chemistry, 54(19), 7047-7061.doi:10.1021/jf060788q. [ Links ]
Di Rienzo, J. A., Casanoves, F., Balzarini, M. G., Gonzalez, L., Tablada, M., & Robledo, C. W. (2008). InfoStat software estadístico, Manual del Usuario (Version 2008). Cordoba, Argentina: Universidad Nacional de Cordoba. [ Links ]
Emechebe, A., Leakey, C. L., & Banage, W. (1971). Verticillium wilt of cacao in Uganda: symptoms and establishment of pathogenicity. Annals of Applied Biology, 69(3), 223-227. doi:10.1111/j.1744-7348.1971.tb04674.x. [ Links ]
Gao, Z.-M., Jun, C., Pan, Y.-M., Wu, X.-H., & Zhang, Z.- H. (2007). Inhibitory Mechanisms of Trichoderma harzianum TH-1 against the Pathogens of Verticillium Wilt and Fusarium Wilt of Cotton [ J]. Cotton Science, 3, 001. [ Links ]
Guedez, C., Cañizales, L., Castillo, C., & Olivar, R. (2009). Efecto antagonico de Trichoderma harzianum sobre algunos hongos patógenos postcosecha de la fresa (Fragaria spp.). Revista de la Sociedad Venezolana de Microbiología, 29, 34-38. [ Links ]
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41(2), 95-98. [ Links ]
Hanson, L. E., & Howell, C. R. (2004). Elicitors of Plant Defense Responses from Biocontrol Strains of Trichoderma virens. Phytopathology, 94(2), 171-176. doi:10.1094/PHYTO.2004.94.2.171. [ Links ]
Harman, G. E. (2006). Overview of mechanisms and uses of Trichoderma spp. Phytopathology, 96(2), 190-194. doi:10.1094/PHYTO-96-0190. [ Links ]
Inderbitzin, P., Bostock, R. M., Davis, R. M., Usami, T., Platt, H. W., & Subbarao, K. V. (2011). Phylogenetics and taxonomy of the fungal vascular wilt pathogen Verticillium, with the descriptions of five new species. PLoS One, 6(12), e28341. doi:10.1371/journal.pone.0028341. [ Links ]
Jabnoun-Khiareddine, H., Daami-Remadi, M., Ayed, F., & El Mahjoub, M. (2009). Biological control of tomato Verticillium wilt by using indigenous Trichoderma spp. The African Journal of Plant Science and Biotechnology, 3(Special issue 1), 26-36. [ Links ]
Junaid, J. M., Dar, N. A., Bhat, T. A., Bhat, A. H., & Bhat, M. A. (2013). Commercial biocontrol agents and their mechanism of action in the management of plant pathogens. International Journal of Modern Plant & Animal Sciences, 1(2), 39-57. [ Links ]
Leakey, C. (1965). Sudden death disease of cacao in Uganda associated with Verticillium dahliae Kleb. East African Agricultural and Forestry Journal, 31(1), 21-24. doi:10.1080/00128325.1965.11662020 [ Links ]
León, B., Arévalo, E., Márquez, K., Bailey, B., Cayotopa, J., & Olivera, D. (2010a). Capacidad antagónica de aislamientos de Trichoderma endófitos de cacao contra Phytophthora palmivora in vitro. Documento presentado en el XXI Congreso Peruano de Fitopatología, Tarapoto, Perú. [ Links ]
León, B., Rojas, M., Rodríguez, G., Arévalo, E., & Márquez, K. (2010b). Antibiosis y micoparasitismo a los principales patógenos de cacao (Theobroma cacao) por hongos endófitos. Documento presentado en el XXI Congreso Peruano de Fitopatología, Tarapoto, Perú. [ Links ]
López-Escudero, F. J., & Mercado-Blanco, J. (2011). Verticillium wilt of olive: a case study to implement an integrated strategy to control a soil-borne pathogen. Plant and Soil, 344(1-2), 1-50. doi:10.1007/s11104-010-0629-2. [ Links ]
Lorito, M., Farkas, V., Rebuffat, S., Bodo, B., & Kubicek, C. P. (1996). Cell wall synthesis is a major target of mycoparasitic antagonism by Trichoderma harzianum. Journal of Bacteriology, 178(21), 6382-6385. [ Links ]
Márquez, D. K., Arévalo, E., León, B., Cayotopa, J., Olivera, D., & Samuels, J. G. (2010). Composición de comunidades de hongos endófitos de cacao nativo en cuencas del alto amazonas del Perú. Documento presentado en el XXI Congreso Peruano de Fitopatología, Tarapoto, Perú. [ Links ]
Martínez, B., Infante, D., & Reyes, Y. (2013). Trichoderma spp. y su funcion en el control de plagas en los cultivos. Revista de Protección Vegetal, 28(1), 1-11. [ Links ]
Mbarga, J. B., Begoude, B., Ambang, Z., Meboma, M., Kuate, J., Schiffers, B., ... Ten Hoopen, G. M. (2014). A new oil-based formulation of Trichoderma asperellum for the biological control of cacao black pod disease caused by Phytophthora megakarya. Biological Control, 77, 15-22. doi:10.1016/j.biocontrol.2014.06.004. [ Links ]
Mejía, L. C., Rojas, E. I., Maynard, Z., Bael, S. V., Arnold, A. E., Hebbar, P., ... Herre, E. A. (2008). Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biological Control, 46(1), 4-14. doi:10.1016/j.biocontrol.2008.01.012. [ Links ]
Meng, N., Tang, B., Huang, X.-D., Ye, S.-M., & Kan, Q.-H. (2007). The inhibition of four Trichoderma species against Verticillium dahliae. Journal of Biology, 4, 016. [ Links ]
Montgomery, D. C. (2008). Design and analysis of experiments, (7.a ed.). Nueva Jersey, EE. UU.: John Wiley & Sons. [ Links ]
Osorio Hernández, E., Hernández Castillo, F. D., Rodríguez Herrera, R., Varela Fuentes, S. E., Estrada Drouaillet, B., & López Santillan, J. A.(2016). Actividad antagónica de Trichoderma spp. sobre Rhizoctonia solani in vitro. Investigación y Ciencia, 24(67), 5-11. [ Links ]
Ormeno-Orrillo, E., & Dávila, D. Z. (1999). Optimizacion del tiempo de esterilizacion de soportes basados en suelo y compost en la producción de inoculentes para leguminosas. Revista Peruana de Biología, 6(2), 181-184. doi:10.15381/rpb.v6i2.8313. [ Links ]
Pegg, G., & Brady, B. (2002). Verticillium wilts. Wallingford, Inglaterra: cabi pub. [ Links ]
Resende, M., Flood, J., & Cooper, R. M. (1994). Host specialization of Verticillium dahliae, with emphasis on isolates from cocoa (Theobroma cacao). Plant Pathology, 43(1), 104-111. doi:10.1111/j.1365-3059.1994.tb00559.x. [ Links ]
Resende, M., Flood, J., & Cooper, R. M. (1995). Effect of method of inoculation, inoculum density and seedling age at inoculation on the expression of resistance of cocoa (Theobroma cacao L.) to Verticillium dahliae Kleb. Plant Pathology, 44(2), 374-383. doi:10.1111/j.1365-3059.1995.tb02790.x. [ Links ]
Schirmbock, M., Lorito, M., Wang, Y.-L., Hayes, C. K., Arisan-Atac, I., Scala, F., ... Kubicek, C. P. (1994). Parallel formation and synergism of hydrolytic enzymes and peptaibol antibiotics, molecular mechanisms involved in the antagonistic action of Trichoderma harzianum against phytopathogenic fungi. Applied and Environmental Microbiology, 60(12), 4364-4370. [ Links ]
Schnathorst, W. (1981). Life cycle and epidemiology of Verticillium. En M. Mace, A. Bell, & C. Beckman (Eds.), Fungal wilt diseases of plants (pp. 81-111). Nueva York, EE. UU.: Academic Press. [ Links ]
Sivasithamparam, K., & Ghisalberti, E. (1998). Secondary metabolism in Trichoderma and Gliocladium. En C. P. Kubicek, & G. E. Harman (Eds.), Trichoderma and Gliocladium basic biology taxonomy and genetics (pp. 139- 191). Londres, Reino Unido: Taylor & Francis. [ Links ]
Toghueo, R. M. K., Eke, P., Zabalgogeazcoa, I., de Aldana, B. R. V., Nana, L. W., & Boyom, F. F. (2016). Biocontrol and growth enhancement potential of two endophytic Trichoderma spp. from Terminalia catappa against the causative agent of Common Bean Root Rot (Fusarium solani). Biological Control, 96, 8-20. doi:10.1016/j.biocontrol.2016.01.008. [ Links ]
Trapero, C., Diez, C. M., Rallo, L., Barranco, D., & López- Escudero, F. J. (2013). Effective inoculation methods to screen for resistance to Verticillium wilt in olive. Scientia Horticulturae, 162, 252-259. doi:10.1016/j.scienta.2013.08.036. [ Links ]
Villarroel-Zeballos, M. I., Feng, C., Iglesias, A., du Toit, L. J., & Correll, J. C. (2012). Screening for resistance to Verticillium wilt in spinach and isolation of Verticillium dahliae from seed of spinach accessions. HortScience, 47(9), 1297-1303. [ Links ]
Wang, Y., Liang, C., Wu, S., Zhang, X., Tang, J., Jian, G., ... Chu, C. (2016). Significant improvement of cotton Verticillium wilt resistance by manipulating the expression of gastrodia antifungal proteins. Molecular plant, 9(10), 1436-1439. doi:10.1016/j.molp.2016.06.013. [ Links ]
Watanabe, T. (2010). Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species (3.a ed.). Boca Raton, EE. UU., Londres, Inglaterra, y Nueva York, EE. UU.: CRC. [ Links ]
White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal rna genes for phylogenetics. En A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), pcr Protocols: A Guide to Methods and Applications (pp. 315-322). San Diego, EE. UU.: Academic Press. [ Links ]
Yildiz, A., & Benlioglu, S. (2010). Effects of soil solarization and some amendments to control verticillium wilt in established olive orchards. African Journal of Biotechnology, 9(40), 6660-6665. doi:10.5897/AJB10.507. [ Links ]
Yuan, Y., Feng, H., Wang, L., Li, Z., Shi, Y., Zhao, L., ... Zhu, H. (2017). Potential of endophytic fungi isolated from cotton roots for biological control against verticillium wilt disease. PLoS One, 12(1), 1-12. doi:10.1371/journal.pone.0170557. [ Links ]
Zeilinger, S., Gruber, S., Bansal, R., & Mukherjee, P. K. (2016). Secondary metabolism in Trichoderma e Chemistry meets genomics. Fungal biology reviews, 30(2), 74-90. doi:10.1016/j.fbr.2016.05.001. [ Links ]
Zhang, D., & Motilal, L. (2016). Origin, dispersal, and current global distribution of cacao genetic diversity. En B. A. Bailey, & L. W. Meinhardt (Eds.), Cacao diseases a history of old enemies and new encounters. Nueva York, EE. UU.: Springer International. [ Links ]
Received: May 03, 2017; Accepted: April 20, 2018











 texto em
texto em