Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ciencia y Tecnología Agropecuaria
Print version ISSN 0122-8706On-line version ISSN 2500-5308
Cienc. Tecnol. Agropecuaria vol.20 no.3 Mosquera Sep./Dec. 2019 Epub Sep 10, 2019
https://doi.org/10.21930/rcta.vol20num3art:1589
Sanidad vegetal y protección de cultivos
Neutral genetic variability and resistance mechanisms present in Myzus persicae (Hemiptera: Aphididae) from different hosts in central Chile
1Investigador PhD, Corporación Colombiana de Investigación Agropecuaria (AGROSAVIA), Centro de Investigación Motilonia. Codazzi, Colombia.
2Académico investigador, Universidad de Talca, Departamento de Producción Agrícola. Talca, Chile.
3Académico investigador, Universidad de Talca, Instituto de Ciencias Biológicas. Talca, Chile
4Corporación Colombiana de Investigación Agropecuaria [AGROSAVIA]
Myzus persicae is considered the third pest of economic importance in Chile affecting several crops. Its genetic variability is influenced by the availability of the primary host. M. persicae has acquired resistance to most of the insecticides used for its control. The current work aimed to evaluate the genetic diversity and the presence of resistance mechanisms of M. persicae in crops, such as peach and sweet pepper. The study was carried out in twelve localities, six in the O'Higgins region and six in the Maule region of central Chile. Monthly collections were performed on the crops and their associated weeds. To evaluate the neutral genetic diversity, seven microsatellite markers were used, and the identification of mechanisms was performed by allelic discrimination tests. According to the results, the highest genetic diversity (> 0.80 on average) was found in peach populations. In sweet pepper and associated weeds, this diversity was 0.36 on average. The frequency of individuals with resistance mechanisms was low and predominantly heterozygous in the hosts assessed, suggesting that the sexual reproduction form of the aphid influences the levels of resistance to the insecticide. The presence of resistance mechanisms in M. persicae depends on the host, the geographical region, and the time in which they are evaluated. There is sufficient evidence that the populations of M. persicae are comprised of extremely few genetic groups, presenting different resistance dynamics to insecticides in the same agricultural season.
Keywords Brassicaceae; insecticides; plant pests; Prunus persica; resistance
Myzus persicae es considerado la tercera plaga de importancia económica de varios cultivos en Chile. Su variabilidad genética es influenciada por la disponibilidad del hospedante primario. Este insecto ha adquirido resistencia a la mayoría de los insecticidas utilizados para su control. El presente trabajo tuvo como objetivo evaluar la diversidad genética y la presencia de mecanismos de resistencia de M. persicae en cultivos como duraznero y pimentón. El estudio se realizó en doce localidades: seis en la región de O'Higgins y seis en la región del Maule en Chile central. Para evaluar la diversidad genética neutral, se utilizaron siete marcadores microsatélites, y la identificación de mecanismos se realizó mediante ensayos de discriminación alélica. La mayor diversidad genética se presentó en poblaciones del duraznero > 0,80 en promedio. En pimentón y arvenses, esta diversidad fue 0,36 en promedio. La frecuencia de individuos con mecanismos de resistencia fue baja y en forma predominantemente heterocigota en los hospedantes evaluados, lo que sugiere que la forma de reproducción sexual del áfido influye en los niveles de resistencia a insecticida. La presencia de mecanismos de resistencia en M. persicae depende del hospedante, región geográfica y momento de muestreo en el que son evaluados. Existe suficiente evidencia de que las poblaciones de M. persicae están compuestas por muy pocos grupos genéticos, presentando diferentes dinámicas de resistencia a insecticidas en la misma temporada agrícola.
Palabras clave Brassicaceae; insecticidas; plagas de plantas; Prunus persica; resistencia
Introduction
Aphids are agricultural pests found worldwide, causing economic damage to many crops —directly— by their feeding activity and —indirectly— by the transmission of viruses to plants (Blackman & Eastop, 2000). Myzus persicae (Sulzer) (Hemiptera: Aphididae) is an extremely polyphagous aphid being able to develop in more than 400 plant species (Blackman & Eastop, 2000). This insect reproduces by cyclic parthenogenesis, which alternates several generations produced by asexual reproduction that occurs on a vast diversity of secondary host plants during spring and summer (Blackman & Eastop, 2007). The green aphid of peach, as it is commonly known, has an annual life cycle, which has a sexual generation in Prunus persica (L.) Batsch (Rosaceae), its primary host, in which sexual hibernation eggs are produced in autumn, alternating with many asexual generations (females only) during spring-summer in several herbaceous plants (secondary hosts) (Charaabi et al., 2008).
Rouger, Reichel, Malrieu, Masson and Stoeckel (2016) pointed out that the genetic diversity of the population in species with complex life cycles is difficult to anticipate; the model developed by these authors demonstrated that cyclic parthenogenesis has specific effects on neutral genetic diversity and are different from other reproductive modes (i.e., complete sexuality, complete cloning). In this sense, and as supported by various studies, the populations of M. persicae that reproduce by cyclic parthenogenesis show greater genetic variability than the populations with obligate parthenogenesis and alternation between the primary and secondary host (Blackman, Malarky, Margaritopoulos, & Tsitsipis, 2007).
The geographical distance between two sampling sites does not influence the genetic variability, but it can be decisive in the genetic differences between the populations analyzed. It has been observed that populations of M. persicae have higher genetic differentiation in sites separated by 120 km or more (Fenton, Malloch, Navajas, Hillier, & Birch, 2003; Guillemaud, Mieuzet, & Simon, 2003; Vorburger, Lancaster, & Sunnucks, 2003; Wilson, Sunnucks, Blackman, & Hales, 2002; Zamoum et al., 2005).
However, the genetic variability of a population of M. persicae can be influenced by other factors, such as the gene flow between populations from one locality to another, which in turn can depend on the environmental conditions of these localities (Vorbuguer et al., 2003); the presence of specialized individuals on a host, and the alternation between the primary and the secondary hosts (Blackman et al., 2007, Kasprowicz, Malloch, Pickup, & Fenton, 2008, Margaritopoulos, Malarky, Tsitsipis, & Blackman, 2007). However, several studies indicate that the availability of P. persica as the primary host influences the genetic variability of M. persicae (Fenton, Kasprowicz, Malloch, & Pickup, 2010; Guillemaud et al., 2003; Margaritopoulos, Malarky, et al., 2007; Wilson et al., 2002; Zamoum et al., 2005) compared to the geographical distance that may exist between two or more study locations (Sánchez, Spina, Guirao, & Cánovas, 2013).
The primary method for the control of aphid populations worldwide is the use of chemical insecticides (Dewar, 2007). However, resistance to different chemical products has already been reported in several countries (Devonshire et al., 1998; Fenton, Margaritopoulos, Malloch, & Foster, 2010; Voudouris et al., 2016). For the control of M. persicae in Chilean agriculture, products of the carbamate, organophosphate, pyrethroid, and neonicotinoid groups are used (Servicio Agrícola y Ganadero, 2012). Studies in Chile on resistance to insecticides in M. persicae have been carried out with genotypes collected sporadically in wide geographical ranges (Castañeda et al., 2011; Fuentes-Contreras et al., 2013), but without considering variables such as plant host, different localities and geographic regions, or the interactions between these. However, genetic variability and the presence of resistance mechanisms of M. persicae have been evaluated at a spatial scale in orchards considering the primary and secondary hosts during an agricultural season (Rubiano-Rodríguez et al., 2014). In this sense, and up to date, there are no studies that allow us to relate the neutral genetic variability and the frequency of resistance mechanisms on a regional scale, considering different hosts (primary and secondary) and different locations.
Accordingly, this study aims at evaluating the neutral genetic diversity and the presence of mutations in the sodium channel (kdr and super kdr) and acetylcholinesterase (MACE) that confer resistance to insecticides in M. persicae, from peach orchards (primary host), sweet pepper Capsicum annuum var. grossum (L.) Sendtn. fields and some associated weeds (secondary hosts) in 12 locations in central Chile: six in the O'Higgins region and six in the Maule region.
Materials and methods
Study site and sampling
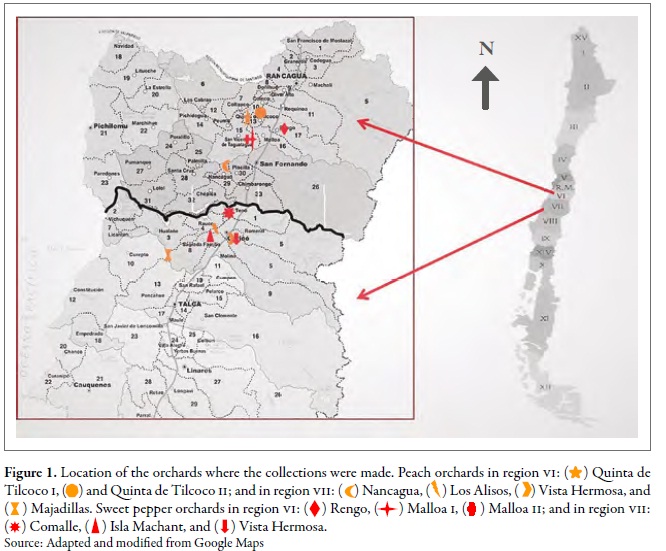
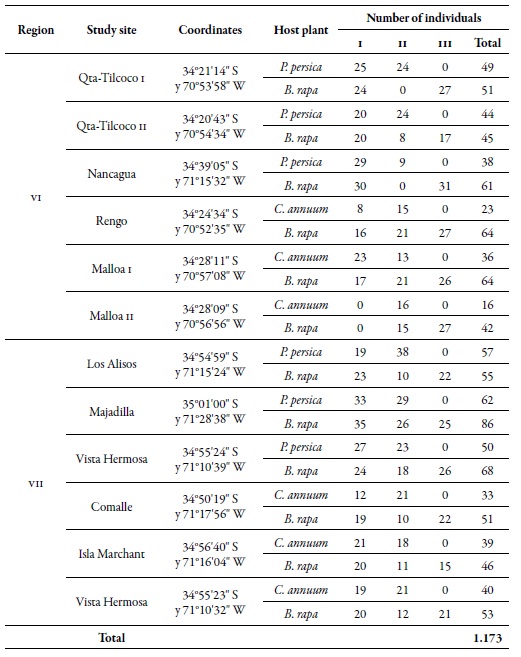
The work was carried out in the regions of O'Higgins (VI) and Maule (VII) in central Chile; six orchards were selected in each region, three for peach trees and three for sweet pepper, under a conventional pest management system (regular application of insecticides). The closest distance of the orchards between regions was found between Comalle, in the Maule region, and Nancagua, in the O'Higgins region, approximately 21 km apart. The orchards of Majadilla, in the Maule region, and Qta-Tilcoco II, in the O'Higgins region, were the most distanced, i.e., 90 km apart (figure 1). At each site, random samples were taken in 20 points; at each point, five apterous or winged individuals of the green aphid of peach were collected in three sampling moments: spring (I), summer-autumn (II) and winter (III), during the agricultural seasons 2010 and 2011 (table 1). Besides, the same number of individuals was collected in Brassica rapa L. (Brassicaceae ), associated in each of the orchards. The samples were preserved in 90 % alcohol, and the individuals were identified using the key of Blackman and Eastop (2007).
Genotyping
The genotyping analysis was carried out in the laboratory of Instituto de Ecología y Evolución of Universidad Austral de Chile. In total, 1,010 individuals were genotyped using the microsatellite marker technique. Seven microsatellite loci were used (Myz2, Myz3, Myz9, Myz25, M35, M37, and M40), which have been described and are widely used for population genetic studies of M. persicae (Fuentes-Contreras, Figueroa, Reyes, Briones, & Niemeyer, 2004; Kasprowicz et al., 2008; Malloch et al., 2006; Margaritopoulos, Malarky et al., 2007; Vorburger, 2006; Wilson et al., 2002). Genomic DNA (gDNA) was obtained from each individual by the salting out method (Sunnucks & Hales, 1996). The gDNA extracted was quantified by spectrophotometry (Nanodrop ND-1000) and its quality was evaluated through electrophoresis in 0.8% agarose gels, stained with ethidium bromide, with which the intensity of the bands could be observed under ultraviolet (UV) light. When the quality of the gDNA was poor, individuals from the same sample that had been stored were taken, and new gDNA was extracted from these.
Genotyping of individuals was performed using the fluorophore labeling technique (Schuelke, 2000), and the determination of allelic sizes was developed by analyzing fragments in an automatic sequencer in Macrogen Inc. (Korea). To establish the width of the bands, the data were analyzed using electropherograms and employing the Genemarker V.1.3 program (Borodovsky & McIninch, 1993). Subsequently, the presence of null alleles or genotyping errors was assessed with the Micro-Checker software V.2.2.3 (Van Oosterhout, Hutchinson, Oills, & Shipley et al., 2004).
Table 1. Location of the study sites, number of individuals of M. persicae per host and sampling moment in the regions of O'Higgins (VI) and Maule (VII), during the 2010-2011 agricultural season1 Sampling moments: spring (I), summer-autumn (II), and winter (III).
Source: Elaborated by the authors
Detection of resistance mutations
The identification of mutations was performed using the PCR technology in real sampling moment (qRT-PCR) by abi TaqMan allelic discrimination trials. The mutations kdr and super kdr were identified by the method described by Anstead, Williamson, Eleftherianos, and Denholm (2004), while the identification of the mace mutation was performed according to the protocol described by Anstead, Williamson and Denholm (2008). Both methods use the 5'-exonuclease activity of the Taq polymerase to quantify specific sequences in a sample. For each mutation, two different probes (with and without mutation), marked with a fluorophore at the 5'' end (fam or hex) were used, as well as a damper (quencher) in the 3' end. Hydrolysis of the probe separates the fluorophore from it, when, by being separated from the quencher, emits a characteristic fluorescence, representing the sequence present in the dna to which it was aligned (cDNA).
The PCR reaction was developed in a total volume of 11.9 μL per individual comprised by 6.25 μL of TaqMan® Universal PCR Master Mix (Applied Biosystems), 1.5 μL of primer F, 1.5 μL of primer R, 0.5 μL of probe S (susceptibility condition), 0.5 μL of probe R (resistance mutation), 0.5 μL of DNA (25 μg/μL), and 1.75 μL of ultrapure H2O. Both for the kdr as well as for the super kdr mutations, the susceptibility probes were labeled with the fam fluorophore, which has an excitation wavelength of 492 nm and an emission wavelength of 517 nm, and this is the reason why it is considered a green emitter. Meanwhile, those of resistance were marked with the hex fluorophore, which has an excitation wavelength of 535 nm and an emission wavelength of 556 nm, so it is considered a yellow emitter. In contrast, for the mace mutation, the susceptibility probe was labeled with the hex fluorophore and the resistance probe with the fam fluorophore. The pcr reactions were carried out in a Stratagene Mx 3000 P thermal cycler, using a thermal profile consisting of an initial stage of 2 min at 50 °C, 16 min-40 s at 95 °C, followed by 40 cycles consisting of 15 s at 92 °C and 1 min at 60 °C; finally, 15 s at 72 °C, measuring the fluorescence of each reaction in this last stage.
Data analysis
All collected individuals were analyzed as multilocus genotypes (MLGs), that is, genotypes that amplified the seven loci used in the genotyping. Genetic diversity was calculated using coarse genetic diversity (dgg) based on the n/N proportion, where n represents the total number of MLGs and N, the total number of individuals analyzed in the samples. The analyses were performed using only one copy of each MLG for all the variables assessed (locality, period and host) because clonal "amplification" is not the same in all genotypes and can lead to deviations from the Hardy-Weinberg equilibrium (hwe) within the samples, thus, distorting the allele frequency estimates (Sunnucks, De Barro, Lushai, Maclean, & Hales, 1997). For example, when the analysis was done for the locality variable, one copy per locality was left of the same mlg, and so on, with all the variables. The allelic frequencies, the proportion of null alleles, the observed (Ho) and the expected (He) heterozygosity, the deviations from the Hardy-Weinberg equilibrium (hwe) and the linkage disequilibrium (Fis) between the data set, were analyzed with Fisher's exact test, implemented in Genepop V.3.2A (Raymond & Rousset, 1995). Allelic richness (Rs = number of alleles regardless of the sample size) was calculated using the FStat program V.2.9.3.2 (Goudet, 1995). Moreover, a molecular analysis of variance (Amova) was performed, using the Arlequin V.2.0 program (Schneider, Roessli, & Excoffier, 2000).
The genetic structure of the data set was generated using a Bayesian cluster analysis employing the program Structure V.2.3.2 (Pritchard, Stephens, & Donnelly, 2000). The number of populations (k) used was in a range of 1 to 10, with 10 runs for each k, 100.000 burn in and 106 iterations in the Markov Monte Carlo chain. The number of genetic groups was established based on the criterion of ΔK of Evanno, Regnaut and Goudet (2005), using Structure Harvester.1
To identify if there were genetic groups that were shared between the different variables, four separate analyzes were made per i) region, ii) sampling moments, iii) host/region, and iv) host/sampling moment. Individuals were assigned to each group when their probability of belonging was > 0.8. The difference in the frequency of insecticide resistance mutations (kdr, super kdr, and MACE) between MLGs (using both single and multiple copies) from different regions, hosts and moment of sampling, was evaluated through the use of contingency tables and the chi-square test (χ2). Multiple comparisons between MLG frequencies were performed by the Marascuilo test using the statistical program XLStat-Pro 7.5 (Addinsoft, New York, U. S. A.).
Results and discussion
Distribution of genotypes and genetic diversity
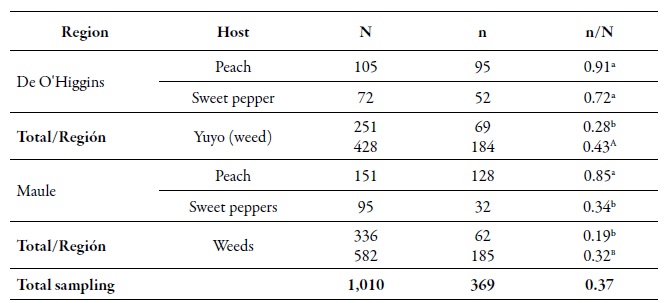
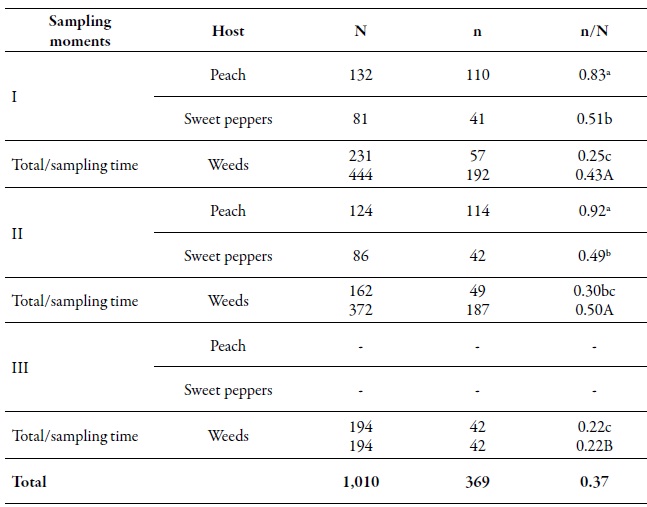
From the total of analyzed individuals, 368 multilocus genotypes (MLGs) were found. At the spatial level, the gross genetic diversity (dgg) was statistically higher in the O'Higgins region (0.43) compared to the Maule region (0.32) (table 2). These results are consistent with those reported by Rubiano-Rodríguez et al. (2014), who evaluated the genetic diversity at the orchard level in the same collection regions. At the temporary level, the dgg of periods I and II (0.43 and 0.50, respectively) was higher compared to period III (tables 2-3). The dgg of P. persicae was statistically superior in its primary host, both spatially and temporally, compared to the other two hosts (tables 2-3), which can be explained in two ways. First, this phenomenon could be related to the holocyclicheteroic reproductive cycle of the green aphid of peach, in which at the beginning of the spring, individuals coming from different origins congregate due to the hatching of founders, the migration of some MLGs that spent the winter in secondary hosts, and the return of the virginouparous females that will give origin to oviparous and unwinged females for sexual reproduction (Blackman & Eastop, 2007). That is why, when sexual reproduction occurs, there is a higher genetic flow, resulting in a more significant presence of different genotypes on peach trees.
A second analysis could be oriented to a lower abundance of M. persicae on peach trees compared to C. annuum and B. rapa. The data of population abundance are not directly comparable between a tree (P. persica) and smaller herbaceous plants (C.annuum and B. rapa), because on the peach tree, it is only possible to find aphid individuals in mid-spring and late autumn, while on secondary hosts, these can be found throughout their vegetative cycle. Moreover, in the case of weeds, such as B.rapa, these can be found throughout the year.
Table 2. Number of aphids analyzed (N), number of MLGs (n) and the gross genetic diversity index (dgg) of Myzus persicae (n/N) in the O'Higgins and Maule regions and on different hosts during the agricultural season 2010-2011Different lowercase letters indicate significant differences according to the χ2 test in Marascuilo’s multiple comparisons between all proportions. Different capital letters indicate significant differences according to the χ2 test in Marascuilo’s multiple comparisons between the total regional proportions.
Source: Elaborated by the authors
Table 3. Number of aphids analyzed (N), number of MLG (n) and the gross genetic diversity index (DGG) of Myzus persicae (n/N) in three sampling moments on different hosts during the 2010-2011 agricultural seasonDifferent lowercase letters indicate significant differences according to the χ2 test in Marascuilo’s multiple comparisons between all proportions. Different capital letters indicate significant differences according to the χ2 test with Marascuilo’s multiple comparisons between the total proportions of the sampling moments.
Source: Elaborated by the authors
At a spatial level, the O'Higgins region showed the highest dgg, which may be related to the fact that this region has a greater cultivated area with peach trees compared to the Maule region. There are several reports that indicate that the highest genetic diversity of M. persicae is found in those areas, or in areas where the cultivation of its primary host predominates; peach is the only plant species where this aphid shows sexual reproduction (Fenton, Kasprowicz et al., 2010; Guillemaud et al., 2003; Margaritopoulos, Malarky et al., 2007; Wilson et al., 2002; Zamoum et al., 2005). Of the total MLGs found, 71 genotypes were multiple, that is, genotypes found more than once in several locations and in different sampling moments, of which 42 % were present in the two regions. Of all the MLGs, 10 were called common, since they are the most frequent, they are located in the two regions, they are found in all three sampling moments, and in the three hosts assessed (table 4). These most frequent MLGs did not show the resistance mechanisms evaluated, except for MLGs G039 and G239 that were resistant heterozygotes (rs) for kdr, G272 which was rs for mace, and G119 that was RS for the three mechanisms assessed (table 4).
The number of MLGs found in this study at the regional level is somewhat higher than the results of the study at the orchard level (Rubiano-Rodríguez et al., 2014), which can be attributed to the fact that in this study a higher number of orchards (three for each crop per region) were assessed. If we compare with studies in other countries, the number of MLGs found in our study is higher, for example, in Spain, studies on sweet pepper, weeds, almonds and a few samples of peaches, found 289 (Sánchez et al., 2013); in Australia, 72 MLGs were found in broccoli (Vorburger, 2006); in New Zealand, in potato, 23 were found (Van Toor et al., 2008), and in Scotland, on potato and broccoli, 21 were found (Kasprowicz et al., 2008). This is also because, in most of these studies, the individuals analyzed came mainly from secondary hosts, in which genetic diversity is generally low.
In studies conducted by Guillemaud et al. (2003) in France, with aphids from peach trees, 100 MLGs were found, and these results were lower than the one reported in this study. This can be because, by using suction traps during the collections, individuals from different hosts can be captured; this could also be associated with the size of the sample, as it was relatively low compared to the sample size of the current study. When samplings or collections are made in the primary host, we can find similar results, as happened in a study conducted in Greece and Italy in areas with and without peach production, in which the mlg number was similar to that of our study, with 482 MLGs (Blackman et al., 2007).
Table 4. Detail of the ten most common MLGs found in this study and their respective resistance profile. Number of aphids characterized for specific MLGs (N) in the regions of O'Higgins and Maule in three sampling moments on different hosts during the 2010-2011 agricultural season1 Hosts: Peach (D), sweet pepper (P), and weeds (M). 2 Sampling moments: spring (I), summer-autumn (II) and winter (III).
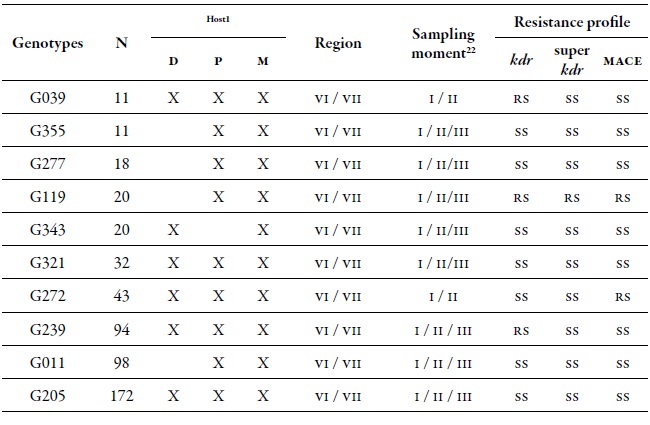
Source: Elaborated by the authors
The seven microsatellite loci in this study were polymorphic, with an average number of alleles ranging from 5.7 to 7.7, in the O'Higgins region, and from 6.4 to 6.9, in the Maule region. The He and Ho were very similar in all the populations of the two regions (table 5), which indicates that these populations were in HWE. The Fis values were significant, but relatively low, which indicated a low level of inbreeding among the populations (table 5). Although these results tend to be frequent in populations with sexual reproduction or with cyclic parthenogenesis, it is also possible to observe strongly positive Fis values for highly clonal species (which occurs in M. persicae during most of its life cycle), if the mutation dominates over the genetic drift (Rouger et al., 2016). In the case of linkage disequilibrium, a few pairs of loci were significant in the samples of P. persica and C. annuum in the two regions. Something similar happened when the variable "sampling moment" was analyzed. In the samples from Brassica rapa, there was a greater number of pairs of loci with significant linkage disequilibrium values (table 5). The presence of the HWE and the low levels of endogamy (Fis), as well as the low levels of linkage disequilibrium, support the theory and importance of sexual reproduction in Chile.
Table 5. Genetic diversity index by region. Average number of alleles (N), allelic richness (Rs = number of alleles independent of the sample size), observed heterozygosity (Ho) and expected heterozygosity (He), Fis value and the proportion of pairs that were significant in the linkage disequilibrium test (DL)* Significant values for p < 0.01.
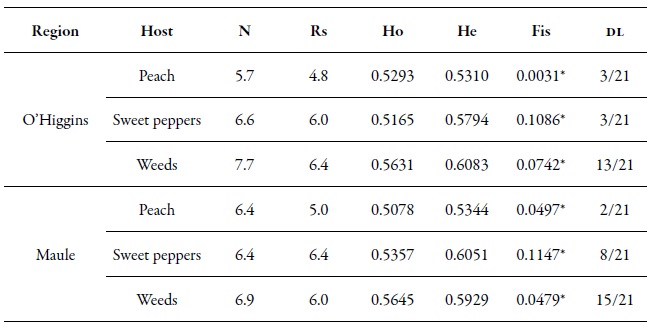
Source: Elaborated by the authors
Other studies carried out in Chile show that populations of M. persicae are in hwe, suspecting the presence of sexual reproduction (Fenton, Margaritopoulos et al., 2010, Rubiano-Rodríguez et al., 2014). Considering what these authors found, during this research, it was possible to observe the presence of sexual morphs and eggs, which were collected and observed in the laboratory, where copulation could also be seen and, subsequently, the emergence of founders. This confirms the positive relationship between the genetic diversity of the green aphid of peach and its primary host, P. persica. According to Fenton, Margaritopoulos et al. (2010), the genetic diversity of the individuals of M. persicae can be maintained during the dispersal to secondary hosts in winter, and their return to the primary host in the spring.
Our results differ with some other studies conducted worldwide with M. persicae that report that the populations studied are not in hwe, for example, in France (Fenton et al., 2003; Guillemaud et al., 2003), Australia (Wilson et al., 2002) and in Greece (Margaritopoulos, Malarky et al., 2007). This could be because the populations analyzed in these studies were collected on secondary hosts mainly, where sexual reproduction does not occur, despite being this type of reproduction one of the assumptions for a population to be in hwe.
Genetic differentiation between populations
The results of the Amova between regions showed that there is significant genetic differentiation (0.28%) between the two regions evaluated (table 6), but there are no significant genetic differences in the populations between the localities of the same region. However, significant genetic differentiation of individuals within localities was found (6.97 %), while the highest genetic variation was found within individuals (92.67 %). The results of the Amova, when the hosts were considered as the main component of the analysis (table 6), indicated a low but significant percentage of genetic variation among hosts (1.37 %). When analyzing the contribution of the localities taking into account the same host, the percentage of variation in this level was not statistically significant. A highly significant percentage of genetic differentiation (6.31%) was observed among individuals within the localities, and the greatest genetic variation continues to occur within individuals (92.34 %). According to the results obtained during the study, the genetic variation among populations of M. persicae that occurs in Chile is more determined by the plant species (host) and not by the place or geographical distance that may exist between the collection sites.
The low but significant percentage of genetic variation found in this study at the regional level may indicate some degree of specialization achieved by some genotypes of M. persicae on certain plants. Previous studies in Chile reported some degree of specialization of M. persicae on tobacco (Fuentes-Contreras et al., 2004, Margaritopoulos, Skouras et al., 2007). Also, in Spain, Sánchez et al. (2013) recorded the abundance of an M persicae genotype on Solanum physalifolium.
The fst values in this study indicate that the populations that were collected on the weeds in the two regions were genetically different to the populations collected in the crops (peach and sweet pepper), which agrees with what was registered by Vialatte, Dedryver, Simon, Galman and Plantegenest (2005). This indicates that the populations of the spike aphid, Sitobion avenae Fabricius (Hemiptera: Aphididae), collected on non-cultivated plants, are generally genetically different from those collected on cultivated plants.
In the analysis of genetic differentiation among pairs of populations, considering regions and hosts, some significant differences were found (table 7). This is how the populations collected on peach trees in the two regions (D-VI and D-VII) and on sweet pepper in the O'Higgins region (P-VI) showed a significant genetic differentiation by pairs (fst) concerning populations collected on weeds (M-VI and M-VII) in the two regions (table 7). Besides, the fst among the populations collected on peach in the Maule region (D-VII) was also significant, compared to the populations collected on sweet pepper (P-VI) in the O'Higgins region (table 7). The Bayesian analysis of the population’s genetic structuring revealed that, when the variables sampling moments, host/region and host/sampling moments were considered, the best partition of the data set was in two genetic groups (K = 2) (figures 2a, b, c); however, when the data were analyzed according to the regions, three genetic groups were found (K = 3) (figure 2d).
Table 6. Analysis of the molecular variance of M. persicae comparing samples between regions and hostsFCT: coefficient of genetic differentiation between regions; FSC: coefficient of genetic differentiation between populations inthe regions; FST: coefficient of genetic differentiation between all populations; FIS: degree of inbreeding; FIT: genetic differentiationbetween subpopulations.
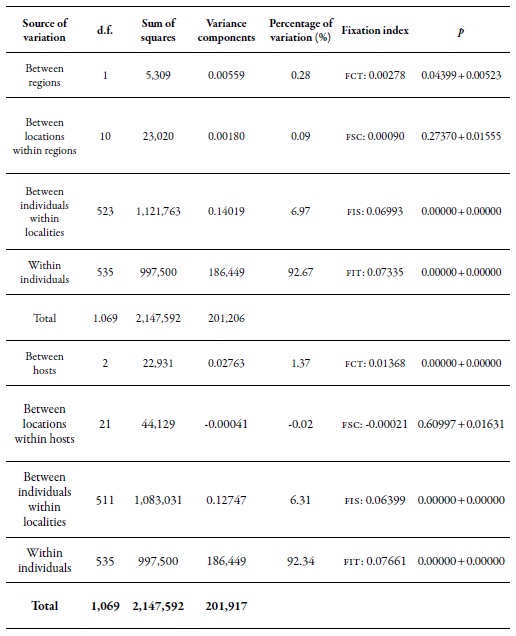
Source: Elaborated by the authors
Table 7. Genetic differentiation among pairs (FST) between samples of M. persicae by region and host1 Peach (D), sweet pepper (P), weeds (M), O'Higgins Region (VI), and Maule Region (VII). The FST values are shown under the diagonal. The probability values based on 999 permutations are shown above the diagonal.
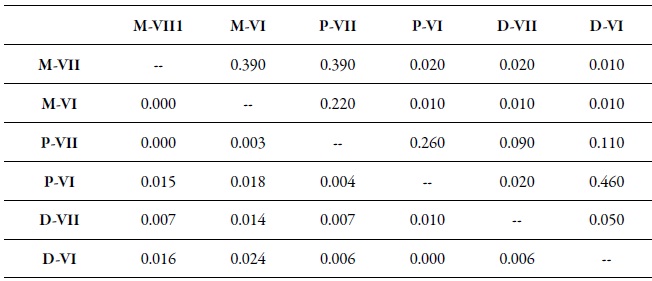
Source: Elaborated by the authors
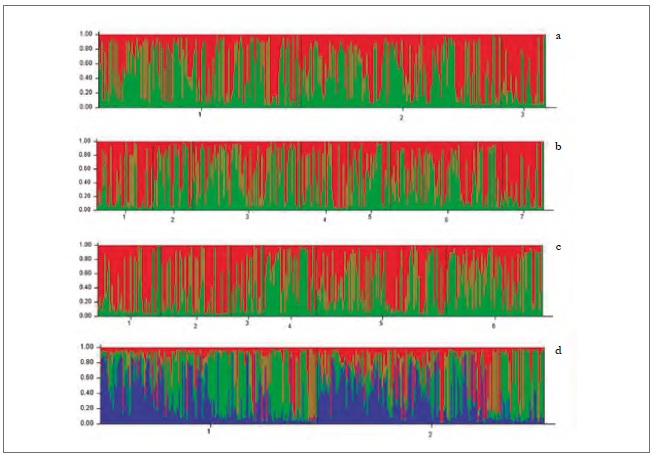
Source: Elaborated by the authors
Figure 2. Genetic groups of M. persicae in central Chile detected using Structure, according to the variables sampling moments (a) host/sampling moments (b), host/region (c), and region (d).
It was not possible to associate all the genetic groups identified for the analyzes performed to any of the evaluated variables and their possible combinations (sampling moments, host/sampling moments, and host/region). This could be due to two facts. First, to the fact that M. persicae is the aphid species with the highest number of reported secondary hosts, which plays an essential role as a source of migration towards the crops, thus, allowing the insect to move from one host to another in search of food and better conditions (Margaritopoulos, Tsourapas, Tzortzi, Kanavaki, & Tsitsipis, 2005).
Second, the existence of sexual reproduction in aphid populations in Chile contributes to high gene flow among populations of M. persicae, both in secondary hosts and in regions throughout sampling moments (Fenton, Margaritopoulos et al., 2010). It seems that there may be more genetic groups in populations with low gene flow and less in populations with high gene flow (Guillemaud et al., 2003; Vialatte et al., 2005; Vorbuger, 2006). This is because the gene flow occurs when there is sexual reproduction, generation a progeny with genetic differentiation; on the contrary, the progeny resulting from asexual reproduction is of low flow, generating identical clones with the same genetic information. Therefore, we could infer that these groups of populations found do not have MLGs with different biological properties and do not provide any apparent biological significance.
Table 8. MLG proportion and its resistance profile in the three mechanisms evaluated in the regions of O'Higgins (VI) and Maule (VII) during spring (I), summer-autumn (II), and winter (III) of the agricultural season 2010-2011
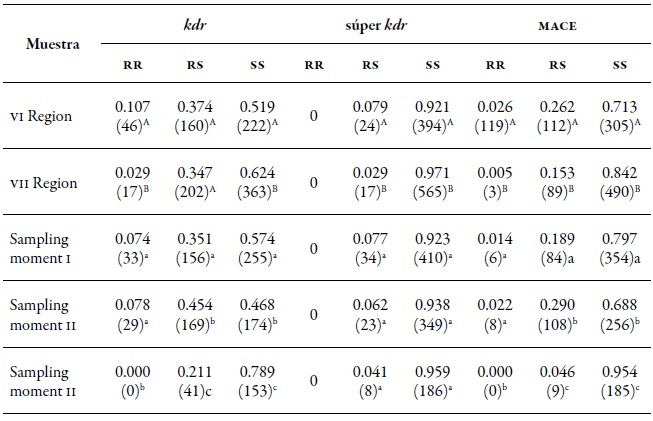
Note:In parentheses the number of MLGs. Different capital letters indicate significant differences according to the χ2 test in Marascuilo’s multiple comparisons between regions. Different lowercase letters indicate significant differences according to the χ2 test in Marascuilo’s multiple comparisons between sampling moments.
Source: Elaborated by the authors Resistance to insecticides
Resistance to insecticides
In the two regions assessed, the three resistance mechanisms evaluated (kdr, super kdr, and mace ) were found. In the O'Higgins region, the three mechanisms had frequencies slightly higher than those of the Maule region (table 8), with kdr standing out with 10.9 % of homozygotes (rr) and 29.4 % of heterozygotes (rs). The super kdr mutation only appeared in rs form in 6 % of the total MLGs identified and, in the case of mace, it was 2.2 % of rr and 18.5 % of rs (figure 3A). At the spatial level, MLGs in the region of O'Higgins tend to show a higher frequency of genotypes with heterozygous resistance in the three mechanisms evaluated, and homozygous resistance in kdr and mace compared to the Maule region (figure 3A, table 8). Meanwhile, in spring (I) and summerautumn (II), the frequencies of the MLGs and the different resistance mechanisms were similar in homozygous form (rr) and being statistically different to the winter frequencies (III).
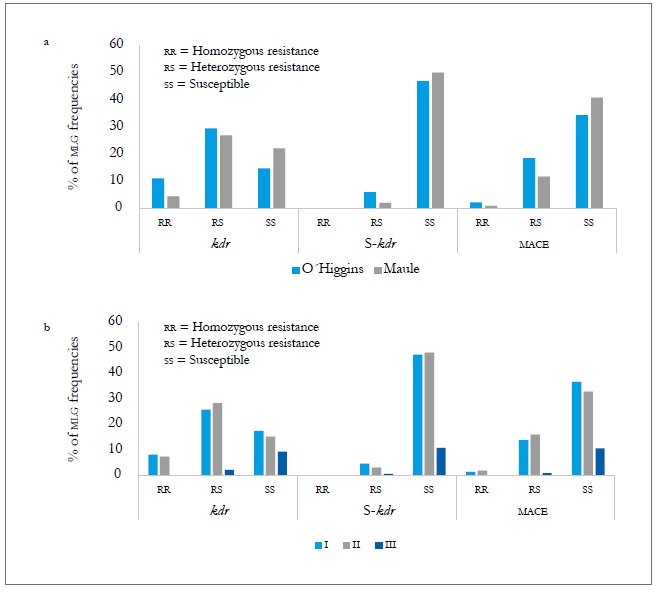
Source: Elaborated by the authors
Figure 3. MLG frequencies with the three resistance mechanisms evaluated according to the region (a) and sampling moments (b) during the 2010-2011 agricultural season. I = Spring-summer, II = Summer-Autumn, and III = Winter.
In the case of the heterozygous form (rs), the frequencies at the three sampling moments showed significant differences in the three mechanisms evaluated (figure 3b and table 8). At the host level, the populations collected on the crops (peach and sweet pepper) were those that showed the highest frequency of genotypes with a mechanism of resistance, either heterozygous or homozygous, both temporally and spatially. Of these two hosts, the peach tree is the one that tends to show genotypes more frequently with a resistance mechanism, but without being significant (figure 4a and b). The presence of the three resistance mechanisms evaluated, either homozygous or heterozygous in several of the MLGs, could be due to insecticide applications in the aerial part of the plants, to which the plantations are subject for the control of agricultural pests. Hence, in the peach orchards, there is higher selection pressure due to the number of applications of insecticides (between nine and 12) for the control of M. persicae and other pests, in comparison to the ones applied in sweet pepper cropping that oscillates between four and six.
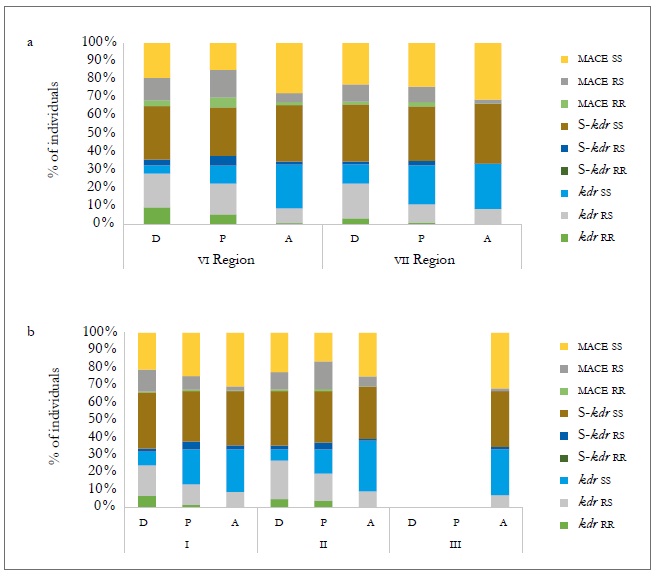
Source: Elaborated by the authors
Figure 4. Percentage composition of individuals in different resistance mechanisms between regions (a) and sampling moment (b) for different hosts: peach (D), sweet pepper (P), and weeds (A) in the 2010-2011 agricultural season.
The groups of insecticides mostly used in these fields and related to these resistance mechanisms are pyrethroids, related to kdr and super kdr (Eleftherianos, Foster, Williamson & Denholm, 2008, Martinez-Torres, Foster, Field, Devonshire & Williamson, 1999), as well as carbamates, related to mace (Moores, Devine & Devonshire, 1994, Nabeshima, Kozaki, Tomita, & Kono, 2003). However, applications of neonicotinoid are also carried out; according to Bass et al. (2011), resistance to this type of insecticide is associated with a mutation in the beta subunit of the nicotinic acetylcholine receptor. Although this resistance mechanism was not evaluated in this study, it is important to consider it in future research.
The three mechanisms studied have been previously identified in populations of M. persicae in Chile (Castañeda et al., 2011) and in other countries such as Greece (Margaritopoulos, Skouras et al., 2007), United States (Srigiriraju, Semtner, Anderson & Bloomquist, 2010), France (Fontaine et al., 2011), and New Zealand (van Toor, Malloch, Anderson, Daoson, & Fenton, 2008). The minimal presence of MLGs with the super kdr resistance mechanism might be because this mechanism only manifests itself in some genotypes that exhibit the kdr type resistance mechanism (Anstead et al., 2008), which was low in this study. The higher presence of RS genotypes, mainly in mace and kdr, may be due to the influence of the holocylic form of reproduction of the green aphid of peach, in which sexual reproduction decreases the proportion of resistant homozygotes and heterozygotes (Fenton, Kasprowicz et al. al., 2010).
The decrease of genotypes with the presence of some resistance mechanism at the end of the agricultural season may be related to the costs of the resistance. As indicated by Fenton, Margaritopoulos et al. (2010), genotypes of M. persicae with the presence of some resistance mechanisms lower their reproductive potential. In addition, there is evidence that several insect species with resistance to insecticides reduce their survival and reproductive ability, as well as being more vulnerable to natural enemies (Abbas, Khan & Shad 2015; Castellanos et al., 2019; Guedes, Smagghe, Stark, & Desneux 2016; van Toor et al., 2013). According to the χ2 test, the frequency of the three resistance mechanisms was dependent on the study region, being more associated with the O'Higgins region for kdr (unique copies χ2=15.9 and df =2, p<0.001, and multiple copies χ2 = 29.4 and df=2, p < 0.001), super kdr (unique copies χ2 = 7.5 and df=1, p < 0.01, and multiple copies χ2 = 12.9 and df = 1, p < 0.001), and mace (unique copies χ2 = 9.9 and df = 2, p < 0.01, and multiple copies χ2 = 27.4 and df = 2, p < 0.001). Regarding the sampling moment, super kdr was not dependent on the sampling moment in the analysis with unique copies (χ2 = 1.7 and df = 2, p = 0.4221), but it was on the sampling moment with multiple copies, being associated mainly with summer-autumn (II) (χ2 = 13.5 and df = 2, p < 0.005), just as in the other two mechanisms, kdr (unique copies, χ2 = 40.8 and df = 4, p < 0.001, and multiple copies, χ2 = 55.9 and df = 4. p < 0.001) and mace (unique copies χ2 = 13.2 and df = 4, p < 0.05, multiple copies χ2 = 56.1 and df = 4, p < 0.001).
A set of 13 MLGs was considered important due to the combination of resistance mechanisms that they showed (table 9). Among these, G119 was highlighted, since it was found in the two regions, in the three sampling moments and on secondary hosts (sweet pepper and B. rapa); moreover, it was also found in a heterozygous form (rs) for the three resistance mechanisms evaluated. This same genotype was highlighted among the 10 most common MLGs (table 4). Another genotype that is important to highlight for its resistance level is G276, which was homozygous resistant (rr) in kdr and mace, although it was collected only on sweet pepper in spring. The genotypes G26, G65, G128, and G353, were rr, rs, and rs in kdr, super kdr and mace, respectively (table 9).
These results indicate the importance of implementing management strategies in Chile that avoid the increase in the frequency of individuals with some type of resistance mechanism, highlighting, among others, the rotation of insecticides with different modes of action.
Table 9. Detail of the genotypes with the highest presence of resistance mechanisms found in this study in the regions of O'Higgins (VI) and Maule (VII)1 Hosts: Peach (D), Sweet pepper (P), and weeds (M). 2 Sampling season: spring (I), summer-autumn (II), and winter (III).
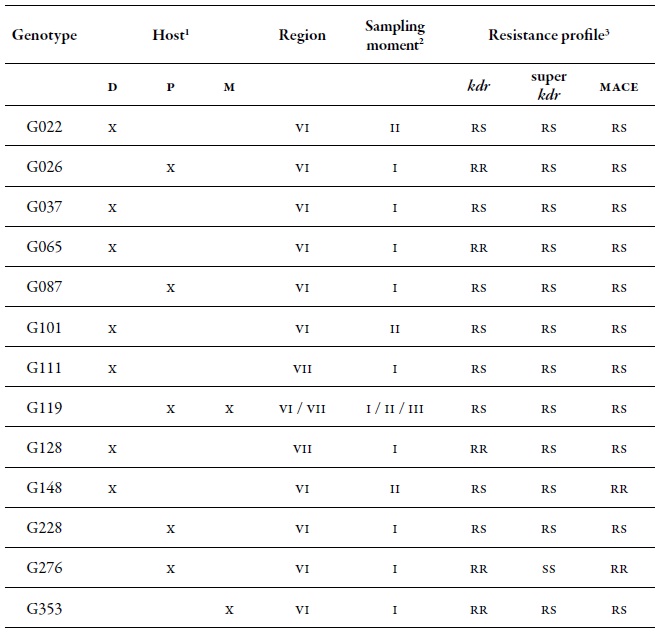
Source: Elaborated by the authors
Likewise, in the implementation of rational integrated pest management (ipm), insecticide applications should be regulated according to the monitoring, thus, decreasing the number of applications. The use of green insecticides and the maintenance of host plants that harbor susceptible individuals free from the action of insecticides can reduce the level of resistance when they migrate to the primary host and participate in sexual reproduction. Last, but not least, it is convenient to increase the beneficial fauna, by conserving natural enemies in biological corridors, since aphids with mutations that confer resistance become easy prey for their natural enemies.
Conclusions
The neutral genetic diversity of Myzus persicae, as recorded in the literature, is still higher in populations collected on its primary host, peach (P. persica), compared to those collected on other secondary hosts (sweet pepper and weeds). This result is closely linked to the occurrence of the sexual reproduction of the insect on the primary host. Due to the high availability of its primary host in the area planted in the O'Higgins region, it was in this region where the highest genetic diversity was found. This allows inferring that several of the genotypes found are closely related to their primary host. In central Chile, there are genotypes with the three resistance mechanisms evaluated, but in a predominantly heterozygous manner. The main host of M. persicae, the peach tree, hosts the most significant number of genotypes with at least one resistance mechanism. This is mainly due to the widespread use of chemical insecticides for the control of the green aphid of peach, which imposes a selection pressure higher than the natural one; hence, they are highly associated with the resistance mechanisms evaluated.
Acknowledgment
The author wishes to thank Christian Figueroa, leader of the laboratory of Instituto de Ecología y Evolución of Universidad Austral de Chile. In particular, to the Organization of American States (oas) and Comisión Nacional de Ciencia y Tecnología (Conicyt) of Chile, for financing their doctorate studies.
REFERENCES
Abbas, N., Khan, H., & Shad, S. A. (2015). Cross-resistance, stability, and fitness cost of resistance to imidacloprid in Musca domestica L., (Diptera: Muscidae). Parasitology Research, 114(1), 247-255. doi:10.1007/s0043 6-014-4186-0. [ Links ]
Anstead, J. A., Williamson, M. S., & Denholm, I. (2008). New methods for the detection of insecticide resistant Myzus persicae in the UK suction trap network. Agricultural and Forest Entomology, 10(3), 291-295. doi:10.1111/ j.1461-9563.2008.00388.x. [ Links ]
Anstead, J. A., Williamson, M. S., Eleftherianos, L., & Denholm, I. (2004). High-throughput detection of knock-down resistance in Myzus persicae using allelic discriminating quantitative PCR. Insect Biochemistry and Molecular Biology, 34(8), 871-877. doi:10.1016/j.ibmb.2004.06.002. [ Links ]
Bass, C., Puinean, A., Andrews, M., Cutler, P., Daniels, M., Elias, J., ... Slater, R. (2011). Mutation of a nicotinic acetylcholine receptor beta subunit is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. BMC Neuroscience, 12(51), 11. doi:10.1186/14712202-12-51. [ Links ]
Blackman, R. L., Malarky, G., Margaritopoulos, J. T., & Tsitsipis, J. A. (2007). Distribution of common genotypes of Myzus persicae (Hemiptera: Aphididae) in Greece, in relation to life cycle and host plant. Bulletin of Entomological Research, 97(3), 253-263. doi:10.1017/ S0007485307004907. [ Links ]
Blackman, R. L., & Eastop, V. F. (2007). Taxonomic Issues. En H. F. Van Emden, & R. Harrington (Eds.), Aphids as Crop Pests (pp. 1-29). Chichester, Inglaterra: CAB International. doi:10.1079/9780851998190.0001. [ Links ]
Blackman, R. L., & Eastop, V. F. (2000). Aphids on the world’s crops. An identification guide, 2.ª ed. Chichester, Inglaterra: John Wiley. [ Links ]
Borodovsky, M., & McIninch, J. (1993). GeneMark: parallel gene recognition for both DNA strands. Computers and Chemistry, 17(2) 123-133. doi:10.1016/00978485(93)85004-V. [ Links ]
Castañeda, L. E., Barrientos, K., Cortes, P. A., Figueroa, C. C., Fuentes-Contreras, E., ... Bacigalupe, L. D. (2011). Evaluating reproductive fitness and metabolic costs for insecticide resistance in Myzus persicae from Chile. Physiological Entomology, 36(3), 253-260. doi:10.1111/ j.1365-3032.2011.00793.x. [ Links ]
Castellanos, N. L., Haddi, K., Gislaine, A., Carvalho. G. A., De Paulo, P. D., ... Oliveira1, E. E. (2019). Imidacloprid resistance in the Neotropical brown stink bug Euschistus heros: selection and fitness costs. Journal of Pest Science, 92(2), 847-860. doi:10.1007/s10340-018-1048-z. [ Links ]
Charaabi, K., Carletto, J., Chavigny, P., Marrakchi, M., Makni, M., & Vanlerberghe-Masutti, F. (2008). Genotypic diversity of the cotton-melon aphid Aphis gossypii (Glover) in Tunisia is structured by host plants. Bulletin of Entomological Research, 98(4), 333-341. doi:10.1017/ S0007485307005585. [ Links ]
Dewar, A. M. (2007). Chemical control. Aphids as crop pests. En H. F. Van Emden, & R. Harrington (Eds.), Aphids as Crop Pests (pp. 391-411). Chichester, Inglaterra: CAB International. [ Links ]
Devonshire, A. L., Field, L. M., Foster, S. P., Moores, G. D., Williamson, M. S., & Blackman, R. L. (1998). The evolution of insecticide resistance in the peach-potato aphid, Myzus persicae. Philosophical Transactions of the Royal Society B-Biological Sciences, 353(1376), 1677-1684. doi:10.1016/j.ibmb.2014.05.003. [ Links ]
Eleftherianos, I., Foster, S. P., Williamson, M. S., & Denholm, I. (2008). Characterization of the M918T sodium channel gene mutation associated with strong resistance to pyrethroid insecticides in the peach-potato aphid, Myzus persicae (Sulzer). Bulletin of Entomological Research, 98(2), 183-191. doi:10.1017/S0007485307005524. [ Links ]
Evanno, G., Regnaut, S., & Goudet, J. (2005). Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology, 14(8), 2611-2620. doi:10.1111/j.1365-294X.2005. 02553.x. [ Links ]
Fenton, B., Kasprowicz, L., Malloch, G., & Pickup, J. (2010). Reproductive performance of asexual clones of the peachpotato aphid, (Myzus persicae, Homoptera: Aphididae), colonising Scotland in relation to host plant and field ecology. Bulletin of Entomological Research, 100(4), 451-460. doi:10.1017/S000748530999044. [ Links ]
Fenton, B., Margaritopoulos, J. T., Malloch, G. L., & Foster, S. P. (2010). Micro-evolutionary change in relation to insecticide resistance in the potato aphid, Myzus persicae. Ecological Entomology, 35(1), 131-146. doi:10.1111/ j.1365-2311.2009.01150.x. [ Links ]
Fenton, B., Malloch, G., Navajas, M., Hillier, J., & Birch, A.N. (2003). Clonal composition of the peach-potato aphid Myzus persicae (Homoptera: Aphididae) in France y Scotland: Comparative analysis with IGS fingerprinting y microsatellite markers. Annals of Applied Biology, 142(3), 255-267. doi:10.1111/j.1744-7348.2003.tb00249.x. [ Links ]
Fontaine, S., Caddoux, L., Brazier, C., Bertho, C., Bertolla, P., ... & Roy, L. (2011). Uncommon associations in target resistance among French populations of Myzus persicae from oilseed rape crops. Pest Management Science, 67(8), 881-885. doi:10.1002/ps.2224. [ Links ]
Fuentes-Contreras, E., Figueroa, C. C., Silva, A. X., Bacigalupe, L. D., Briones, L. M., Foster, S. P., & Unruh, T. R. (2013). Survey of resistance to four insecticides and their associated mechanisms in different genotypes of the green peach aphid (Hemiptera: Aphididae) from Chile. Journal of Economic Entomology, 106(1), 400-407. doi:10.1603/ ec12176. [ Links ]
Fuentes-Contreras, E., Figueroa, C. C., Reyes, M., Briones, L. M., & Niemeyer, H. M. (2004). Genetic diversity and insecticide resistance of Myzus persicae (Hemiptera: Aphididae) populations from tobacco in Chile: evidence for the existence of a single predominant clone. Bulletin of Entomological Research, 94(1), 11-18. doi:10.1079/ BER2003275. [ Links ]
Guedes, R. N. C., Smagghe, G., Stark, J. D., & Desneux, N. (2016). Pesticide induced stress in arthropod pests for optimized integrated pest management programs. Annual Review of Entomology, 61(1), 43-62. doi:10.1146/annurevento-01071 5-023646. [ Links ]
Goudet, J. (1995). FStat (version.2.9.3.2): a computer program to calculate F-statistics. Journal of Heredity, 86(6), 485-486. doi:10.1093/oxfordjournals.jhered.a111627. [ Links ]
Guillemaud, T., Mieuzet, L., & Simon, J. C. (2003). Spatial and temporal genetic variability in French populations of the peach-potato aphid, Myzus persicae. Heredity, 91(2), 143-152. doi:10.1038/sj.hdy.6800292. [ Links ]
Kasprowicz, L., Malloch, G., Pickup, J., & Fenton. B. (2008). Spatial and temporal dynamics of Myzus persicae clones in fields and suction traps. Agricultural and Forest Entomology, 10(2), 91-100. doi:10.1111/j.1461-9563.2008.00365.x. [ Links ]
Malloch, G., Highet, F., Kasprooicz, L., Pickup, J., Neilson, R., & Fenton, B. (2006). Microsatellite marker analysis of peach-potato aphids (Myzus persicae, Homoptera: Aphididae) from Scottish suction traps. Bulletin of Entomological Research, 96(6), 573-582. doi:10.1079/BER2006459. [ Links ]
Margaritopoulos, J. T., Malarky, G., Tsitsipis, J. A., & Blackman, R. L. (2007). Microsatellite DNA and behavioural studies provide evidence of host-mediated speciation in Myzus persicae (Hemiptera: Aphididae). Biological Journal of the Linnean Society, 91(4), 687-702. doi:10.1111/j.10958312.2007.00828.x. [ Links ]
Margaritopoulos, J. T., Skouras, P. J., Nikolaidou, P., Manolikaki, J., Maritsa, K., ... Tsitsipis, J. A. (2007). Insecticide resistance status of Myzus persicae (Hemiptera: Aphididae) populations from peach and tobacco in mainland Greece. Pest Management Science, 63(8), 821-829. doi:10.1002/ps.1409. [ Links ]
Margaritopoulos, J. T., Tsourapas, C., Tzortzi, M., Kanavaki, O. M., & Tsitsipis, J. A. (2005). Host selection by winged colonisers within the Myzus persicae group: a contribution towards understanding host specialisation. Ecological Entomology, 30(4), 406-418. doi:10.1111/j.0307-6946. 2005.00700.x. [ Links ]
Martínez-Torres, D., Foster, S. P., Field, L. M., Devonshire, A. L., & Williamson, M. S. (1999). A sodium channel point mutation is associated with resistance to DDT and pyrethroid insecticides in the peach-potato aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae). Insect Molecular Biology, 8(3), 339-346. doi:10.1046/j.13652583.1999.83121.x. [ Links ]
Moores, G. D., Devine, G. J., & Devonshire, A. L. (1994). Insecticide-insensitive acetylcholinesterase can enhance esterase-based resistance in Myzus persicae and Myzus nicotianae. Pesticide Biochemistry and Physiology, 49(2), 114-120. doi:10.1006/pest.1994.1038. [ Links ]
Nabeshima, T., Kozaki, T., Tomita, T., & Kono, Y. (2003). An amino acid substitution on the second acetylcholinesterase in the pirimicarb-resistant strains of the peach potato aphid, Myzus persicae. Biochemical and Biophysical Research Communications, 307(1), 15-22. doi:10.1016/s0006-291x (03)01101-x. [ Links ]
Pritchard, J. K., Stephens, M., & Donnelly, P. (2000). Inference of population structure using multilocus genotype data. Genetics, 155(2), 945-959. [ Links ]
Raymond, M., & Rousset, F. (1995). Population genetics software for exact tests and ecumenicism. Journal of Heredity, 86(3), 248-249. doi:10.1093/oxfordjournals.jhered.a111573. [ Links ]
Rouger, R., Reichel, K., Malrieu. F., Masson, J. P., & Stoeckel, S. (2016). Effects of complex life cycles on genetic diversity: cyclical parthenogenesis. Heredity, 117(5), 336-347. doi: 10.1038/hdy.2016.52. [ Links ]
Rubiano-Rodríguez, J. A., Fuentes-Contreras, E., Figueroa, C. C., Margaritopoulos, J. T., Briones, L. M., & Ramírez, C. C. (2014). Genetic diversity and insecticide resistance during the growing season in the green peach aphid (Hemiptera: Aphididae) on primary and secondary hosts: a farm-scale study in central. Bulletin of Entomological Research,104(2), 182-194. doi:10.1017/S00074853 1300062X. [ Links ]
Sánchez, J. A., Spina M. L., Guirao, P., & Cánovas, F. (2013). Inferring the population structure of Myzus persicae in diverse agroecosystems using microsatellite markers. Bulletin of Entomological Research, 103(4), 473-484. doi:10.1017/S0007485313000059. [ Links ]
Schneider, S., Roessli, D., & Excoffier, L. (2000). Arlequin. A software for population genetic data analysis, Version 2.0. Geneva, Switzerland: Genetics and Biometry Laboratory, Universidad de Ginebra. [ Links ]
Schuelke, M. (2000). An economic method for the fluorescent labeling of PCR fragments. Nature Biotechnology, 18(2), 233-234. doi:10.1038/72708. [ Links ]
Servicio Agrícola y Ganadero. (2012). Lista de plaguicidas autorizados. Recuperado de https://www.sag.gob.cl/OpenDocs/asp/pagDefault.asp?boton=Doc51&argInstanciaId=51&argCarpetaId=327&argTreeNodosAbiertos=(327)(-51)&argTreeNodoActual=327&argTreeNodoSel=7 [ Links ]
Srigiriraju, L., Semtner, P. J., Anderson, T. D., & Bloomquist, J. R. (2010). Monitoring for MACE resistance in the tobacco-adapted form of the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae) in the eastern United States. Crop Protection, 29(2), 197-202. doi:10. 1016/j.cropro.2009.11.006. [ Links ]
Sunnucks, P., De Barro, P. J., Lushai, G., Maclean, N. & Hales, D. F. (1997). Genetic structure of an aphid studied using microsatellite: cyclic parthenogenesis, differentiated lineages, and host specialization. Molecular Ecology, 6(11), 1059-1073. doi:10.1046/j.1365-294X.1997.00280.x. [ Links ]
Sunnucks, P., & Hales, D. F. (1996). Numerous transposed sequences of mitochondrial cytochrome oxidase I-II in aphids of the genus Sitobion (Hemiptera: Aphididae). Molecular Biology and Evolution, 13(3), 510-524. doi:10.1093/oxfordjournals.molbev.a025612. [ Links ]
Van Toor, R. F., Foster, S. P., Anstead, J. A., Mitchinson, S., Fenton, B., & Kasprowicz, L. (2008). Insecticide resistance and genetic composition of Myzus persicae (Hemiptera: Aphididae) on field potatoes in New Zealand. Crop Protection, 27(2), 236-247. doi:10.1016/j.cropro.2007.05.015. [ Links ]
Van Toor, R. F., Malloch, G.L., Anderson, E. A., Daoson, G., & Fenton, B. (2013). Insecticide resistance profiles can be misleading in predicting the survival of Myzus persicae genotypes on potato crops following the application of different insecticide classes. Pest Management Science, 69(1), 93-103. doi:10.1002/ps.3370. [ Links ]
Van Oosterhout, C., Hutchinson, O. F., Oills, D. P. M., & Shipley, P. (2004). Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Molecular Ecology, 4(3), 535-538. doi:10.1111/j.14718286.2004.00684.x. [ Links ]
Vialatte, A., Dedryver, C. A., Simon, J. C., Galman, M., & Plantegenest, M. (2005). Limited genetic exchanges between populations of an insect pest living on uncultivated and related cultivated host plants. Proceedings of the Royal Society B-Biological Sciences, 272(1567), 1075-1082. doi:10.1098/rspb.2004.3033. [ Links ]
Vorburger, C. (2006). Temporal dynamics of genotypic diversity reveal strong clonal selection in the aphid Myzus persicae. Journal of Evolutionary Biology, 19(1), 97-107. doi:10.1111/j.1420-9101.2005.00985.x. [ Links ]
Vorburger, C., Lancaster. M., & Sunnucks, P. (2003). Environmentally related patterns of reproductive modes in the aphid Myzus persicae and the predominance of two ‘superclones’ in Victoria, Australia. Molecular Ecology, 12(12), 3493-3504. doi:10.1046/j.1365-294X.2003.01998.x. [ Links ]
Voudouris, C. C., Kati, A. N., Sadikoglou, E., Williamson, M., Skouras, P. J., ... Margaritopoulos, J. T. (2016). Insecticide resistance status of Myzus persicae in Greece: long-term surveys and new diagnostics for resistance mechanisms. Pest Management Science, 72(4), 671-683. doi:10.1002/ ps.4036. [ Links ]
Wilson, A. C. Sunnucks, C., P., Blackman, R. L., & Hales, D. F. (2002). Microsatellite variation in cyclically parthenogenetic populations of Myzus persicae in southeastern Australia. Heredity, 88(4), 258-266. doi:10.1038/sj.hdy. 6800037. [ Links ]
Zamoum, T., Simon, J. C., Crochard, D., Ballanger, Y., Lapchin, L., ... Guillemaud, T. (2005). Does insecticide resistance alone account for the low genetic variability of asexually reproducing populations of the peach-potato aphid Myzus persicae. Heredity, 94(6), 630-639. doi:10.1038/ sj.hdy.6800673. [ Links ]
Received: July 24, 2018; Accepted: May 14, 2019











 text in
text in