Introduction
To improve crop profitability, agricultural workers frequently use pesticides to combat pests that threaten crop loss (Cotton et al., 2018; Lutovac et al., 2017). Among the pesticides, there is a group of organophosphate compounds whose use can cause poisoning with adverse effects on the health of people who are occupationally exposed to these substances (Díaz et al., 2017). Organophosphate pesticide (OP) poisoning manifests with inhibition of the cholinesterase enzyme activity (Carmona, 2007) and its degree of severity is associated with the biological condition of the exposed individual, the toxicity of the manipulated pesticide and the characteristics of the exposure, in terms of duration and frequency (Butinof et al., 2017).
In the context of occupational health, to reduce the health risks of individuals occupationally exposed to pesticides, one of the measures proposed by the Food and Agriculture Organization of the United Nations (FAO) is the implementation of a surveillance program. In countries such as the United States and Colombia, this control is carried out using the activity of cholinesterase enzymes as biological indicators by which exposures that represent a danger to these workers are monitored and detected. The parameters of how and when to measure cholinesterase activity are stated in guidelines, such as the one developed for physicians who supervise workers exposed to cholinesterase inhibitor pesticides (Office of Environmental Health Hazard Assessment [OEHHA], 2015) published by the Office of Environmental and Health Risk Assessment of the Environmental Protection Agency of California (United States). In this guide, the measurement of the activity of erythrocyte cholinesterase (EC) and plasma cholinesterase (PC) is recommended in persons who use OP for more than six days in a month, and whose label contains the words “danger” or “warning.” The establishment of cholinesterase enzymes should be done 30 days before the exposure of the worker and once exposed, periodically using Ellman’s technique (OEHHA, 2015).
Another guide is the one published by the Colombian Ministry of Social Protection (MPS), which describes the mechanism of comprehensive occupational health care for workers exposed to cholinesterase inhibitor pesticides (MPS, 2008a). The guide indicates that the surveillance of workers should be done by establishing the EC levels before their exposure, periodically and after their retirement, using Michel’s method. The pre-exposure sample must be taken when the worker has not been exposed in a previous period of 30 days. In addition, periodic checks should be carried out every three months in case a worker has permanent exposure, or EC levels should be determined before and immediately after exposure, in case of occasional contact. A worker is considered intoxicated with OP when comparing the EC activity values before exposure with those obtained in the periodic controls, the latter showing a decrease of over 25%, and must be temporarily withdrawn from exposure if the enzyme activity drops more than 30% (MPS, 2008a).
In Colombia, the cases of individuals who show EC activity values equal to or less than 50% and who show signs and symptoms are registered and notified to Sistema Nacional de Vigilancia en Salud Pública [National Public Health Surveillance System] (Sivigila) (Instituto Nacional de Salud [INS], 2018). Considering the events reported by Sivigila for poisonings with chemical substances, for every 100,000 inhabitants in 2016, 1,764 cases (25.2%) of pesticide poisonings were registered, of which 294 (16.7%) occurred via respiratory exposure largely associated with occupational poisonings, and 308 (17.5%) showed OP poisonings (INS, 2016b).
The aim of this review is to describe the use of the activity of EC and PC enzymes as biomarkers to monitor the health status of workers exposed to OP, to disclose how their effectiveness has been in practice in terms of prevention, protection, and reduction of the risk of affecting the health.
Materials and methods
A systematic literature search was conducted in seven online databases: BioMed, DialNet, DOAJ, Medline, PubMed, Redalyc, and SciELO, following the items proposed by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), which include the identification, selection, and inclusion of the reviewed literature. The publication years included from the year 2003 to the year 2019. The descriptors used were Organophosphate poisoning, cholinesterase activity as a biomarker, and cholinesterase determination in farmers. The articles were selected considering the following inclusion criteria: studies with population occupationally exposed to pesticides that used cholinesterase as a biomarker to assess exposure, and articles in Spanish and English. Studies of poisonings due to suicidal purposes mostly reported in the clinical records of health centers, hospitals, or toxicology laboratories were excluded.
For this review, a total of 200 articles were identified, which were evaluated by reviewing their titles and abstracts; after the removal of duplicate articles and review of studies, 100 articles underwent full-text review. According to the inclusion and exclusion criteria, data from a total of 49 scientific articles were entered into this synthesis. The results are exposed in different sections that include cholinesterases as exposure and effect biomarkers to organophosphate pesticides; the biological role of cholinesterase enzymes; organophosphate pesticides (OP) as anticholinesterase substances; laboratory methods to determine the activity of cholinesterase enzymes; the influence of biological, chemical and environmental factors on cholinesterase values; cholinesterase evaluations in populations exposed to OP; evaluations of pre- and post-exposure cholinesterases to OP, and measurement of cholinesterase levels between individuals exposed and not exposed to OP.
Development of the topic
Cholinesterases as exposure and effect biomarkers to organophosphate pesticides
A biomarker is an objectively measurable and evaluable characteristic, such as an indicator of normal biological and pathogenic processes, or responses to therapeutic pharmacological interventions (Ptolemy & Rifai, 2010). A biomarker can represent a variety of agents that serve as disease prognosis and diagnosis, or as a specific sensitive tool for risk assessment. The markers can be biological, physical, or molecular in nature. A biomarker must have high accuracy, precision, sensitivity, and specificity; furthermore, it must contemplate the analytical variables and their effects on the results such as sampling, handling, storage, processing, and levels of concentration of the biomarker (Ptolemy & Rifai, 2010).
On one side, the biomarker is considered to be of exposure when it accounts for the presence of the chemical in the body, while, on the other side, a biomarker is of effect, when it reports on the physiological changes produced by the substance in the body (Ríos & Solari, 2010). In this sense, to assess the exposure of workers who occupationally manipulate OP, the decrease in the activity of cholinesterase enzymes is used as a biomarker.
There are two types of cholinesterases: plasma and erythrocyte. The terms nonspecific cholinesterase, serum or s-type cholinesterase, pseudocholinesterase, butyrylcholinesterase, BChE, or EC 3.1.1.8 are also used to mention the PC; the latter term comes from the nomenclature of the International Union of Biochemistry and Molecular Biology (UIB). Likewise, the EC is also called E-type orspecific cholinesterase, true cholinesterase, acetylcholinesterase, AChE, or EC 3.1.1.7 (Carmona, 2006, 2007; Férnandez et al., 2011; Jaga & Dharmani, 2007; Medina et al., 2015; Restrepo et al., 2017).
Specifically, PC measured in serum or blood plasma is used as an exposure biomarker for acute poisoning, while EC measured in red blood cells is used as a biomarker for chronic exposure effect and biomarker (Lu, 2007; Jaga & Dharmani, 2007; Jors et al., 2006; Restrepo et al., 2017; Sapbamrer & Nata, 2014).
The biological role of cholinesterase enzymes
PC is formed in the liver and is found in plasma. Its function is not clearly known, but it is presumed to be involved in lipid metabolism; besides, it controls the concentration of choline in the plasma and prevents the accumulation of butyrylcholine. EC is found in erythrocytes and cholinergic synapses located at neuromuscular junctions and in central nervous system (CNS) connections, interneuronal connections of the peripheral nervous system, and neuroglandular and neuromuscular junctions of the parasympathetic nervous system. Its function is to inactivate the neurotransmitter acetylcholine through its biotransformation into choline and acetic acid, which regulates the transmission of the nerve impulse (Bohórquez et al., 2012; Carmona, 2006; Férnandez et al., 2011; Medina et al., 2015).
Figure 1a illustrates the activity of acetylcholinesterase (AChE) when hydrolyzing the neurotransmitter acetylcholine (ACh) in the synaptic space. This reaction allows the choline to be reabsorbed by the presynaptic cholinergic neuron to produce the neurotransmitter acetylcholine again, by choline acetyltransferase (ChAT), which is responsible for binding the choline with the acetate obtained from acetyl coenzyme A (AcCoA). Once the neurotransmitter acetylcholine is formed, it is deposited in the synaptic vesicles from where it is expelled by exocytosis to bind to postsynaptic muscarinic or nicotinic type receptors, allowing the propagation of the nerve impulse (Ferrer, 2003; Hurtado & Gutiérrez, 2005).
The activity of the enzyme acetylcholinesterase in the body is crucial because it breaks down the neurotransmitter acetylcholine; this reaction makes neurotransmission momentary, as neurotransmitter-receptor interaction is disrupted. However, the functioning of acetylcholinesterase can be altered by the presence of anticholinesterase substances that block the enzyme and prevent hydrolysis of the neurotransmitter, which, in turn, causes an accumulation of acetylcholine in the synaptic space and increases the duration of the nerve impulse. Subsequently, overstimulation of postsynaptic neurons is observed because they cannot return to their resting state (Fernández et al., 2010; Ferrer, 2003; Restrepo et al., 2017) (figure 1b).
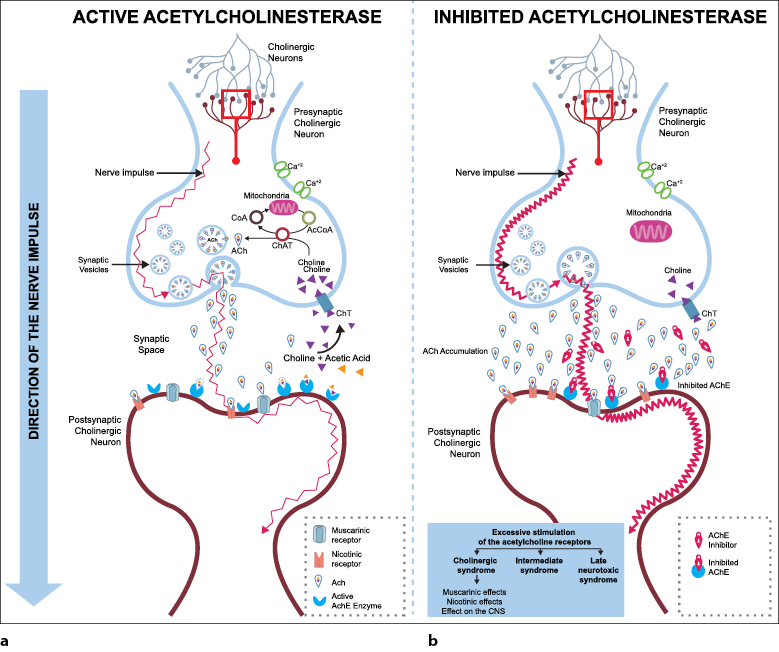
Source: elaborated by the authors
Figure 1 Acetylcholinesterase activityPart a) the biological action mechanism of the acetylcholinesterase enzyme in the active state, and part b), the effect of this mechanism when the enzyme is inhibited. Abbreviations: AChE: acetylcholinesterase, ACh: acetylcholine neurotransmitter, Ca+2: calcium ion, CoA: acetyltransferase enzyme, AcCoA: acetyl coenzyme A, ChAT: choline acetyltransferase enzyme, ChT: choline transporter
Organophosphate pesticides (OP) as anticholinesterase substances
Organophosphate pesticides are toxic substances that are chemically classified as phosphoric acid esters (Lutovac et al., 2017); moreover, they are characterized by being volatile and fat-soluble compounds, qualities that allow them to overcome the biological barriers of humans, including the blood-brain barrier. OP can be absorbed into the body by the conjunctival, oral, cutaneous and inhalation routes, after being used mainly as insecticides, but also as acaricides, nematicides and fungicides (Bohórquez et al., 2012; Fernández et al., 2010; Hurtado & Gutiérrez, 2005; MPS, 2008b).
A person can come into direct contact with these substances in an occupational context (agricultural or livestock work), domestic (tasks such as gardening and cleaning), voluntary (suicide attempt), or accidental (consumption of food with pesticide residues). Indirectly, contact occurs in an environmental context when the person lives or frequents nearby places where these pesticides are applied (Bohórquez et al., 2012; INS, 2010).
The toxic effect produced by exposure to OPs in the human body is the inhibition of the functioning of cholinesterase enzymes, and this is the reason why these types of pesticides are considered as anticholinesterase substances (Hanna & Orozco, 2014; Toro et al., 2017). Figure 1b shows the case of EC, whose inactivity occurs when the organophosphate pesticide joins it causing the enzyme to be phosphorylated, and blocking its catalytic function (Cuaspud & Vargas, 2010). In days, the possibility that the EC is dephosphorylated (reactivated), a process known as aging, decreases; thus, OPs are irreversible inhibitors (Cotton et al., 2018; Lutovac et al., 2017). The recovery of EC activity occurs with the replacement by a new enzyme related to the formation of erythrocytes in a period of 120 days (Cotton et al., 2018).
OP poisoning can affect the function of the eyes, exocrine glands, and skeletal muscles, as well as the digestive, respiratory, cardiovascular, urinary, and nervous systems. Specifically, within the muscarinic effects, miosis, sweating, blurred vision, conjunctival hyperemia, lacrimation or tearing, bronchial secretions, bronchoconstriction, vomiting, abdominal colic, diarrhea, rhinorrhea, sialorrhea, bradycardia, and urinary incontinence are found. Among the nicotinic effects, tachycardia, hypertension, peripheral vasoconstriction, myocardial hyperexcitability, mydriasis, asthenia, muscle weakness, and muscle fasciculations, among others, are observed. Central nervous system effects include headache, agitation, psychosis, mental confusion, seizures, coma, and respiratory depression (Lu, 2007; Férnandez et al., 2011; Hurtado & Gutiérrez, 2005; Lutovac et al., 2017; Sapbamrer & Nata, 2014).
The symptoms found in agricultural workers exposed to OP are documented in several national (Amaya et al., 2008; Díaz et al., 2017; Hanna & Orozco, 2014; Rodríguez et al., 2010; Toro et al., 2017; Varona et al., 2007) and international studies (Lu, 2007; Marrero et al., 2017; Neupane et al., 2014; Nganchamung et al., 2017; Palacios & Paz, 2011; Sapbamrer & Nata, 2014) that have measured the activity of cholinesterase enzymes, which are detailed in table 1.
Table 1 Symptoms reported in agricultural workers exposed to organophosphate pesticides
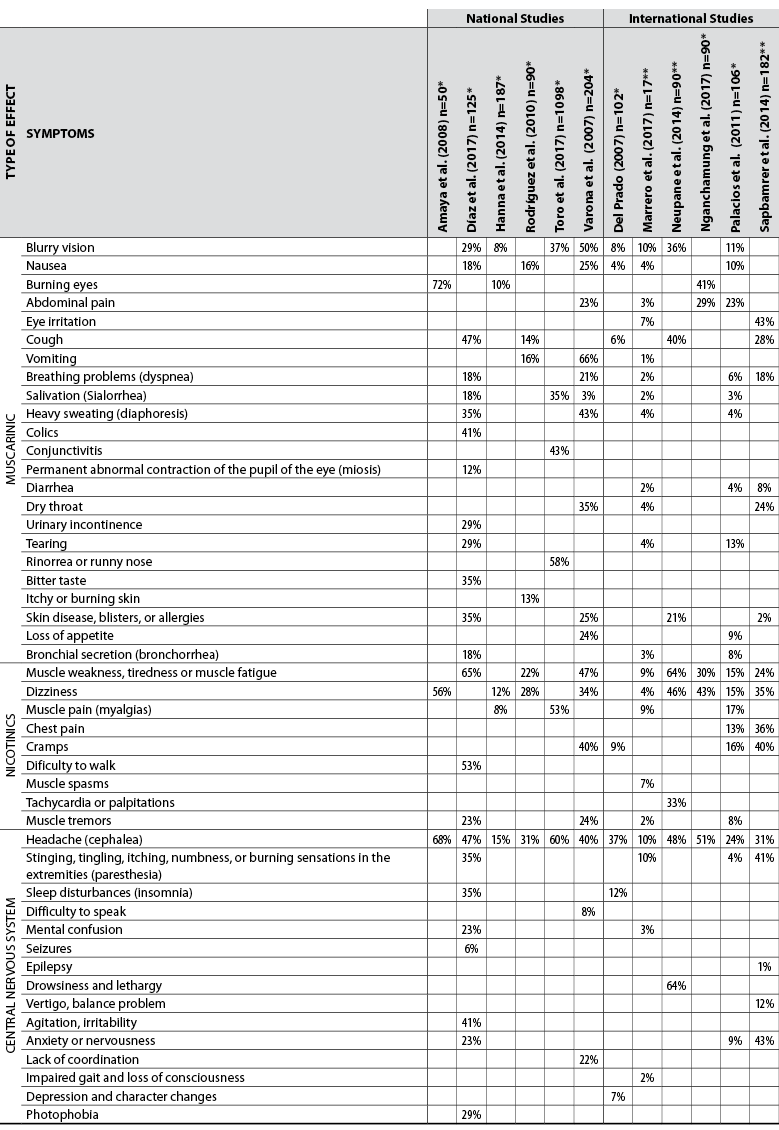
*n = number of total study individuals
**n = number of individuals in the exposed group
Source: Elaborated by the authors
It should be noted that the signs and symptoms manifested in poisoning depend on the type of OP to which the worker is exposed (Lu, 2007); similarly, it has been observed that some of the signs or symptoms are associated with cholinesterase inhibition, such as blurred vision, which has been related to abnormal EC values (p = 0.008) in tomato producers under greenhouse conditions exposed to OP from the department of Boyacá (Rodríguez et al., 2010). However, symptoms have also occurred in exposed farmers without a decrease in cholinesterase levels, as observed in the study by Palacios and Paz (2011) carried out in the State of Sinaloa (Mexico). Therefore, it is important to observe which are the normal activity intervals of the enzyme, according to the implemented technique. If the range is wide, it is possible to mask an intoxication with normality that does not show decreased activity levels but shows symptoms in individuals (Cuaspud & Vargas, 2010).
Laboratory methods to establish the cholinesterase enzymes activity
There are various laboratory methods to establish the activity of PC and EC in workers exposed to OP, which were used in national studies carried out in the departments of Boyacá (Rodríguez et al., 2010), Caldas (Toro et al., 2017; Varona et al., 2012), Cauca (Díaz et al., 2017), Córdoba (Hanna, & Orozco, 2014), Cundinamarca (Amaya et al., 2008), Magdalena (Lozano, 2015), and Putumayo (Varona et al., 2007), as well as in international studies carried out in Argentina (Butinof et al., 2017; Simoniello et al., 2010), Australia (Cotton et al., 2018), Bolivia (Jors et al., 2006), Brazil (Nerilo et al., 2014), Ecuador (Cuaspud & Vargas, 2010; Silverio et al., 2015), the Philippines (Lu, 2007, 2009), Honduras (Blanco et al., 2016), Indonesia (Rahman et al ., 2015), Iran (Jalilian et al., 2016), Mexico (Alvarado et al., 2019; Palacios et al., 2009; Palacios & Paz, 2011; Ortega et al., 2016), Nepal (Neupane et al., 2014, 2017), Peru (Rosales, 2015), Russia (Lutovac et al., 2017), Thailand (Nganchamung et al., 2017; Sapbamrer & Nata, 2014), and Venezuela (Marrero et al., 2017, 2018).
Ellman's method
Ellman's method is quantitative and colorimetric; besides, it is fast and is used to measure EC and PC separately, expressed in units per liter (U/L). It is based on the measurement of the thiocholine production rate resulting from the hydrolysis of the cholinesterase enzyme (Ellman et al., 1961): the higher the activity of the enzymes present in the blood sample, the amount of reaction product increases (Palacios et al., 2009; Tecles & Cerón, 2003; Toro et al., 2017).
For the PC analysis, Ellman's method uses butyrylthiocholine as a substrate: if cholinesterase is activated, butyrylthiocholine breaks down to thiocholine and butyrate. The resulting thiocholine reacts with Ellman's reagent, 2-nitrobenzoic acid (DTNB), generating as a product, a yellow carboxylic acid, which is measured spectrophotometrically at a wavelength of 412 nm; the increase in yellow color is proportional to the amount of cholinesterase (Ellman et al., 1961; Neupane et al., 2014). In the case of EC, after separating the red blood cells from the blood sample, an aliquot of the erythrocyte supernatant is taken and placed to react with the acetylthiocholine substrate, which by the action of the enzyme, will be hydrolyzed into acetate and thiocholine. The thiocholine from this reaction follows the same procedure described above with the PC.
The use of the Ellman’s method is reported in investigations with occupationally exposed populations to OP (Alvarado et al., 2019; Lozano, 2015; Nerilo et al., 2014; Neupane et al., 2017; Nganchamung et al., 2017; Rodríguez et al., 2010; Sapbamrer & Nata, 2014; Silverio et al., 2015; Toro et al., 2017).
Michel's method
Michel's method is a quantitative and electrometric technique used to determine the activity of EC and PC (Fernández et al., 2010). The enzyme is reacted with an acetylcholine substrate producing a certain amount of acid in a standard buffer solution to quantify cholinesterase levels. The value of the activity of cholinesterase enzymes is measured by spectrophotometry in reference to the change in pH per hour (∆ pH/hour or UpH/hour) (Palacios et al., 2009). This method is reported in different studies of workers exposed to OP (Amaya et al., 2008; Blanco et al., 2016; Lu, 2007, 2009; Varona et al., 2007; Varona et al., 2012).
Lovibond's method
Lovibond’s method is a semiquantitative and colorimetric technique that is used to establish the activity of the EC and PC, which has a low cost and requires a minimum blood sample (Jalilian et al., 2016). It is based on a color change that occurs at a certain time due to a variation in pH. The activity of cholinesterase enzymes is expressed as a percentage in intervals of 12.5%, assuming that less than 75% are abnormal values (Hanna & Orozco, 2014; Neupane et al., 2017). For EC, a blood sample is hemolyzed in the laboratory according to the protocols established; subsequently, the enzyme is reacted using acetylcholine perchlorate, and, as an indicator solution, bromothymol blue is used. By the action of the enzyme, the substrate is hydrolyzed to choline and acetic acid. Acetic acid reacts with the indicator inducing a change in color and pH over a time interval (INS, 2016a; Neupane et al., 2014). This method was reported in the study by Rahman et al. (2015) with workers exposed to OP.
Influence of biological, chemical and environmental factors on cholinesterase values
Cholinesterase activity can fluctuate in humans according to interindividual variability, such as ethnic and genetic traits, and other aspects of intraindividual variability, such as age, sex, and reproductive and health status in terms of the use of medications and the presence of some diseases. This is how PC activity values can increase with high blood pressure, thyroid disorders, arthritis, asthma, obesity, alcoholism, hepatitis, and diabetes. On the other hand, they can decrease with cirrhosis, tuberculosis, cancer, epilepsy, malnutrition, anemia, liver failure, and intestinal parasitism. Further, EC levels are depressed by hemolytic anemia (Bohórquez et al., 2012; Fernández et al., 2011; Medina et al., 2015). Particularly in women, variations in cholinesterase levels are related to several states, including pregnancy, menstruation, menopause, abortion, and consumption of hormonal contraceptives (Carmona, 2006; Férnandez et al., 2011).
Since these factors, as well as OPs, can cause a decrease in the activity of cholinesterase enzymes, they have been considered as exclusion criteria in several studies with populations occupationally exposed to OP to obtain unbiased values. In this sense, the studies by Butinof et al. (2017) and Nganchamung et al. (2017) used liver failure, the use of medications, and habits such as alcoholism and drug addiction as exclusion criteria. For their part, Rosales (2015) and Ortega et al. (2016) did not consider pregnant women (Butinof et al., 2017; Nganchamung et al., 2017; Ortega et al., 2016; Rosales, 2015).
Conversely, the type of cholinesterase and the level to which its activity may decrease depends on the chemical composition of the pesticide and the solvents used in its preparation (INS, 2016b, 2016c). Likewise, it is known that the activity of the PC or EC enzymes can decrease preferentially due to some pesticides; such is the case, for example, of dimethoate and phosmet that preferentially reduces the activity of EC, while mevinphos and chlorpyrifos do the same with that of PC (OEHHA, 2015). However, there is no clarity about the responsible biological mechanisms that cause this effect on the activity of one or another cholinesterase. Nonetheless, the lower the cholinesterase activity values, there is a higher risk of OP poisoning (Marrero et al., 2017), and the symptoms that deteriorate health are manifested (Sapbamrer & Nata, 2014).
Cholinesterase evaluations in populations exposed to OP
According to time, context, and amount of pesticide, OP exposure is classified as acute and chronic. An exposure is acute when the contact occurs in a short time interval (days), in an occupational, domestic, voluntary or accidental context, and involves high amounts of the pesticide. An exposure is chronic if the contact occurs over a long period (months and years) in an occupational and environmental context, and with low amounts of the pesticide (INS, 2010).
It is possible to identify acute poisoning when depressed values of PC activity are reported, and adverse health symptoms appear (Neupane et al., 2017). The PC is used because it takes less time to lower its levels, as well as to recover them (between days and weeks). Because EC requires more time to become depressed and return to normal levels, between one and three months are needed to determine chronic poisoning (Carmona, 2006; Cotton et al., 2018; Restrepo et al., 2017). Furthermore, the activity of this enzyme is more susceptible to being inhibited by multiple organophosphates, and its activity is highly related to cholinesterase in the nervous system (Cotton et al., 2018; Cuaspud & Vargas, 2010; Lu, 2007; Neupane et al., 2017).
A person after acute or chronic exposure to OP may have low levels of PC or EC, respectively (Jaga & Dharmani, 2007; Lutovac et al., 2017), associated with different factors, such as the inappropriate use of these compounds due to ignorance of the adverse effects on human health, the agricultural work carried out, the exposure time, inadequate implementation of personal protection measures and the inappropriate storage of pesticides in the housing. These factors are reported in studies that have analyzed cholinesterase concentrations in persons occupationally exposed to OPs (Cuaspud & Vargas, 2010; Díaz et al., 2017; Hanna & Orozco, 2014).
The national study carried out by Varona et al. (2007) determined EC levels within three days after exposure to pesticides using Michel’s method in 204 workers from the Putumayo department. For this, the normal ranges used for men were 0.804 to 0.992 ∆ pH/hour, and 0.822 to 0.99 ∆ pH/hour for women. Thus, they observed inhibition of the EC enzyme in 18 % (36/204) of the population. These authors observed a statistically significant difference between the EC levels of 47 % (95/204) of the farmers who indicated having had previous pesticide poisoning, compared to 53 % (109/204), who stated that they had not had it. Conversely, this variable was not related to decreased EC values (Varona et al., 2007).
Amaya et al. (2008) evaluated the EC activity in 50 farmers in the department of Cundinamarca using Michel’s method, with reference values for men from 0.855 to 0.881 ∆ pH/hour and from 0.836 to 0.859 ∆ pH/hour for women. As a result, they found low levels of EC activity in 100 % (50/50) of the farmers. Among the risk factors identified in this population through the Odds Radio (OR) measurement, such as the value considered to present exposure or poisoning by pesticides, the authors reported that the inappropriate use of personal protection elements (OR = 5.71) and the lack of evacuation prior to fumigation (OR = 5.72) were the risk factors most related to pesticide exposure and poisoning (Amaya et al., 2008).
Varona et al. (2012) analyzed EC and PC using Michel’s method in 132 farmers in the department of Caldas. For this, they used as ranges of normality those established by the Environmental Health Group of INS: EC = 0.91-1.64 ∆ pH/hour, and PC = 0.71-1.17 ∆ pH/hour. Hence, they found inhibition of the EC enzyme in 34.1% (45/132) of the workers. Regarding the risk factors for this population, the authors report that the average time of exposure to pesticides was nine years; 78 % (104/132) fumigated at least once a week with an average of 5 hours per day; 49 % (65/132) reported consuming food in the plot, and 74.2 % (98/132) were never trained in the use of pesticides (Varona et al., 2012).
Hanna and Orozco (2014) evaluated the EC using Lovibond’s method in 187 farmers in the department of Córdoba, where they observed that 9.6 % (18/187) of the farmers showed levels below normal. The farmers who presented inhibited levels carried out tasks that involved direct manipulation of the pesticides and increased exposure; 4.8 % were fumigators, and 3.8 % were collectors (Hanna & Orozco, 2014).
Nevertheless, Lozano (2015) analyzed 80 records of the PC level of banana workers in the department of Magdalena employing Ellman’s method, using 3,200 to 9,000 U/L as a normal range at 25 °C and 405 nm. On the contrary, the abnormal levels were < 3,200 U/L. He obtained abnormal PC values in 11 % (10/80) of the workers associated with the age of the individual and the time of exposure to pesticides (p = 0.005). Of the ten individuals with inhibited PC activity, six had an average PC value of 2,898 U/L, with ages between 19 and 34 years, and an average exposure time of four years; three workers registered an average PC level of 2,958 U/L, with ages between 35 and 44 years, and with one year of exposure; further, only a worker registered an average PC activity of 2,952 U/L, with 47 years of age and seven years of exposure (Lozano, 2015).
Díaz et al. (2017) evaluated the activity of the EC and PC by Michel’s method in 125 potato producers in the department of Cauca, where they observed that the farmers presented an average EC value of 1.2455 ∆ pH/hour (0.530-1.831) and 1.65217 ∆ pH/hour (0.960-1.962) for PC. About 8 % (10/125) of the farmers had EC inhibition, and none showed PC inhibition. Of the 8 % reported with EC inhibition, 5 % corresponded to individuals between 40 and 44 years of age, 60 % (6/10) to men, 50 % (5/10) used elements of personal protection, and 6 % (7/10) did not receive training on pesticide management (Díaz et al., 2017).
Toro et al. (2017) established the PC levels in 1,098 coffee growers in the department of Caldas by Ellman’s method at 37 °C and 405 nm; for this, they used a PC reference range from 4,659 to 14,443 U/L. They found that 3.8 % (42/1,098) of the PC values had decreased. The PC inhibition could be related to the fact that 76 % of the coffee farmers mixed the insecticides, 22 % applied the pesticides in the plot more than twice a week, and 38 % never used body protection equipment during a fumigation (Toro et al., 2017).
On the other hand, at the international level, Lu (2009) observed decreased levels of EC in 40.6 % (94/232) of the farmers in the province of Benguet (Philippines), as did Silverio et al. (2015) in 70 farmers in the province of Oro (Ecuador), who detected a low EC level of 44.4 % (20/45). Similarly, Nganchamung et al. (2017) analyzed the activity of the two cholinesterase enzymes, EC and PC, from 90 farmers in the province of Ratchathani (Thailand), where they found that 50.0 % (45/90) of the farmers showed abnormal values in the activity of EC and 51.1 % (46/90) registered abnormal values in PC activity (Lu, 2009; Nganchamung et al., 2017; Silverio et al., 2015).
Cholinesterase evaluations to pre- and post-exposure to OP
According to the surveillance guidelines, a baseline value of the exposed population is required to have comparison values of the activity of the cholinesterase enzyme that favors the understanding of the test results. However, it is not always possible to obtain the value of the activity of the enzyme before exposure, because, when studying populations, in most cases, individuals have already been exposed (Marrero et al., 2017; Palacios et al., 2009; Ramírez et al., 2015). Therefore, some researchers have proposed comparing pre- and post-exposure values. Such is the case of Jalilian et al. (2016), who established the activity of the EC before and after fumigation in 21 farmers in the province of Ilam (Iran). These authors found that, before starting work with OP (Diazinon), all 21 workers had normal cholinesterase activity values, three with 100 % and 18 with 87.5 %. After the exposure, EC activity decreased to 75 % in 13 workers and up to 67.5 % in five workers (Jalilian et al., 2016).
Neupane et al. (2017), measured the PC activity of 25 farmers in the Chitwan district (Nepal) before and immediately after exposure to OP using the portable field tester Test-mate ChE (model 470) at 30 °C, performed readings at a wavelength of 450 nm. The test uses the value of 2.03 U/mL in a range between 1.35 to 3.23 U/mL as normal parameters for the PC. The authors obtained a mean PC value before exposure of 1.41 U/L, and after exposure, the value was much lower (1.29 U/L) compared to the normal range, evidencing a decrease of 8.51% (Neupane et al., 2017). It should be noted that this analyzed sample used –for an average of 9.48 years– the OPs for their agricultural work.
Measurement of cholinesterase levels between exposed and unexposed individuals to OP
Other studies have analyzed samples taken between exposed workers and individuals not directly exposed, some linked to the environment where pesticides are applied and others unrelated, to improve the interpretation of the results of the test for cholinesterase enzymes and favor their usefulness as a biomarker (Butinof et al., 2017; Cotton et al., 2018; Lutovac et al., 2017; Marrero et al., 2017; Marrero et al., 2018; Neupane et al., 2014; Ortega et al., 2016; Rosales, 2015; Simoniello et al., 2010).
It must be pointed out that in the study by Nerilo et al. (2014) carried out in the municipality of Maringa (Brazil), researchers observed a high inhibition in EC activity (> 30 %) in 4.6 % of the exposed workers (8/173) compared to the control group (p = 0.003), whereas no group evidenced changes in PC activity (Nerilo et al., 2014). In contrast, Rosales (2015) determined the activity of the PC and the EC in a population of 109 individuals, 59 exposed farmers and 50 persons without exposure from the Virú district (Peru), where they found that the inhibition in the PC was significant (p < 0.001) between the exposed (4,733 ± 1,350.1 U/L) and the control groups (7,075 ± 1,674 U/L); nonetheless, there was no difference for EC between the exposed (4,867 ± 632.2 U/L) and the control groups (5,051 ± 505.5 U/L) (p > 0.05). Considering the reference values (3,269 U/L for PC and 4,395 U/L for EC) of the total number of persons in the exposed group, 15.3 % (n = 9) showed values below these parameters, that is, inhibition was evidenced in both enzymes (Rosales, 2015).
For their part, the study by Marrero et al. (2017), carried out in the Aragua State (Venezuela), determined that 11.7 % of the PC activity levels reported by the workers in the exposed group was significantly lower compared to the control group (p < 0.05) (Marrero et al., 2017). These same authors confirmed these findings in 2018 in 30 agricultural workers of the Aragua State (Venezuela), where 20 individuals were part of the exposed group, and 10 belonged to the control group, establishing a significant reduction in the average PC activity value in the exposed group (6.4350 ± 1.2465 U/L) compared to the control group (8.2000 ± 1.8749 U/L) (p < 0.05). The mean PC activity value for both groups was registered within the normal range (4,970 to 13,977 U/L); however, 15 % (n = 3) of the exposed workers showed enzyme values below the standard parameters (Marrero et al., 2018).
Contrary to previous studies, Butinof et al. (2017) reported no inhibition in the activity of the PC in any of the groups; the values for the exposed group were between 3,349.58 and 8,886.56 U/L, and for the control group, the values ranged between 3,292.10 and 7,289.48 U/L (reference value: 3,200 to 9,000 U/L) (p = 0.11). Similarly, Ortega et al. (2016) did not find statistically significant differences in EC activity between the group of exposed and unexposed farmers (p = 0.339).
Simoniello et al. (2010) carried out a two-stage study that was differentiated by the number of biomarkers used with 145 persons from the city of Santa Fe (Argentina), who were analyzed for the activity of both cholinesterases, comparing direct versus indirect exposure including a control group. Enzyme activity was analyzed with Ellman's reagent (DTNB) at pH 7.6; both determinations were made at 25 °C and a wavelength of 405 nm. EC was measured as U/L erythrocytes and PC as KU/L. The study was carried out in two stages: in the first stage (group A), 84 farmers participated; they were divided into three subgroups, 27 pesticide applicators (directly exposed), 27 farmers that did not fumigate (indirectly exposed), and 30 individuals with no history of occupational exposure to pesticides (control group). In the second stage (group B), 61 workers were included, and these were divided into three subgroups: 18 applicators (directly exposed), 23 rural workers (indirectly exposed), and 20 unexposed persons (control group). The results of group A showed a significant decrease in EC compared to the control group, both in persons with direct (33 %, p < 0.001) and indirect (23 %, p < 0.001) exposure. Likewise, the authors evidenced a decrease in the PC of 9.8 % in the directly (p = 0.003) and 14% in the indirectly exposed persons (p = 0.08). In group B, when both exposure subgroups were compared with the control group, a 34 % inhibition of EC was reported in the directly exposed persons, and 22 % in the indirectly exposed individuals (p < 0.001); further, a significant decrease in PC of 8.5 % (p = 0.03) was registered only in the indirectly exposed group. There were no statistically significant differences (p > 0.05) when comparing the subgroups of exposed individuals (direct and indirect exposure) in groups A and B for the reported cholinesterase data.
Cuaspud and Vargas (2010) analyzed the EC in 145 workers from the city of Tulcán (Ecuador), of which 95 farmers belonged to the exposed group, and 55 individuals were not exposed to pesticides, but carried out different agricultural jobs and inhabited the study area (control group). The authors used Ellman’s method, employing 6-6-dithiodinicotinic acid (DTNA) as a reagent instead of Ellman's reagent (DTNB). The reference values ranged between 3,081 and 4,745 U/L, established from the average EC value of the control group (3,625.41 U/L), in a range of 3,081 to 4,745 U/L. These authors observed that of the total percentage of the exposed farmers, 44.21 % (n = 42) showed depressed EC activity values. When comparing the exposed group with the control group, they presented a statistically significant difference (p < 0.05) in the mean EC value, showing a value of 3,154.99 U/L in farmers, and 3,625.41 U/L in non-farmers. Further, they reported a lower average EC activity value (2,994.3 U/L) in farmers (exposed group) who had a working time of more than nine years (n = 80) compared to the average value (3,257.2 U/L) of those who had a lower working time than nine years (n = 15). Similarly, they obtained lower values in the EC activity (3,066.9 U/L) in farmers who stored the pesticides at home compared to the farmers who stored them outside their housing (3,180.4 U/L), as well as those who deposited them in an exclusive area (3,212.75 U/L) (Cuaspud & Vargas, 2010).
Sapbamrer and Nata (2014) determined the activity of the EC through a modified procedure of Ellman’s method in 304 workers from the Ban Tom sub-district (Thailand), including 182 rice producers (exposed group) and 122 non-farmers (control group). The absorbance rate was measured at 405 nm, and at 30, 60, 90, and 120 s at 30 °C. The results indicated that the mean EC activity was lower in the farmers compared to the controls, i.e., 9,594 U/L vs. 10,530 U/L (reference values from 6,400 to 8,200 U/L). In the exposed group, 3.3 % (n = 6) of the farmers showed EC levels lower than 6,400 U/L, which was the lower limit of the normal range (Sapbamrer & Nata, 2014).
Cotton et al. (2018) compared EC values between 41 Australian farmers and 14 non-farmers before and after exposure, taking samples at four moments in time: the first measurement was carried out after three to four weeks; the second, between six to seven weeks; the third, from nine to 12 weeks, and the fourth, from 10 to 12 weeks. The authors used the Test-mate ChE cholinesterase test system (Model 400), which is based on Ellman’s method and the EAChE field test kit, to establish as a reference value 3.66 ± 0.54 U/ml. Furthermore, these authors reported that there was no significant difference in the average activity of the EC between agricultural workers and the control group; however, there was a significant reduction in EC between the follow-up period of three to six weeks (p = 0.015) (Cotton et al., 2018).
Lutovac et al. (2017) studied 175 workers in the chemical industry and agricultural production in the Rasina district (Serbia), including 78 workers of the pesticide production process, 50 agricultural workers, and 47 persons who were not exposed to pesticides; in the study, they carried out the EC analysis using Ellman’s method and employing propionaldehyde as the substrate. The results of the study showed that of the 128 workers exposed to pesticides, EC activity was within the reference range. Moreover, when reviewing the medical records, it was evident that in 72 % of the individuals, there was a slight decrease in the EC activity per year (Lutovac et al., 2017).
Neupane et al. (2014) determined the EC in a sample of 180 individuals, 90 farmers (exposed group), and 90 blood donors (control group) from the Chitwan district (Nepal). To do this, the authors used the Test-mate ChE cholinesterase test system (model 400) developed by EQM Research Inc., considering the reading of the EC level and the EC adjusted according to the hemoglobin level (Q); the laboratory test was carried out in the field below 30 °C. They found EC and Q levels significantly lower (p = 0.01) among farmers compared to the controls. The mean Q value in farmers was 28.92 U/g and, in the control group, the value vas 30.05 U/g (Neupane et al., 2014).
Finally, some studies have verified the impact that training has provided to improve OP use and management. Such is the case of the study by Rodríguez et al. (2010), in which they measured the EC activity in 90 farmers using Ellman’s method (normal ranges from 3,000 to 9,300 U/L, at 25 °C, and at a wavelength of 405 nm; Spinreact), where they took an initial sample and, two months later, a second sample after carrying out training on pesticide management by agricultural engineers. In the first measurement, 22 farmers (24.4 %) with decreased EC levels were registered, and in the second sample, 90 farmers (100%) showed normal EC values (Rodríguez et al., 2010).
Discussion
The reviewed studies show the validity and utility of the use of cholinesterase activity as biomarkers to monitor occupationally exposed populations to OP, which is essential when making decisions about administrative controls in the medium and at the source, such as reduction of the exposure time, permanence or change of the type of pesticide, assessment of the appropriate use of personal protective equipment, good fumigation practices, correct storage, packaging and transport of products, and ventilation control at the storage site.
When comparing the different studies carried out in relation to the measurement of PC or EC levels, it is important to bear in mind that the ranges of the normal reference values may show analytical variations due to the method used between laboratories. Therefore, it is crucial to know the type of technique employed and its measurement scope, as well as the commercial kit used and the criteria under which reference values and ranges of normality are assigned according to the type of population, age, and sex. Considering these parameters, studies that can be compared with the results obtained in a measurement of cholinesterase activity in populations of agricultural workers occupationally exposed to OP can be reliably established.
It is evident that by detecting depressed levels of CP and EC enzymes early, it is possible to implement corrective measures for health care before adverse effects, typical of this type of exposure, appear. However, control is more easily carried out in company workers who, being formally employed under a contract, have legal protection that covers them in occupational surveillance programs, in which the recommendations of the guidelines that promote and prevent pesticide poisonings are implemented. The situation is different in informal and independent farmers for whom monitoring the cholinesterase enzymes is a limitation, due to the lack of knowledge of the risks posed to their health by the handling of OPs and the lack of access to laboratory tests of this biomarker, either because they are not linked to healthcare providers and occupational risk managers, or because they do not have the availability of laboratories to perform them.
Among the recommendations of the studies, it should be noted that the populations of farmers in urban and rural areas should have access within their health service to periodic controls of cholinesterase levels, so that they can have a medical record of the activity values of the enzyme and, with this compilation of pre-existing values, carry out the comparison in the search for inhibition of the activity of the enzymes. A comparison between intra-individual values of cholinesterase activity is essential to implement effective surveillance.
The publications also indicate that it is relevant to measure the levels of the enzyme in unexposed populations, to contrast them with the exposed populations, and improve the utility of these biomarkers as a tool to identify persons at risk of exposure to OP. For this reason, in countries like Colombia, it is vital to promote studies that allow establishing reference levels of national cholinesterase activity per department, according to the sociodemographic characteristics of the population. In this review, national studies carried out in seven of the 32 departments of the country, including Boyacá, Caldas, Cauca, Córdoba, Cundinamarca, Magdalena, and Putumayo, were described. Efforts need to be made to complete a national overview of OP exposure characteristics in other agricultural populations.
Considering that farmers require attention and education because they are a vulnerable population due to occupational exposure to OP, farmers must be able to perform the cholinesterase enzyme test as a control test. Moreover, it is also necessary to continue implementing interventions such as training that make the information visible, allowing agricultural workers to understand the risk of poisoning, that they are aware of the figures reported in the country for pesticide poisonings, and that they can relate a decrease in the activity of cholinesterase enzymes with impacts on health due to the inappropriate handling for this type of pesticide.
Finally, it is necessary to promote a culture of self-care in farmers, aimed at avoiding and mitigating risks and damage to health, by adopting behaviors, such as reading safety and technical sheets, the use of adequate personal protection implements for oral, inhalation, ocular and dermal routes, and regular visits to the doctor, especially when signs or symptoms associated with OP exposure are evident.
Conclusions
Cholinesterases are used as biological biomarkers to detect the inactivity of enzyme function, reflecting adverse effects on the health of workers; furthermore, they are effective and improve their usefulness to the extent that comparisons are made with pre-existing values and in persons without exposure. Studies show that there are populations that have begun to give greater importance to monitoring the effects of organophosphates on health; however, it must be strengthened in rural, informal, and independent farming populations.











 text in
text in 



