Introduction
In the last three decades, consumers worldwide have become increasingly concerned about the quality and safety of the market’s food (Soleno, 2015). Fruit and vegetable consumption have increased substantially in recent years because of their well-recognized nutritional value. They are also essential foods because they provide fiber and antioxidant compounds to the human diet (Tzortzakis & Chrysargyris, 2016).
Asparagus is a perennial, rustic vegetable with a short post-harvest life (Pascualetti et al., 2013). According to statistics from the Food and Drug Administration (FDA), Peru is the second-largest producer of asparagus worldwide (4%). It is surpassed only by China (89%) -which produces it for domestic consumption mainly- and followed by Mexico (3%), Germany (1%), and Spain (1%). The main Peruvian producing areas for 2018 were Ica (53%) and La Libertad (37%), as per figures from the Ministry of Agriculture and Risk (Agronline, 2019). It is known that the shelf life of asparagus (the period elapsing between harvest and end-use) is fifteen days. After that, the degradation or senescence process accelerates and is no longer suitable for human consumption (Cosio, 2017).
Green asparagus (Asparagus officinalis L.) is a trendy vegetable for its turgid texture and nutritional value. However, freshly harvested, it is very susceptible to shoot yellowing and decay that is mainly related to its high respiration rate and microbial contamination. Shoots undergo several considerable physiological changes during storage, including loss of water, degradation of chlorophyll and ascorbic acid, and alterations in phenolic compounds. Meanwhile, the degree of lignification is also a significant determining factor of this product quality (Wang & Fang, 2019). Such changes can be reduced by combining rapid post-harvest cooling, low-temperature storage, chemical treatments, and the use of modified or controlled atmospheres, which have been used for reducing asparagus spoilage during cold storage, although they have added few benefits (Mercado-Ruiz et al., 2013).
The fresh and minimally processed fruit and vegetable industry commonly uses chlorinated water and organic acids for cleaning and disinfection. However, they may not be effective against some pathogenic microorganisms and viruses (Aziz & Ding, 2018). Besides, the formation of trihalomethanes and chloramines from the reaction of chlorine to soluble organic compounds can threaten human food safety or cause environmental contamination. Therefore, different authors suggest the use of alternative disinfectant agents (Gutiérrez et al., 2016b), which is how the latest disinfectant technologies, such as UV light, ultrasound, ozone, irradiation, and organic acids, inactivate pathogens and maintain product quality (Bermúdez-Aguirre & Barbosa-Cánovas, 2013).
Ozone (O3) naturally decomposes into oxygen and has been reviewed for its safety as a disinfectant. This substance was declared GRAS (generally recognized as safe), and, in 2003, it received formal approval from the US FDA for food contact. For this reason, and considering that it has no carcinogenic effect on human health, the use of ozone for food processing increased around the world (Aziz & Ding, 2018). Hormesis involves stimulating a plant using low or sublethal doses of an inducer/agent, such as a chemical inducer or physical stress, to obtain a beneficial or protective response (Ribeiro et al., 2012). Ozone can induce hormesis in fruit and vegetables, promoting various positive physiological responses, including the synthesis of antioxidants, polyamines, ethylene, phenolic compounds, and other secondary metabolites (Pretell et al., 2016).
The use of ozone for post-harvest handling is currently growing since recent studies, especially on the main vegetables for export, indicate that the right application dose can delay senescence and extend the shelf life of certain types of vegetables such as broccoli, lettuce, and cabbage (Aziz & Ding, 2018).
Experiment design plays a vital role in process and product optimization, particularly manufacturing and continual improvement (Arias-Nava et al., 2015). Frequently, these models’ goal is to optimize the simulated actual production system (Dellino et al., 2010). The response surface methodology is a set of statistical techniques employed to find the optimal product processing conditions, without increasing operating costs and experimentation times, and improve systems in which one or more responses of interest are influenced by various independent variables (Torres et al., 2018).
In this sense, it is quite feasible to use the potentialities of ozone for the post-harvest treatment of vegetables, especially green asparagus. This research aims to determine, through the response surface methodology, the required gaseous ozone concentration, and storage time to obtain minimally processed green asparagus with the best physicochemical characteristics.
Materials and methods
Raw material
For this study, shoots of green asparagus Asparagus officinalis L. (Asparagaceae), UC157 F1 variety, from the province of Chao (coordinates: 08°32'25" S and 78°40'39" W) in La Libertad, Peru. We worked with fresh green asparagus (within 7 hours of being harvested) using shoots free of pests and smells that were not physically damaged (bumps, bruises, or otherwise). According to NTP 011.109:2008 (Indecopi, 2008), they were classified with AB tip quality and 14–20 mm caliber.
Treatment with minimal processing
The shoots were sprayed with potable water to eliminate surface impurities and immersed in a solution of 100 ppm chlorine dioxide for 5 minutes at room temperature. They were cut to 17 cm, considering the measurement from the base to the tip. Then, excess moisture was removed, and shoots were packaged in expanded polystyrene trays weighing 150 g ± 3 g to be transferred to the treatment chamber. There, the gaseous ozone generated by an ozonating machine (Ozonomatic OZ-500) was injected at a flow of 500 mg/h through a 7 mm diameter hose, subjecting the shoots to concentrations between 0-10 ppm, controlled by a digital gaseous ozone meter (Crowcon Gasman O3, range: 0-100 ppm, sensitivity: ± 0.1 ppm). Finally, the shoot trays were removed from the chamber, wrapped with polyvinyl chloride (PVC) film, and stored in a temperature-controlled refrigeration chamber for 30 days at 4 °C ± 1 °C and 85–90% ± 0.1% relative humidity. These conditions were monitored using a thermohygrometer (Fluke 971, sensitivity: ± 2.5%).
Analytical techniques to evaluate the physicochemical characteristics
The techniques employed to determine the physicochemical characteristics of materials are as follows:
Weight loss. The green asparagus trays’ weight before and after each established storage period was established. The results were expressed as a percentage of weight loss compared with the baseline weight (Wang & Fang, 2019).
Color. Following the CIELAB system, the Kónica-Minolta CR-400 colorimeter was calibrated with a standard blank. Then, the luminosity or L* value (L* = 0 for black and L* = 100 for white) was determined with ten samples for each treatment. The results were expressed as the average of all the values obtained (Albanese et al., 2007).
Firmness. An Instron 3342 texture analyzer with a 50 N cell was used. The tissue’s breaking stress was measured with a 3.18 mm diameter crosshead and a speed of 1 mm/s, as recorded by Bluehill 2.0 (Albanese et al., 2007). The results were expressed as the average of ten samples per treatment.
Lignin content. Lignin is a cross-linked phenolic polymer insoluble in all solvents; therefore, it is necessary to break down its structural components chemically. Thioacidolysis is a method used to hydrolyze lignin. The tissue was dehydrated; then, 0.5 g were homogenized with 150 mL of 80% ethanol for four minutes. The mixture was vacuum filtered; the residue was washed with 20 mL of 80% ethanol and dried at 50 °C for 24 hours. For drying, 15 mL of 2 mol/L HCl and 1 mL of thioglycolic acid were added, cooked for 4 hours, and centrifuged at 10,000 rpm for 15 minutes. The residue (lignin-thioglycollate) was washed with 10 mL of water, suspended again in 20 mL of 0.5 mol/L NaOH with a magnetic stirrer for 18 hours at room temperature, and centrifuged; 4 mL of concentrated HCl were added to the supernatant liquid. Thioglycolic acid lignin was precipitated at 4 °C for four hours and centrifuged; the residue was dissolved in 10 mL of 0.5 mol/L NaOH; absorbance was read at 280 nm. The quantification was carried out in mg/100 g sample using a coumaric acid standard curve (An et al., 2007; Wang & Fang, 2019).
Chlorophyll content. We followed the method proposed by Ruiz Santiago et al. (2019) and Wang and Fang (2019). For this, the sample was finely cut into 0.5 cm2 strips weighing 0.5 g and macerated in a mortar adding 5 mL of 80% acetone solution until all the pigment was extracted from the sample. Subsequently, the sample was placed in a tube and centrifuged at 2,000 rpm for ten minutes. The supernatant containing the pigments was separated, adjusting each tube to 6 mL with 80% acetone; 0.5 mL of each extract’s supernatant was taken and diluted to 5 mL with 80% acetone. A Thermo Spectronic Genesys 6 spectrophotometer measured the absorbance at wavelengths of 645 and 663 nm using a sample of 80% acetone as a blank. The results were expressed as the average of three samples per treatment. For the quantification of total chlorophyll, the following equation was used: Total chlorophyll = (20.2 × Abs645nm) + (8.02 × Abs663nm).
Statistical analysis
Rotatable central composite design (RCCD)
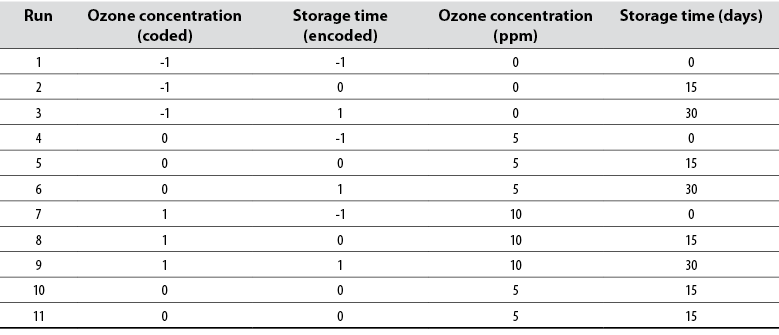
The rotatable central composite design of the response surface methodology was used with a 24 array. Data were analyzed with Minitab 17. In table 1 is shown the coded experimental levels of the independent variables.
Results and discussion
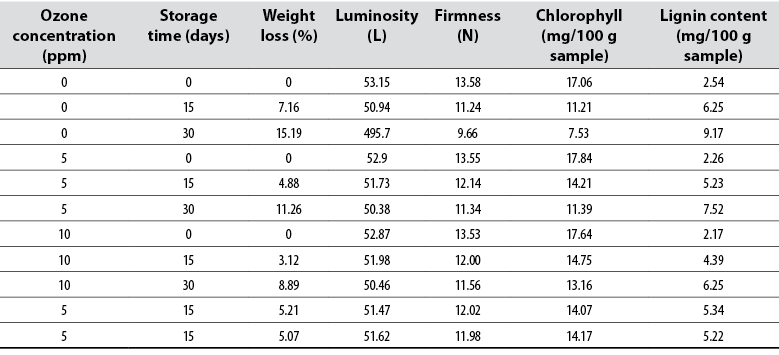
The results of the physicochemical characteristics obtained after applying the RCCD of the response surface methodology, which allowed modeling the behavior of these dependent variables as a function of gaseous ozone concentration and storage time are presented in table 2.
Effect of ozone concentration and storage time on weight loss
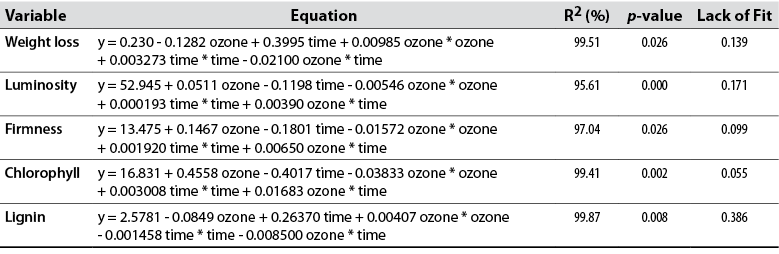
With gaseous ozone concentrations of 6-10 ppm for a storage time of 20-25 days, weight loss reached acceptable levels for fresh and minimally processed products (less than 10%), compared to samples at lower concentrations, including non-ozonized shoots (figure 1). Variance analysis indicated that the quadratic model had a significant effect (p < 0.05), a high coefficient of determination (R2 = 99.51%), and a lack of fit (p > 0.05), which accounts for the good model of the data (table 3).
The effect of gaseous ozone on weight loss in other plant products presented similar results. For instance, Glowacs and Rees (2016) reported a decrease in weight loss to 3.70% in green and red peppers with the continuous application at 0.045-0.2 ppm for 14 days of storage at 10 °C, and Glowacs et al. (2015) reported a reduction in weight loss by 5% in zucchini (Curcubita pepo) with the continuous application at 0.01 ppm for 17 days of storage at 8 °C.
Respiration, perspiration, and exudate due to the cutting and handling of vegetables are possible causes of water loss and consequent weight loss in minimally processed vegetables (Glowacz et al., 2015). Ozone could have a limiting effect on some of these factors and thus restrict weight loss. Stomata in plants could close in response to oxidative stress caused by ozone, and, as a result, the amount of water retained in ozone-treated samples is higher than in non-ozone-exposed samples (Tabakoglu & Karaca, 2018). For their part, Pretell et al. (2016) mentioned that the beneficial impact of applying ozone to plants could be observed, for example, in the inhibition of damage to cell membranes and a delay in membrane-associated functions, which would explain the lower weight loss values of the ozonated fruit, compared with the control sample.

Source: Elaborated by the authors
Figure 1 Contour and surface blanket response of (a) weight loss, (b) luminosity, and (c) firmness for ozone concentration and storage time
During partial post-harvest water loss, water stress causes oxidative stress with increased lipoxygenase and peroxidase activities. Plant cells have a system of enzymatic and non-enzymatic antioxidants that maintain ROS (reactive oxygen species) at the proper levels (Chkaiban et al., 2007). The enzymes involved in ROS elimination, such as CAT (catalase), APX (ascorbate peroxidase), GPX (guaiac peroxidase), and SOD (superoxide dismutase), show more significant activity during water loss, suggesting that the antioxidant system is acting as a consequence of water stress (Fan et al., 2016). The effect of ozone depends much on concentration and application time; a combination of low concentration and short application time, therefore, could act as a light oxidizing agent, stimulating the activities of these enzymes to protect plant cells and reducing water loss (Modesti et al., 2018).
Effect of ozone concentration and storage time on color
Figure 1 shows that, with gaseous ozone concentrations of 6–10 ppm for a storage time of 25-27 days, luminosity decreased slightly in the shoots, compared to the samples treated at a lower concentration—including the non-ozonated ones—, in which intense darkening was noted. Variance analysis indicated that the quadratic model had a significant effect (p < 0.05), a high coefficient of determination (R2 = 95.61%), and a lack of fit (p > 0.05), which accounts for the good model of the data (table 3).
Gutiérrez et al. (2016a) studied gaseous ozone application at 1, 2, and 5 ppm for 12 days of storage at 5 °C in arugula (Eruca sativa Mill.), achieving less darkening of the leaves, compared to the samples without ozonation. Glowacs et al. (2015) evaluated the continuous application of gaseous ozone at 0.01 ppm for 17 days of storage at 8 °C in zucchini, obtaining similar results. Likewise, Han et al. (2017) evaluated the effect of pre-cooling and initial application of gaseous ozone at 2 ppm for 15 days of storage at 0 °C on the luminosity of blackberries, achieving less darkening than the sample without ozonation.
Bermúdez-Aguirre and Barbosa-Cánovas (2013) state that vegetables such as lettuce represent, in terms of color, a complex system made up of enzymes (peroxidase and polyphenol oxidase), pigments (carotenoids and chlorophyll) and ascorbic acid. Santisteban (2016) describes that another cause of quality deterioration is the change in the natural color, such as fresh green asparagus going dark due to the loss of chlorophyll during the storage period.
Also, Glowacs and Rees (2016) mention that exposure to gaseous ozone decreases the activity of catalase and peroxidase, an enzyme that catalyzes phenol oxidation typical of quinones that cause brown colors or darkening in plants.
Effect of ozone concentration and storage time on firmness
With gaseous ozone concentrations of 5–10 ppm up for a storage time of 25–30 days, the firmness of the shoots decreased slightly, compared to the samples treated at a lower concentration, including the non-ozonized ones (figure 1). Variance analysis indicated that the quadratic model had a significant effect (p < 0.05), a high coefficient of determination (R2 = 97.04%), and a lack of fit (p > 0.05), which accounts for the good model of the data (table 3).
Glowacs et al. (2015) researched the continuous application of gaseous ozone at 0.01 ppm for 17 days of storage at 8 °C in zucchini, obtaining a similar trend. Han et al. (2017) evaluated the effect of pre-cooling and initial application of gaseous ozone at 2 ppm for 15 days of storage at 0 °C in blackberries, reporting greater firmness in the treated berries than the sample without ozonation. Ali et al. (2014) studied the continuous application of gaseous ozone at 1.5, 2.5, 3.5, and 5.0 ppm for 14 days of storage at 25 °C in papaya (plus a control sample without ozonation), obtaining obtained similar results for firmness. Goffi et al. (2018) reported that the continuous application of gaseous ozone at 150 ppb in the morning and 180 ppb at night for six weeks of storage at 0 °C in kiwi allowed higher firmness retention, compared to the sample control.
The ozone treatment delayed the hardening of the tissue in carrot sticks. These modifications were associated with changes in the cellulose, hemicellulose, and lignin content, mainly due to the reduction of cell wall lignification. However, each vegetable’s cuticle thickness and composition often depend on cultivar and maturity, making it even more challenging to select an optimal dose of ozone (De Souza et al., 2018). The decrease in vegetables’ firmness is a problem because it reduces their commercial value (Glowacs et al., 2015). Glowacs and Rees (2016) suggested that vegetables exposed to continuous gaseous ozone can reduce water loss during storage, improving firmness maintenance.
Effect of ozone concentration and storage time on chlorophyll content
Gaseous ozone concentrations of 4-8 ppm produced a slight effect on chlorophyll content retention in the shoots with a tendency to decrease over 30 days of storage, compared to the non-ozonized sample (figure 2). Variance analysis indicated that the quadratic model had a significant effect (p < 0.05), a high coefficient of determination (R2 = 99.41%), and a lack of fit (p > 0.05), which accounts for the good model of the data (table 3).

Source: Elaborated by the authors
Figure 2 Contour and surface blanket response of (a) chlorophyll content and (b) lignin content for ozone concentration and storage time
Gutiérrez et al. (2016a) studied gaseous ozone application at 1, 2, and 5 ppm for 12 days of storage at 5 °C in arugula, stating that there were no significant changes in chlorophyll content. Karaca and Velioglu (2014) did not find a substantial decrease in the chlorophyll content of parsley using gaseous ozone at 0.95 ppm for 20 minutes at room temperature. However, ozone was reported to cause slight discolorations in lettuce and spinach.
Effect of ozone concentration and storage time on lignin content
Between gaseous ozone concentrations of 7-10 ppm for a storage time of 20-30 days, the lignin content increased slightly in the shoots, compared to the samples treated at a lower concentration, including control (not ozonized) ones (figure 2). Variance analysis indicated that the quadratic model had a significant effect (p < 0.05), a high coefficient of determination (R2 = 99.87%), and a lack of fit (p > 0,05), which accounts for the good model of the data (table 3).
An et al. (2007) explored the application of a 1 ppm ozone solution for 30 minutes in green asparagus stored for 25 days at 3 °C, reporting a similar trend. Chauhan et al. (2011) evaluated the initial application of 10 ppm gaseous ozone for 30 days of storage at 6 °C, where the lignin content in carrot pieces was lower than the sample without ozonation.
An et al. (2007) point out that the hardening of green asparagus a few days after harvest is attributed to increased lignin levels during storage. Furthermore, the authors suggest that one of the determining factors of hardening is the thickening of cell walls’ polysaccharide structural components. Qiu et al. (2013) and Wang and Fang (2019) reveal that lignification in asparagus is controlled by different enzymes, including phenylalanine ammonia-lyase (PAL), which is critical to the lignin biosynthetic pathway; therefore, the application of ozone could delay the biosynthesis of this component of insoluble dietary fiber.
Optimization
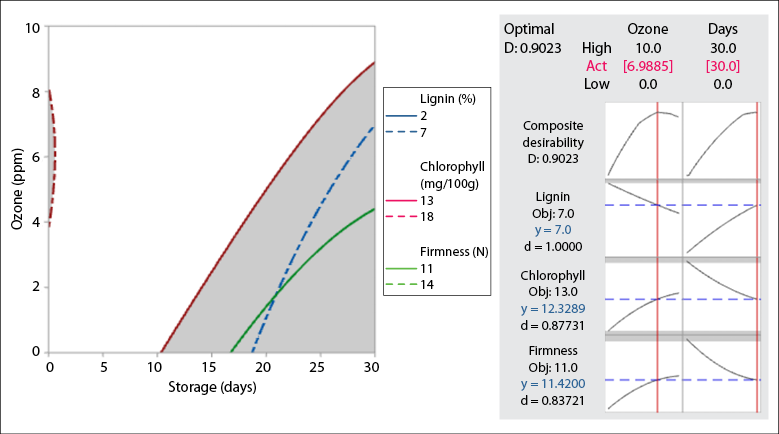
For optimizing the independent variables, we used the technique of overlapping the contours of the studied variables firmness, chlorophyll content, and lignin content, considered as the most significant to maintain the quality of green asparagus shoots during the logistics and marketing chain of this minimally processed product (figure 3). We found that the regions of interest determined by the optimal area are a concentration of 6.98 ppm gaseous ozone for 30 days of storage, with the following as the best quality characteristics: 11.42 N, firmness; 12.33 mg/100 g, chlorophyll sample, and 7 mg/100 g, lignin sample.
Conclusions
Through the response surface methodology, we determined the region of interest for optimal firmness, chlorophyll content, and lignin content retention in green asparagus during post-harvest handling. Based on this, the initial application of gaseous ozone should be between 4-7 ppm for 30 days of storage at 1 °C. The results suggest that this technology is a good alternative for the preservation of fresh vegetables.











 text in
text in