Introduction
The northern area of Lake Titicaca is one of the centers of origin and diversity of Solanum tuberosum L. In this place, wild species such as Solanum bukasovii Juz. and Solanum bukasovii var. multidissectum (Hawkes) Ochoa gave rise to Solanum stenotomum Juz. et Buk., listed as the first domesticated potato. Solanum stenotomum originated Solanum andigenum Juz. et Buk., which by polyploidization and interspecific hybridization processes resulted in a great diversity of potatoes. Ancient Peruvians were responsible for scattering them across the center, south, and north of Peru. Then, the Spanish colonizers spread them throughout Europe and the world (Centro Internacional de la Papa & Federación Departamental de Comunidades Campesinas, 2006; Gil-Rivero et al., 2019; Gómez et al., 2012; Rodríguez, 2010).
Currently, more than 5,000 varieties of potato are known in Peru, including wild, native, and commercial varieties. Potato is considered the world’s fourth food staple of the human diet due to its nutritional and culinary characteristics (Gómez et al., 2012; Kramm, 2017). In the culinary field, potato can be eaten in various preparations such as soups, mashed potatoes, French fries, dried potatoes, chuño, and tocosh. The last two are the most important ethnomedicinally for the Andean populations due to their content of natural antibiotics (Álvarez, 2001; Centro Internacional de la Papa, Asociación Pataz, & Instituto Nacional de Innovacion Agraria, 2015; Pesantes, 2015).
Solanum tuberosum is propagated vegetatively using cuttings, tubers, and in vitro cultures, and sexually through botanical seeds, which are the most used form because it preserves the agronomic attributes of the species. According to the principle of cell totipotential, plants retain the ability to regenerate and rebuild their tissues until they become once again whole plants with the same genotype (Araújo et al., 2009; Hartmann & Kester, 1995; Rojas et al., 2004). Therefore, farmers use seed tubers to obtain plants identical to the mother plant, although this method allows the transmission of diseases that, together with poor agricultural practices and genetic potential, reduce yields.
This problem resulted in the implementation of new propagation alternatives in production systems, such as in vitro plant tissue cultures. Meristem culture, somatic embryogenesis, and in vitro tuberization are the most common techniques in plant biotechnology since they ensure better genetic and phytosanitary quality of the crop due to a lower viral and bacterial load. Thus, the production and profitability of crops are maximized (Araque et al., 2018; Hernández & Díaz, 2019; Tacoronte et al., 2017).
Propagation by young stem cuttings is useful in pre-basic and basic seed programs. This technique lies in selecting young greenhouse plants that contain between five and six nodes and cutting them at the base of the shoot, leaving a basal leaf with its bud. Then, the apical bud is removed, and portions of the stem are sectioned to obtain cuttings consisting of a leaf and its axillary bud, which will induce rooting when treated with hormones and sown in the sand (Cotes & Ñustez, 2001; Ramírez et al., 2011b).
The rooting of any cutting requires the right hormonal balance of phytohormones or phytoregulators. In agriculture, the most widely used phytohormone is indole-3-butyric acid (IBA), and the most widely used phytoregulator is 1-naphthaleneacetic acid (ANA); these compounds are not affordable for small and medium farmers and agricultural technicians (García et al., 2001; Moreno et al., 2009; Uribe et al., 2012). Although it is known that 2,4-dichlorophenoxyacetic acid (2,4-D) is the most used synthetic auxin herbicide in agriculture to control weeds because it is cheap and easy to buy, it reportedly induces the rooting of cuttings at low concentrations (De la Cruz et al., 2014). Then, the aim of this research is to evaluate the rooting of young stem cuttings of S. tuberosum var. yungay by applying different 2,4-D concentrations.
Materials and methods
We selected Solanum tuberosum var. yungay (Solanaceae) plants with five to six nodes from the Biotechnology Laboratory of the Institute for Potato and Andean Crops, Universidad Nacional de Trujillo, and cultivated them in pots of 10 cm in diameter with a substrate consisting of a mixture of sand, humus, and moss in a 1:1:1 ratio under greenhouse conditions.
Seedbed preparation
In the greenhouse, we prepared a seedbed consisting of two layers of sand: the first, 4 cm thick with coarse sand of 1 cm in diameter at the bottom, and the second, 10 cm thick with fine sand of 0.05 cm in diameter on the top. This substrate was previously disinfected with 2% sodium hypochlorite for 24 hours and solarization for one week.
Sowing cuttings of Solanum tuberosum var. yungay
When the mother plants were able to acclimatize and had between five and six leaves, young stem cuttings consisting of a shoot and its leaf were sectioned (figure 1). The method was to moisten the basal part of the cuttings with water and then impregnating them with the 2,4-D hormone powder according to the indicated treatments: T1: 0%; T2: 0.3%; T3: 0.5%; and T4: 0.8%.
Finally, the plants were sown in rooting beds at field capacity and watered twice a week with sterile water added with liquid NPK-based fertilizer (12-14-12) at a rate of 5 g/L. The greenhouse was kept at an average temperature of 25 °C ± 2 °C, relative humidity of 80%, and a photoperiod of 16 hours of light and 8 hours of darkness.
Statistical design
A completely randomized design was used, consisting of 4 treatments, 24 repetitions, and 96 experimental units. Twenty days after sowing, the number of roots, their length, and seedling height were measured. The recorded data were analyzed with the RStudio integrated development environment, version 1.2.5033. The presence of statistically significant differences was evaluated using Welch’s Anova test because no variable met the homoscedasticity, and the Games-Howell post hoc test, with a 5% significance level, for selecting the best treatment.
Results and discussion
The number of roots, the maximum root length, and the seedling height produced by the different 2,4-D concentrations 20 days after sowing clearly show the action of synthetic auxin, with significant differences between treatments and evaluated parameters (table 1). Ramírez et al. (2011a) have shown that, in Solanaceae, it is necessary to use auxins for the rooting of young cuttings, while in other plant species, they are not essential since water is enough to induce rooting. However, it is crucial to consider that the type of substrate used influences the quality and quantity of roots formed due to more aeration and water retention (López et al., 2008). For their part, Ludwig-Müller and Cohen (2002) reported that auxins regulate plant development, including rooting, since the hormone concentration used is responsible for inducing or inhibiting radical development of future plants.
Table 1 Average values of the variables measured for each treatment
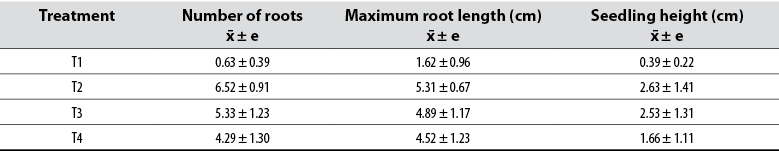
Note: x̄ ± e: mean estimate
Source: Elaborated by the authors
When 0.3% 2,4-D was used, better results were obtained in terms of the number of roots, maximum root length, and seedling height (table 1). When analyzing the Games-Howell post hoc test (table 2), we noted that the T2 treatment is the best as it achieved a more significant number of cell divisions that promoted root growth (figure 2). De la Cruz et al. (2014) corroborate this finding by stating that the 0.3% concentration of 2,4-D exerts a positive effect on the rooting of young cuttings of Rosa canina L. (Rosaceae).
Table 2 Homogeneous subsets obtained from the Games-Howell test for each treatment according to the variables analyzed
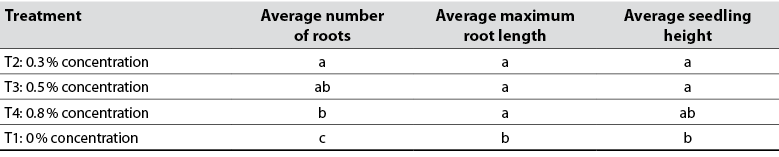
Note: Distinct letters in subsets have a significant difference at 95 % confidence.
Source: Elaborated by the authors

Photo: Segundo Eloy López Medina
Figure 2 Rooted seedling of S. tuberosum var. yungay obtained from young stem cuttings with 2,4-D application 20 days after sowing
Moreover, we observed that, when increasing the 2,4-D dose from 0.3% to 0.5% and 0.8%, there was no rise in the number of roots, maximum root length, and seedling height that exceeded the results of the T2 treatment. It can be inferred that a higher 2,4-D concentration causes a hormonal imbalance that ends up inhibiting rooting. Hartmann and Kester (1995) reported that volumes of growth regulators should be optimal to induce rooting. Also, Moreno et al. (2004) argued that by using ANA, good results are obtained in rooting potato cuttings. Ramírez et al. (2011a) reported that the use of ANA at a 0.4% concentration induces 100% rooting and survival of cuttings. For their part, López et al. (2016) indicated that IBA at a 0.0001% concentration has a positive effect on the rooting of cuttings.
As a result of good root development, a more significant increase in the length of the plants was observed (table 1, figure 2). Likewise, the action of auxins, which improves the plasticity of the cell wall, generated a greater deposit of cellulose, which promotes longitudinal growth (Hager, 2003; Henríquez, 2004). Giraldo et al. (2009) reported that the presence of auxins induces the synthesis of gibberellins, which are the hormones responsible for cell elongation.











 text in
text in 




