Introduction
One of the most obtained by-products in the dairy industry is whey (green-yellow liquid), which results from milk coagulation during cheese making. It represents between 85 and 90% of milk volume and has more than 50% of the milk nutrients (Almécija, 2007; Guerrero-Rodríguez et al., 2012). Acid whey is a type of whey obtained from acid milk coagulation (pH between 4.5 and 5.0) during the stretched-curd cheese production (Jelen, 2003). In Colombia, this type of cheese is widespread and makes part of the most produced in this country, therefore, its production is considerable (Vásquez, 2017).
According to Liu et al. (2005), 9 kg of whey are obtained per 1 kg of curd. Considering that ratio, and a Colombian cheese production of 50,000 tons (Escobar, 2015), whey production is nearly 440 million liters. However, this by-product is not completely valued and is intended mainly for animal feed. In many cases, it is released into soils and water sources due to a lack of awareness of how to take advantage of this raw material (Londoño et al., 2008), becoming a significant pollutant because of its organic matter content (fat, lactose, proteins, among others) (Ghaly & Kamal, 2004; Mukhopadhyay et al., 2005). In comparison with traditional whey, the acidity of this whey prevents the proliferation of microorganisms and favors its conservation (Simanca et al., 2010) and processing.
Falling film vacuum evaporation is a technique that can be used for the processing of dairy products. It allows reducing significant product volumes, concentrating high-value components, and removing as much water content as possible, without thermal degradation of the raw material due to vacuum conditions (reduced boiling point). With this technique, it is possible to treat heat-sensitive materials due to its high heat transfer coefficients (2000-4000 W/m2∙°C), thereby reducing thermal degradation of products, power consumption, and residence times, in comparison with traditional evaporation (Chen & Jebson, 1997; Li et al., 2011). This evaporation stage will improve and enhance the characteristics of this by-product, becoming a raw material for other processes to obtain high-quality finished products (Bimbenet et al., 2007; Zhu et al., 2011).
Pereira (2015) has used this technique to concentrate skimmed milk and acid whey at 200 mbar and 60°C, achieving a volume reduction factor (VRF) of nearly 2 for both cases. Despite this, the studies reporting this process technique for acid whey concentration are few. Most studies have focused on heat transfer coefficient calculation and optimization for different industrial solutions, where milk is commonly the product of interest (Jebson & Iyer, 1991; Monnier et al., 2012). Arias and Espinel (2006) worked with traditional evaporation as an intermediate stage for whey concentration using stockpots. However, there is no detailed study of this technique’s effect on the concentrated product’s characteristics. More importantly, none of these studies analyzed the effect of a higher VRF on the product characteristics. Therefore, this study aims to evaluate the effect of falling film vacuum evaporation on the number of cycles and concentrate characteristics reaching a VRF of 4 in the batch configuration.
Material and methods
Raw material pretreatment
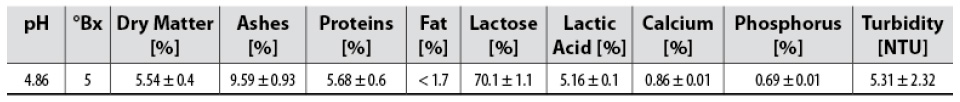
The acid whey used was clarified previously by membrane filtration. The clarification was performed with skimmed and deproteinized whey (at 100°C, 15 min), pre-filtered with canvas. The filtration process was conducted using a 1.0 m-long membrane module, six mono-channel tubular ceramic membranes with a 0.2 µm membrane cut-off (TAMI® Industries, Nyons, France), a surface area of 0.132 m2 (six membranes), filtration conditions at 70°C, and transmembrane pressure gradient (ΔTMP) of 2 bar. The physicochemical characteristics of the whey used in this study are shown in table 1.
Falling film vacuum evaporation procedure
Five lots of acid whey were processed (19 L each lot). Muñoz and Solano (2014) designed and reported the evaporator used (figure 1), which consists of a 1.4 m-long stainless-steel tube (4) with a diameter of
3.8 cm and a 1.2 m-long, 3 mm-thick transparent acrylic tube with a diameter of 5 cm (figure 1). This evaporator has a heating system formed by two 1600 W resistors located inside the evaporator tube and connected to 220 V for a better temperature control system. The acid whey (4-5 °Bx) was fed by suction through a vacuum pump (working pressure of 200 mbar) (9) at temperatures between 60 and 65°C. At the evaporator outlet, the concentrated whey was separated from water vapor streams and stored in different storage tanks (7, 8). Once a cycle was over, the concentrated whey was fed again to the feeding tank (3) to begin a new cycle. The process was finished upon achieving a VRF of 4.
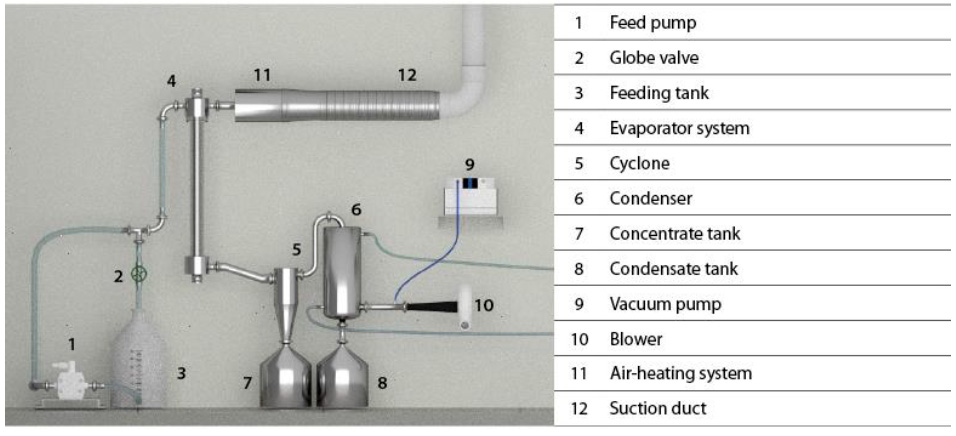
Source: Muñoz and Solano (2014)
Figure 1 Diagram of the falling film vacuum evaporator used in this study
Quantification methods
Samples were characterized by dry matter % (Association of Official Analytical Communities International [AOAC International], 2006a); protein % (AOAC International, 2006b), ash % (AOAC International, 2005a), fat % (AOAC International, 2005b), calcium % (AOAC International, 2000a), phosphorus % (AOAC International, 2000b), lactic acid % (Association of Official Analytical Chemists, 1984), °Brix with Fisher No. 13 964 74C portable refractometer (0-90 °Bx) and turbidity with Hanna® HI 98703 turbidimeter. Samples were studied for color analysis through visible spectrophotometry at 440 nm, similar to Benavides-Prada and Muvdi-Nova (2014). HPLC was used for lactose % with a 7.8 x 300 mm COREGEL 107-H Column (8 µm) at 30 °C and 0.6 mL/min. The mobile phase was an aqueous solution of 8 mM H2SO4 using the isocratic mode. Tukey’s test was carried out to verify statistical differences among mean values obtained from the physicochemical analysis using Minitab statistical software, v. 19.
Results and discussion
The VRF achieved in this study was 4 (around 18 °Bx) due to a very stable foam, which stopped water removal from the whey solution. Nicorescu et al. (2009) attributed this phenomenon to the protein in treated whey, which has foaming properties that can be amplified by heat denaturation (Hui, 1993). The number of cycles used to achieve each VRF are shown in figure 2 —a cycle corresponds to one pass of the solution through the evaporator system—. It indicates that the number of cycles increases linearly with VRF. Tukey’s test confirms this linear behavior because the mean values among VRFs are different from each other. It was also challenging to keep falling film uniformity constant, which made the standard deviation of the number of cycles increased with the VRF.
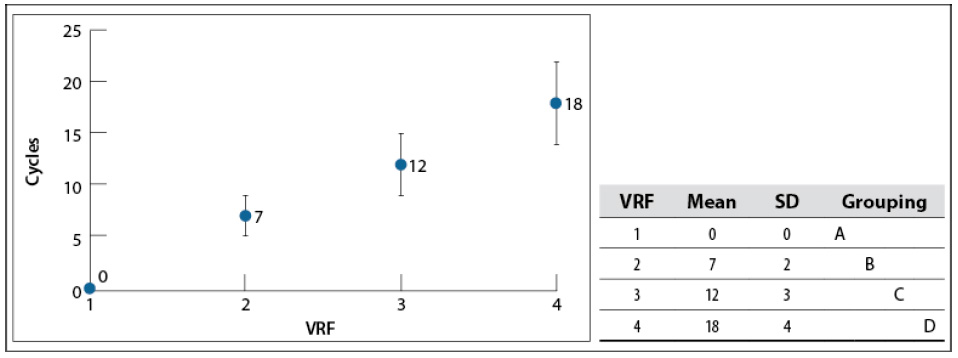
Source: Elaborated by the authors
Figure 2 The number of cycles used for each VRF in the concentration process at 60–65 °C and 200 mbar. SD: standard deviationNote: Mean values that do not share the same letter are significantly different. Information was grouped using Tukey’s method and 95% CI.
°Bx, proteins, and ashes contents increased with the VRF (figure 3). It is noteworthy that they were not affected by the deviation in the number of cycles for each VRF.
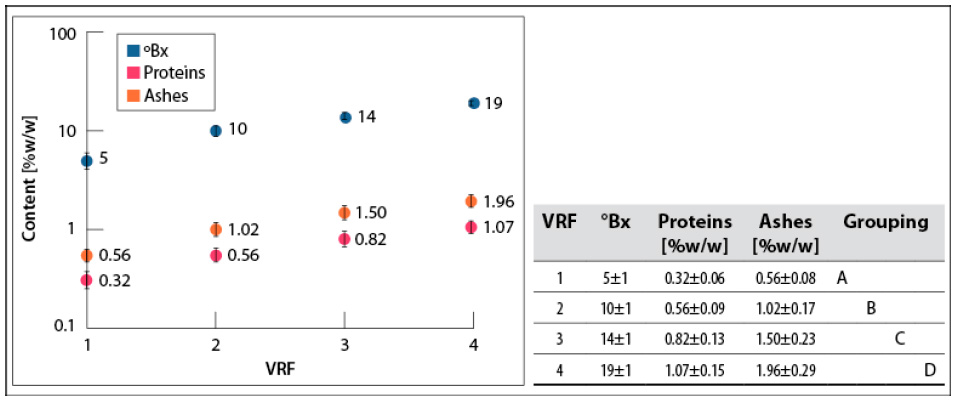
Source: Elaborated by the authors
Figure 3 °Bx, proteins, and ashes contents in concentrates obtained for each VRF at 200 mbar and 60- 65°C. SD: standard deviationNote: Mean values that do not share a letter are significantly different. Information was grouped using Tukey’s method and 95% CI.
Whey turbidity as a function of the VRF is shown in figure 4. All the samples were diluted to the initial concentration (4–5 °Bx; VRF = 1 as a baseline), to find variations in turbidity due to the concentration process.
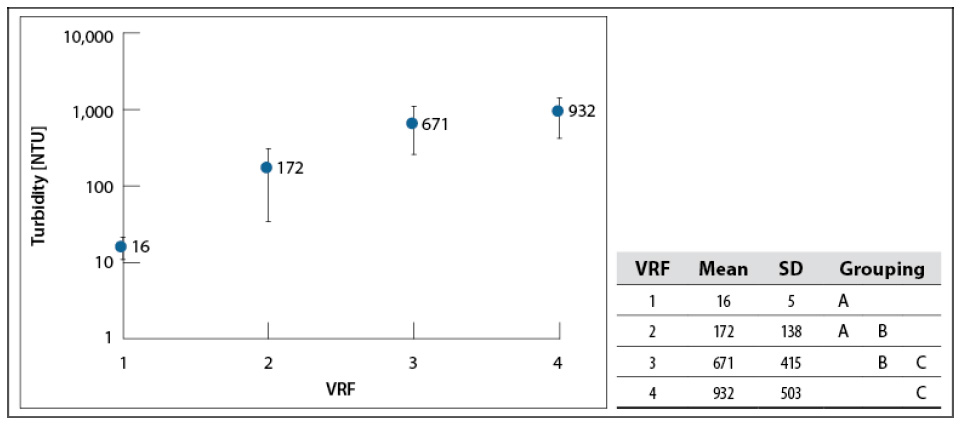
Source: Elaborated by the authors
Figure 4 Turbidity in whey concentrates for each VRF at 200 mbar and 60–65 °C. SD: standard deviationNote: Mean values that do not share a letter are significantly different. Information was grouped using Tukey’s method and 95% CI.
These results show that turbidity increases with whey concentration —mean values are different from each other, according to Tukey’s test—, suggesting that part of the soluble serum protein became insoluble due to irreversible protein denaturation produced by heat treatment (above 60 °C) (Álvarez, 2013; Parris et al., 1991). Muñoz and Solano (2014) and Anaya and Bueno (2015) reported the results for cassava starch hydrolysate concentration using the same equipment, showing that their samples’ turbidity was not affected.
The effect of VRF on sample color was analyzed by absorbance at 440 nm, similar to Benavides-Prada and Muvdi-Nova (2014); at this wavelength, the transmitted or reflexed color is yellow (Harris, 2007). All the samples also were diluted to the initial value (VRF = 1 as a baseline). As whey is concentrated, absorbance increased slightly; after running an average of 18 cycles (figure 5). However, Tukey’s test shows that there is not a significant difference among mean values. Absorbance changes in the visible spectrum are related to color alteration and product thermal degradation (browning degradation).
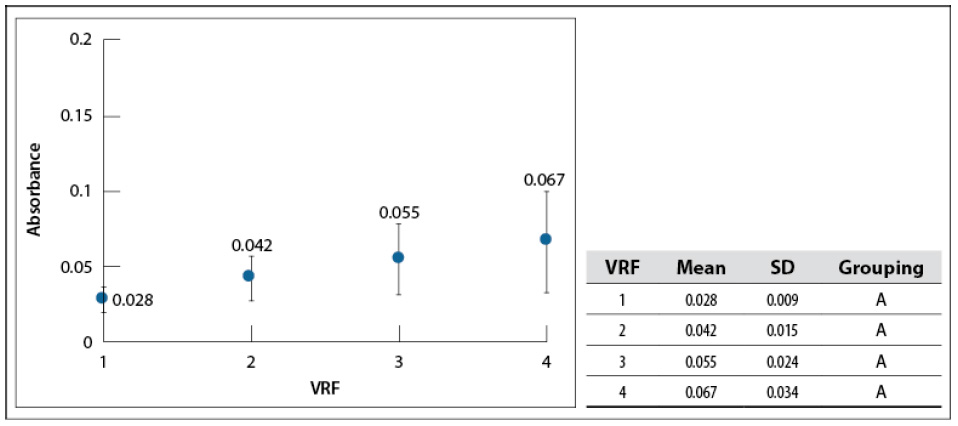
Source: Elaborated by the authors
Figure 5 The sample’s absorbance concentrates for each VRF at 200 mbar, 60–65 °C, and L=440 nm. SD: standard deviationNote: Mean values that do not share a letter are significantly different. Information was grouped using Tukey’s test and 95% CI.
Benavides-Prada and Muvdi-Nova (2014) studied glucose syrups concentration using a rising film evaporator. They reported absorbance increased from 0.5 to 1.3 when running between 1 and 3 cycles, showing the low influence of falling film evaporation on whey color (associated with low browning degradation). One highlight of the obtained concentrates was the crystallization of some solids during storage at 4 °C. According to Luquet (1993), it is due to the formation of α-lactose monohydrate (crystals) at temperatures below 15 °C. This characteristic would facilitate further treatment stages, such as separation and drying.
Conclusions
The effect of falling film vacuum evaporation on acid whey permeate was analyzed, observing a linear behavior of the number of cycles regarding VRF, despite the difficulty for falling film formation. There was not browning degradation during the process, showing the low influence of this technique on the product. °Brix, protein, and ashes contents were not affected by the process and were directly proportional to the VRF. Turbidity changed considerably with the VRF due to the presence of insoluble denatured protein formed during the process; for this reason, it would be advisable to consider thermal treatment after ricotta preparation to remove the remaining protein. The use of anti-foaming agents is recommended to obtain a higher VRF.











 texto en
texto en