Introduction
Livestock breeding in the high Andean areas in Nariño, Colombia, is characterized by the base of its meadows made up of kikuyu grass Pennisetum clandestinum Hoechst Ex Chiov. (Poaceae), mainly in higher altitude zones where crops are restricted or impossible to establish (León-Guevara et al., 2014). Livestock management practices such as overgrazing and deficient fertilization cause high degradation of the pastures; so, establishing livestock systems affects biodiversity, modifies the balance of nutrients, increases compaction in a relatively short time (less than 2-3 years), reduces the volume of pore spaces, contributes to decreased biota, reduces the speed of water flow, and promotes erosion (Steinfeld et al., 2009).
Feng et al. (2003) reported that agricultural management practices affect soil quality and productivity. Their effect on edaphic microbial communities and impact on soil functioning is little known. Two approaches can be used to evaluate their impact on edaphic microorganisms: 1) monitoring the structural and functional diversity in the whole edaphic microbial community and 2) evaluating the specific functional groups connected with processes involved in the nutrient cycle, such as nitrogen-fixing bacteria, ammonium oxidizing bacteria (AOB), nitrite-oxidizing bacteria (NOB), and denitrifying bacteria (DB) (Coleman & Whitman, 2005). These studies provide information about the relationship between microbial diversity, ecosystem functions, and ecological interactions between functional groups, a priority in microbial ecology studies (Colloff et al., 2008; Malchair et al., 2010).
Moreover, De la Paz-Jiménez et al.’s (2002) previous studies have shown that microorganisms are highly sensitive to anthropogenic disturbances, responding in much shorter time scales than to physical or chemical ones. Some of the microbial parameters used include the functional groups of nitrifying bacteria; thus, the presence, absence, density, and activity of these organisms make them an excellent indicator to determine soil quality.
Therefore, the research objective is to quantify nitrifying bacteria as indicators of soil quality due to their high sensitivity to environmental changes and anthropogenic activities. They are evaluated in secondary forest (SG), traditional pasture (TP), and silvopastoral system (SP) using isolation, presence, and quantification methods to determine which land use has more significant presence of both AOB and NOB.
Materials and methods
Location
We conducted the research at the El Rincón farm in the Catambuco township, Pasto, at an altitude of 2,820 m a.s.l., an average temperature of 12 °C, and an average annual rainfall of 806 mm (Instituto de Hidrología, Meteorología y Estudios Ambientales [Ideam], 2016). Geographically, it is located at 01°08′12″N and 77°18′57″W. Its relief is mountainous and volcanic represented by high slopes and well- formed watersheds. Its soils are classified as Typic melanudands, developed from volcanic ash, very deep, well-drained, with a loamy to sandy-loam texture, whose pH varies between 4 and 5.7; besides, they have high organic carbon content, high cation exchange capacity, low phosphorus content, and moderate fertility (Corporación Autónoma Regional de Nariño [Corponariño], 2011; Instituto Geográfico Agustín Codazzi [IGAC], 2004).
Land uses
The soils in the high Andean region of Nariño are used differently; we prioritized three uses of the livestock area in this research, as described below.
Secondary forest (SF)
The nearly-27-year-old SF is characterized by native species such as chilca (Baccharis odorata Kunth (Asteraceae)), amarillo (Miconia polyneura Triana (Melastomataceae)), encino liso (Weinmannia rollottii Killip (Cunoniaceae)), pucasacha (Tibouchina mollis (Bonpl.) Cogn. (Melastomataceae)), and laurel de cera (Morella pubescens (Humb. & Bonpl. ex Willd.) Wilbur (Myricaceae)) in different successional stages and introduced species such as pine (Pinus patula Schltdl. & Cham. (Pinaceae)), Acacia decurrens Willd., Acacia melanoxylon R.Br. (Fabaceae), and alder (Alnus acuminata Kunth (Betulaceae)). No extraction or management practice was carried out since it is entirely intended for restoration and preservation.
Traditional pasture (TP)
TP comprises natural grasses such as kikuyu (Pennisetum clandestinum Hochst. ex Chiov. (Poaceae)) and improved grasses such as broadleaf plantain (Plantago major L. (Plantaginaceae)), red clover (Trifolium pratense L.), and Brazilian grass (Phalaris tuberosa L. (Fabaceae)). A living fence of A. acuminata bounds the area. The total pasture area is 12 ha, divided into 2,000 m2 lots, where agroecological practices such as applying biopreparations, molasses, and cattle manure take place. The pasture has been run for seven years and cattle enters every 40 days.
Silvopastoral system (SP)
The nearly-12-year-old SP occupies an area of 3.30 ha. It has a silvopastoral arrangement with A. acuminata sown at 3 × 3 m and natural grasses such as P. clandestinumand forage grasses such as P. major, T. pratense, dandelion (Taraxacum officinale F.H. Wigg. (Asteraceae)), and Lotus sp. (Fabaceae). The pastures are rotated every 40 days, and agroecological management practices such as applying organic compost, molasses, and cattle manure, are followed.
Experimental design
We employed a completely randomized design (CRD) in a 3 × 2 factorial arrangement with six repetitions, where Factor A was land uses (SF, TP, and SP) and Factor B sampling depth (0-10 cm and 10-20 cm).
Field phase
The sampling for the microbiological analyses involved selecting three points at random for each land use, in which we extracted a 20 cm long × 20 cm wide x 20 cm deep soil monolith using a shovel, separated into two sections (0-10 cm and 10-20 cm). From each section, the portion of the middle third (100 g of soil) was extracted. We placed the samples in self-seal pouches and transported them in polystyrene coolers to the Universidad de Nariño Biology Laboratory. They were kept at 4-6 °C for 24 hours, where serial dilutions and inoculation were performed in selective culture media (Gómez, 2008).
For the physical-chemical soil analysis, 15 sites were randomly located for each land use following a zigzag pattern. The subsamples were extracted with an auger, selecting the soil portion of the middle, and homogenized. We took, a representative sample of 1 kg, place it in a self-seal pouch, and took it to the Universidad de Nariño Soil Laboratory for analysis.
Isolating nitrifying bacteria
For isolation, 100 g of soil were used for each treatment. In isolating nitrifying bacteria, we employed two culture media: ammonium broth and nitrite broth, following Schmidt and Belser’s (1994) most probable number (MPN) method. This technique involves determining the presence or absence (positive or negative) of particular attributes of microorganisms in soil samples or other environments in replicates of consecutive dilutions, as modified by Moreno et al. (2007). For this purpose, dilutions were made until the microorganism concerned was not detected. We used three tubes as replicates for each dilution and seeded them in a liquid culture medium. The bacteria were incubated for 15 days in the Universidad de Nariño Biology Laboratory, a period with remarkable growth; they were subsequently determined and quantified.
Quantifying nitrifying bacteria
A test with the Griess-Ilosvay reagent, which uses diazotizing reagent and coupling reagent, was performed to detect NO2- caused by the oxidative action of AOB from NH4+; this indicator turns fuchsia for positive tubes. The nitrate dye test or diphenylamine test was performed to observe NO3-, derived from the oxidative activity of NOB from NO2-, adding a nitrate dye reagent, whose indicator is dark blue in the form of a precipitate for positive tubes (Gallego, 2012).
The evaluation method considers that too pale colors in both tests may appear negative due to nitrite in insufficient trace amounts to develop the Azo dye’s stable and robust coloration. Similarly, in some cases, the absence or paleness of color is because the AOB oxidize ammonia into NO2-, but in some cases, the NOB can survive in the same culture medium, taking this ion and oxidizing it into NO3-, which limits the traces that can react with the Azo dye (Gallego, 2012).
The relevant readings and quantification of populations were expressed in CFU g/moist soil through humidity corrections; finally, we obtained the data expressed in CFU g/dry soil (Gallego, 2012) based on the guide table to quantify populations of nitrifying bacteria.
Results and discussion
Presence and quantification of AOB and NOB
We performed biochemical tests to isolate and incubate bacteria in differential media; we could determine AOB and NOB according to the resulting coloration, where violet or purple indicates AOB and blue NOB. The Andeva (table 1) showed highly significant differences (p < 0.005) for the three land uses (SF, TP, and SP), the two soil depths (0-10 cm and 10-20 cm), and the interaction between uses at different depths.
Table 1. ANOVA for AOB quantification, Pasto, Nariño, 2019

Note.** Highly significant statistical differences, p < 0.01
Source: Elaborated by the authors
In the case of NOB, highly significant statistical differences were found for the various land uses and depths (p = 0.0001); no statistical differences were found for the interactions (table 2).
Table 2. ANOVA for NOB quantification, Pasto, Nariño, 2019

Note.* Significant differences, p < 0.05; ** Highly significant differences, p < 0.01; ns: not significant
Source: Elaborated by the authors
Regarding land uses, we found the highest presence and density of AOB and NOB in TP and SP, with SF being the least abundant (table 3), possibly due to fertilization and the beneficial action of the organic fertilizer made of cattle manure and molasses. The latter become an essential pathway in recycling nutrients under suitable management conditions through their mineralization dynamics in the soil (Gaviria et al., 2015; Murgueitio & Calle, 1998). There was also a relationship with the results obtained by Enwall et al. (2007), who demonstrated that different types of fertilization have apparent effects on the activity and composition of nitrifying bacteria, resulting in a more active and diverse community.
Table 3. Tukey’s multiple comparison for AOB and NOB land uses (CFU/g), Pasto, Nariño, 2019
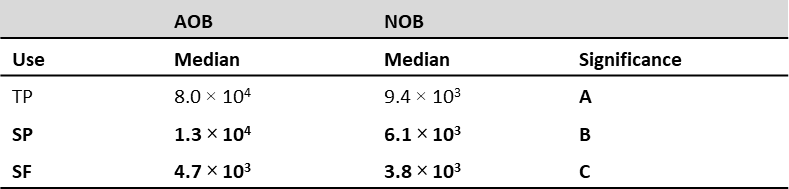
Note.Means with the same letter are not significantly different (p > 0.05)
Source: Elaborated by the authors
The Tukey’s multiple comparison test for both depths (table 4) indicates that the depth with the highest abundance of AOB was 0-10 cm, with 3.8 × 104 CFU/g, and the lowest 10-20 cm, with 2.8 × 104 CFU/g.
Table 4. Tukey’s multiple comparison for AOB and NOB soil depth (CFU/g), Pasto, Nariño, 2019

Note.Values with the same letter are not different (Tukey’s test p > 0.05)
Source: Elaborated by the authors
When comparing the population density of nitrifying bacteria (AOB and NOB) at both depths of land uses evaluated, we could determine that there was a greater number of nitrifying bacteria at 0-10 cm deep for the different land uses, results similar to those found by McNeill and Unkovich (2007). They stated that in the first 5 cm of soil depth, the mineralization process is carried out more efficiently thanks to the diffusion of oxygen; therefore, this surface is the most biologically active layer of the soil.
The soils where the research was carried out come from volcanic ash and, due to local climatic conditions, the values of organic material are higher in the surface horizon than in the subsurface ones. This creates ideal conditions for agricultural production, guaranteeing a good structure and important levels. The structure of their granules also benefits the aeration process, favoring microorganism growth and development (López, 2001).
The population density of AOB is higher at 0-10 cm deep as nitrification processes are carried out in this layer of soil due to oxygen; besides, because of the land slope, there are no water puddles. Agrochemicals are not applied, and paddock rotation is expected. Lata et al. (2004) indicated that the survival of nitrifying bacteria in environments with low concentrations of oxygen would depend on their ability to compete with plant roots and heterotrophic organisms, the addition of pesticides, and intensive management practices, altering the physicochemical and biological properties of the soil.
The highest population densities of NOB occurred in TP and SP. These land uses are characterized by high anthropogenic activity, mainly livestock breeding, in which the application of organic fertilizers is common. According to Murgueitio (2003), monocultures, chemical (NPK) and organic fertilizers such as chicken manure, and tillage are used in the pastures for massive production of livestock feed, encouraging microbial activity.
Nonetheless, nitrification rates have increased in agroecosystems such as grasslands, where the pressure for crop productivity demands an intensive use of fertilizers, leading to consequences such as nitrogen leaching, water and atmosphere pollution (Liu et al., 2013; Subbarao et al., 2012).
According to the results obtained, the SP land use exhibited a high presence and quantity of AOB and NOB, probably because this type of soil, unlike conventional production systems, is highly diversified and self-sufficient. It also produces positive impacts on soil quality that, together with high tree cover and scarce conventional agricultural production practices such as tillage and burning, favor natural processes and biological interactions. In turn, fundamental ecosystem processes, such as the nutrient cycle, biological control, C sequestration, edaphic structure maintenance, fertility, and productivity, are benefited (Gómez, 2008).
Meanwhile, when comparing the populations of AOB and NOB in the evaluated land uses, we determined that SF had the lowest populations; the low counts of nitrifying bacteria in this cover could be because these sites are characterized by a greater amount of organic matter, which can indirectly inhibit nitrifying bacteria (Bruns et al., 1999). These bacteria are inhibited for being chemoautotrophs, which have high specificity for the carbon source and available inorganic energy.
This finding agrees with Brouwer et al.’s (2006) research in the thick forests of Falmouth, Massachusetts (USA), who observed lower nitrification rates in comparison to agricultural soils, possibly due to a high concentration of carbon in the fallen leaves and lignin in the woody material. Lignin is structurally resistant to decomposition, thus allowing the carbon content not to vary in forest soils.
The lower population of nitrifying bacteria in forests, as reported in this study, may also be caused by the allelopathy process characteristic of these covers, in which compounds such as tannins that influence forest development and prevent nitrate loss in the ecosystem are released (Blanco, 2006). The NOB reported in this research had low population density in the SF, which may be related mainly to the low amount of substrate from AOB’s oxidation of NH4+ in this cover.
Due to the great diversity of woody and semi-woody species and the complexity of interactions within the ecosystem, SFs may be inhibiting nitrifying bacteria, as affirmed by Lata et al. (2004). There is also a direct relationship between the release of allelopathic compounds and the inhibition of the growth of nitrifying bacteria in forests.
The results reported in the literature are related to those obtained in the TP and SP covers, in which organic fertilization is carried out with compost of cattle manure and biofertilizers applied by the producer. This agronomic practice contributes to high densities of nitrifying bacteria.
The interaction between land use and the sampling depth of AOB was evaluated through the Tukey’s test (table 5); note that the land uses at both sampling depths show statistical differences. The highest abundance is reported in TP, with 8.8 × 104 CFU/g, at a sampling depth of 0-10 cm, followed by the same land use but at 10-20 cm deep, with 7.2 × 104 CFU/g, while SP exhibited an abundance of 1.7 × 104 CFU/g at 0-10 cm deep. On the contrary, SF at a depth of 10-20 cm had the lowest abundance of AOB, with 1.3 × 103 CFU/g.
Table 5. Tukey’s multiple comparison for land use * depth of AOB (CFU/g), Pasto, Nariño, 2019

Note.Values with the same letter are not different (Tukey’s test p > 0.05)
Source: Elaborated by the authors
These results agree with Murgueitio’s (2003) studies, in which the highest AOB counts were found in pastures; this behavior could be associated with modifications in the environment due to agricultural activities. Accordingly, soils with agronomic use show high nitrification rates, which can be attributed to the high use of external inputs, mainly nitrogen fertilizers. When added to the soils, they accelerate C transformation during the oxidation of organic matter into carbon dioxide (CO2) and increase microorganisms’ mineralization of organic nitrogen into mineral nitrogen (NH4+), offering a favorable environment for the growth of nitrifying bacteria (Bruns et al., 1999).
The changes in the microbial communities of functional groups can be explained by the effects produced by the metabolic products of cattle urine and feces. They possibly favor the growth of nitrifying bacteria because animal urine contains urea, which is hydrolyzed into ammonium carbonate, and the feces contain large amounts of protein material, which are converted into NH4+ by saprophytic bacteria under aerobic or anaerobic conditions (Murgueitio, 2003). Urine and feces promote bacterial population growth since they are a necessary input for the growth of AOB, being their primary energy source (Pacheco et al., 2002). Therefore, the ammonium concentration is one factor that most affects the community of nitrifying bacteria (Le Roux et al., 2008).
Conclusions
The population density of AOB and NOB at different soil depths is affected by the vegetation cover and its management. The highest values of CFU gram-1 for the two biological groups studied occurred in TP and SP with A. acuminata and P. clandestinum. Furthermore, nitrifying bacteria (AOB and NOB) are found in a more significant proportion at a depth of 0-10 cm since the most biologically active layer is in the surface soil. In the forest cover, the density of AOB and NOB was low; besides, the bacteria’s sensitivity to humidity, poor aeration, and high organic matter content became evident.











 texto em
texto em 



