Introduction
The use of organic and inorganic products as soil amendments in agricultural production is a strategy to turn waste into resources. The addition of organic matter amendments such as compost, fertilizers, agricultural residues, biochar, and lime is a common practice for the immobilization of heavy metals and improve contaminated soils (Angelova et al., 2010).
In Colombia, mainly in the departments of Antioquia, Bolívar, Chocó, and Córdoba, there are a large number of sites highly contaminated by heavy metals from precious metal mining, with more than 12,400 mines in operation and around 50,000 people who are influenced directly or indirectly by this work (Agencia Nacional de Minería, 2017). The amount of mercury released into the environment from this activity has been estimated at about 80-100 tons per year (Unidad de Planeación Minero Energética, 2014).
These pollutants need to be treated as they generate changes in the soil that affect its quality and productivity, and they can transform into more toxic species when entering the trophic chain causing severe problems to human and environmental health (Marrugo et al., 2010). In situ stabilization of heavy metals is one of the most common, most efficient, and low-cost soil recovery methods. In this method, additives are applied to the soil to reduce the mobility and bioavailability of heavy metals (Madejón et al., 2018).
The consumption and marketing of vegetables play an important role in the local family basket; these are used for self-consumption and constitute one of the most common food sources (Instituto Colombiano Agropecuario, n. d.). However, horticultural crops in the Colombian Caribbean region are more susceptible to damage due to the influence of heavy metals (Agencia Nacional de Minería, 2017).
Accordingly, there is a need to mitigate the toxicological risk of heavy metals in crops susceptible to them by applying amendments to soils contaminated by mining. In this study, the evaluation of amendments (biochar, vermicompost, and lime) was carried out in contaminated soils in the south of the department of Bolívar (Colombia), with the aim of minimizing the mobility of mercury, lead, cadmium, and arsenic in these soils.
Materials and methods
Soil sampling
The soils for the tests were obtained in the mining district of Barranco de Loba, south of Bolívar, at coordinates 08°56′44″ N and 74°06′21″ W. The mining soil was obtained through random sampling in an approximate area of 50 m2 located 3 km from the mining area. Sample collection was carried out according to the parameters established by Instituto Geográfico Agustín Codazzi (2007).
Physicochemical analysis of the soil
The samples were dried at room temperature (32 °C), crushed, sieved, and mixed. A sample of the processed soil was taken for its chemical characterization, to determine its physicochemical properties (Cao et al., 2010). The pH analysis was carried out following the potentiometric method (Rosas-Patiño et al., 2017). The analysis of the physicochemical properties and the classification of the soil type were carried out as follows: texture by the Bouyoucos method; organic matter (OM) through the Walkley-Black method; total phosphorus (P) employing Bray II; sulfur (S) by extraction with monobasic calcium phosphate and determination by colorimetry; exchangeable bases (Ca, Mg, Na, and K) utilizing extraction with standard ammonium acetate with a pH of 7.0; Ca and Mg were quantified by atomic absorption, and Na and K through atomic emission spectrophotometry (Cao et al., 2010). Heavy metals (Cu, Fe, Zn, Mn, Pb, Cd, As, and Hg) were analyzed using the EPA 3051ª method (United States Environmental Protection Agency, 1997).
Bioavailability of metals in soils
The extraction of heavy metals was carried out in the soil samples without separating the particles according to their diameters. The dry soil was pulverized (in a ball mill) and weighed in duplicate (1 g) in a 125 mL Erlenmeyer flask with a 0.01 M CaCl2 extracting solution (Vázquez et al., 2016). Metal extraction was carried out on a mechanical shaker at 250 rpm for two hours. Subsequently, it was centrifuged, taking the supernatant and filtering it in plastic funnels with Watman paper in 25 mL volumetric balloons, which were transferred to plastic vials and refrigerated at 4 °C. Quality control was carried out by preparing three reagent blanks under the same conditions as the samples. Lead, cadmium, arsenic, and mercury readings were taken on a Thermo Electron Corporation, ICE-3500 atomic absorption spectrometer with airflow-acetylene, acetylene/nitrous oxide, and Zeeman background corrector when operating in the graphite oven mode (United States Environmental Protection Agency, 1997).
Experimental design
This research was carried out between the months of January and December 2017 in a mesh house located at Universidad de Córdoba (Colombia), with an average temperature of 30.2 °C and relative humidity of 78- 88 %. The amendments and their doses were applied based on the soil analysis and the references of studies conducted with these types of amendments (Lemtiri et al., 2015); the soils without amendment were used as a control. Each system was established in pots of 2 L capacity and stabilized for 30 days, to later sow cucumber seeds. The treatments were carried out in triplicate using a completely randomized block design with two factors (amendment and dose) and three levels, for a total of 30 experimental units, including the control replicas.
Three types of amendments (biochar, vermicompost, and lime) were used; these were obtained from commercial products. The characterization of their pH, electrical conductivity and concentrations of heavy metals (Hg, Pb, As, and Cd) was carried out. The doses of the amendments were: biochar: D1 = 0.5 %, D2 = 2 %, D3 = 4 %; vermicompost: D1 = 5 %, D2 = 10 %, D3 = 15 %, and lime: D1 = 0.5 %, D2 = 1 %, D3 = 2 %. Once the treatments were established and the stabilization period had elapsed (30 days), soil samples were taken from each, dried at room temperature and homogenized to later determine the physicochemical parameters (pH, OM, cation exchange capacity [CEC], texture, and heavy metals).
Quality control
For the analyzed metals, the relative standard deviation was below 6 % in all cases; the coefficient of determination of the quantification systems remained above 0.995, and the analysis of the controls for each sample batch recorded absorbances with exponential figures of 10-3 and 10-4. The certified reference material SQC001-30G (metals in soils) was analyzed in triplicate to verify the traceability of the methodologies (Pb = 145 ± 3.81 mg/kg; Cd = 224 ± 3.64 mg/kg; As = 123 ± 1.90 mg/kg; and Hg = 4.59 ± 0.0778 mg/kg). Recovery percentages achieved ranged between 90 and 110 %.
Statistical analysis
The results of the analyzes are presented as the arithmetic mean of the determinations in duplicate ± standard deviation. When it was necessary to involve concentrations below the detection limit in the statistical analysis, a value equal to half of this value was used (Smeti & Golfinopoulos, 2015). To evaluate the existence of significant differences between the mean concentrations of metals in the soil, an analysis of variance (Anova) was used. When the Anova yielded statistically significant differences, Dunnett's test was used, according to the treatments at different doses (Miller & Miller, 2002). The significant difference (p < 0.05) between the metal values in different amendments and doses was calculated using the Statgraphics Plus software, version 5.0.
Results and discussion
Physicochemical properties of the experimental soil and effect of the application of amendments
The soils obtained in the Barranco de Loba municipality, Bolívar department, are characterized by suffering high erosion and are classified as oxisols and ultisols of sedimentary origin. The physicochemical properties of the original soils are shown in table 1. The data show that the original soil (control) presents a silty clay texture, and therefore, it is considered a heavy soil with poor structural development, where root growth and the root exploration area is highly restricted (Orozco-Rodríguez & Muñoz-Hernández, 2012). Likewise, a moderately acidic reaction is evidenced, with a pH slightly above 5.5 due to the presence of aluminum and high sulfur content. This is why it has a high tendency to mobilize nutrients and other chemical species, such as the heavy metals analyzed and reported by Anjanappa et al. (2012).
In these soils, the OM content is low (Orozco-Rodríguez & Muñoz-Hernández, 2012), so the CEC is due to the presence of a high percentage of clays, normal percentages of interchangeable bases of Ca, Na and Mg, and Ca/Mg ratio from normal to broad. However, the Mg/K ratio is higher than 18, reflecting a K deficit (Orozco-Rodríguez & Muñoz-Hernández, 2012). A moderate content of P and levels of microelements with excessive amounts of Fe and Zn are also observed; this characteristic agrees with the pH values, since it is possible to find a high solubility of minor elements in these soils. From the mineralogy standpoint, significant fractions of apatites and hematites are identified, reflected in the levels of Ca and P recorded in the analysis.
Once the soils were amended, and after a 30-day stabilization period, the physicochemical analyzes showed an improvement in their conditions. One of the parameters with the highest change was pH, reaching values above 6 in all treatments, and around neutrality when it was amended with a higher percentage of material.
Table 1. Physicochemical soil parameters and treatments in Barranco de Loba, Bolívar
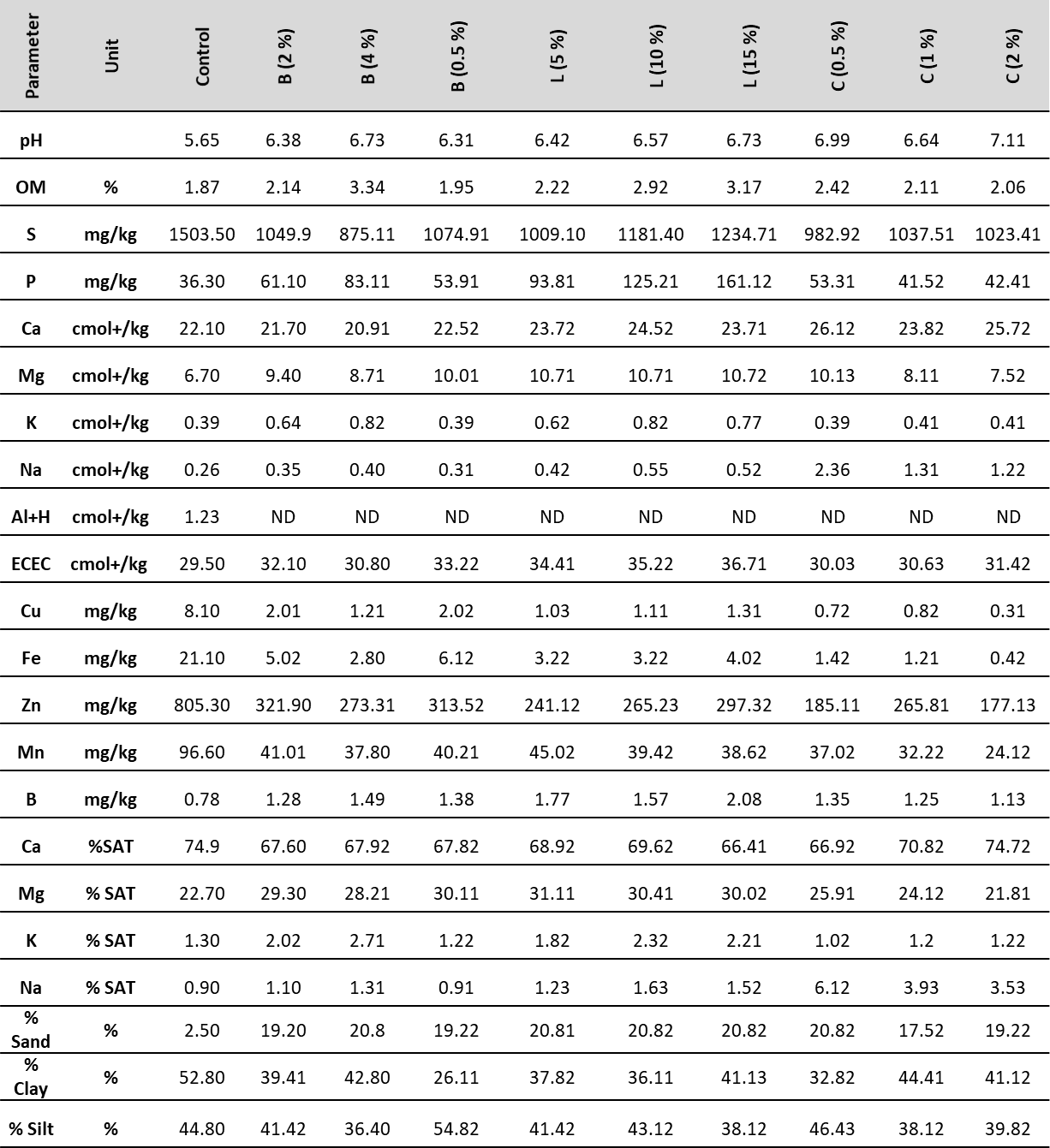
Note:B: biochar; L: vermicompost; C: lime; OM: organic matter; ECEC: effective cation exchange capacity; ND: not determined
Source: Elaborated by the authors
It should be noted that with the use of lime, the soil showed the highest rise in pH, due to the alkaline nature of this material. In addition to being a regulator par excellence of soil pH, lime facilitates the use of nitrogen fertilizers, corrects acidity, provides the necessary calcium for the growth and development of crops, increases soil fertility, and neutralizes the toxic and acidifying effect of some mineral elements such as iron and aluminum (Lehoux et al., 2013).
On the other hand, various studies indicate that just like lime, the use of organic amendments tends to increase pH levels. For vermicompost, this increase is associated with its use in large masses and the power of attraction of H3O+ ions by negatively charged chemical groups (Mahar et al., 2015). In the case of biochar, its alkalizing effect on soils is due to the low presence of functional groups with H and O, and its high aromaticity (Ahmad et al., 2014).
Another noticeable effect after the addition of amendments was the increase in OM, mainly when vermicompost and biochar were used, due to their high percentages of organic compounds such as humic and fulvic acids. This increase in OM content is also reflected in the increase in CEC, reaching values higher than 35 meq/100 g in treatments with higher vermicompost doses. In general terms, the treated soils show a good nutritional offer and, according to the physical and chemical characteristics, it can be inferred that they are suitable for agricultural use. The use of amendments, especially organic, seems to be a good option considering that their properties contribute to improving the physical and chemical conditions of the soils.
Total concentration of heavy metals in the soil and their treatments
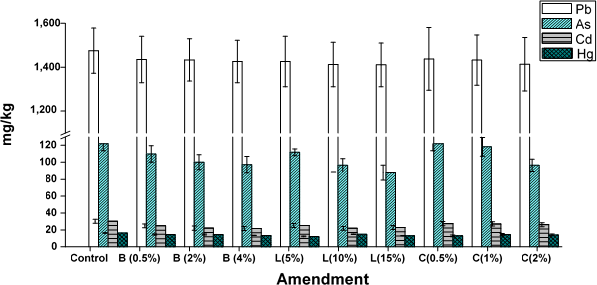
The total concentrations of heavy metals (Pb, Cd, As, and Hg) in the evaluated soils and treatments are shown in figure 1. As these soils are exposed to artisanal gold mining, they show high contents of heavy metals, whose accumulation is influenced by physicochemical properties such as soil acidity, OM composition, and texture (Guzmán & Barreto, 2011). It is highlighted that textures with high percentages of clay favor ion exchange in the colloidal micelle.
In general, the concentrations of heavy metals in the different treatments were slightly lower than those found in the control soil. This difference can be attributed to the metal leaching process during the stabilization stage or its dilution during the preparation of different treatments, evidenced in the slight tendency of the metal concentration to decrease as the dose of the amendment increases.
A statistical analysis was performed using Dunnett's test to assess whether there were significant differences between the treatments and the control. Differences were only established between the control and the concentration in the treatments for Cd and As. Obaji and Romero (2017) found that, for the mining soils of the Barranco de Loba district, Cd and As are the metals with the highest mobility, even after the addition of organic amendments, such as biochar and vermicompost.
Source: Elaborated by the authors
Figure 1. Heavy metal concentrations in the control soil and in those with amendments, Barranco de Loba. B: biochar; L: vermicompost; C: cal.
The heavy metal concentrations presented the following sequential order: Pb > As > Cd > Hg, and were well above the values found in other studies carried out in Colombia. Hg concentrations were higher than those found in other studies carried out in southern Bolívar by Pérez-Vargas et al. (2014) in the Santa Cruz mine (7.81 and 3.55 mg/kg) and by Argumedo et al. (2013) in Barranco de Loba (6.91 and 3.13 mg/kg). According to the work of Yacomelo (2014), in several municipalities of the Atlántico department, the concentrations of As in the current study were much higher than the maximum values recorded in the municipality of Santa Lucía (29.63 mg/kg) and also the Pb and Cd concentrations in the municipality of Campo Cruz (17.00 mg/kg and 8.77 mg/kg, respectively). In the department of Córdoba, in the middle and lower valley of the Sinú River, the concentrations ranged between 0.023 and 0.132 mg/kg for Pb and from 0.008 to 0.077 mg/kg for Cd (Roqueme et al., 2014); these ranges are very low in comparison with those recorded in the present study. These soils can be considered dangerous due to their metal content.
Conversely, heavy metal concentrations are high compared to the reference values established by the Canadian Environmental Quality Guidelines for soils in different uses (Hg: 6.6 mg/kg, Cd: 1.4 mg/kg, As: 12 mg/kg, and Pb: 70 mg/kg, for agricultural use), and even higher than those established by the German federal law through Ordinance BBoSchG/1999 (Martínez & González, 2017) for clayey soils (Hg: 1 mg/kg, Cd: 1.5 mg/kg, As: 20 mg/kg, and Pb: 100 mg/kg).
In general, the vast majority of metals were retained considerably after the stabilization period with the amendments used. In the case of vermicompost and biochar, this may be because these materials are known for their high capacity to accumulate large amounts of organic and inorganic contaminants (Kushwaha et al., 2016).
Vermicompost generates dissolved organic matter (DOM), which undergoes a process similar to that of oxides and silicates present in the clayey fraction (an effect due to the pH) by developing a negative charge thanks to the dissociation of acid groups in the polymer molecules that compose it. This negative charge is capable of retaining cations through electrostatic forces. The functional groups present in humic polymers are capable of complexing transition metal cations. Similarly, the carboxyl, amino, phenolic, and sulfhydryl groups present in biochar have the facility to donate electrons and act as transition metal ligands —that are electron acceptors— to form very stable coordination compounds. Thus, polydentate bonds that decrease the bioavailability of heavy metals for plants can be established (Dunham-Cheatham et al., 2015).
Lime amendment, on the other hand, has been used for centuries to increase soil pH and, in this way, reduce the absorption of metals by crops (Derakhshan et al., 2018). Reports of success in liming with 1 % calcium hydroxide are available for field studies. In experiments with pots, Lehoux et al. (2013) found a significant reduction in Cd and Zn concentrations. However, Sagardoy (2011) observed a decrease in Cd and Zn concentrations in spinach by 80 % and 75 %, respectively.
Effects of amendments on the bioavailability of heavy metals in the soil
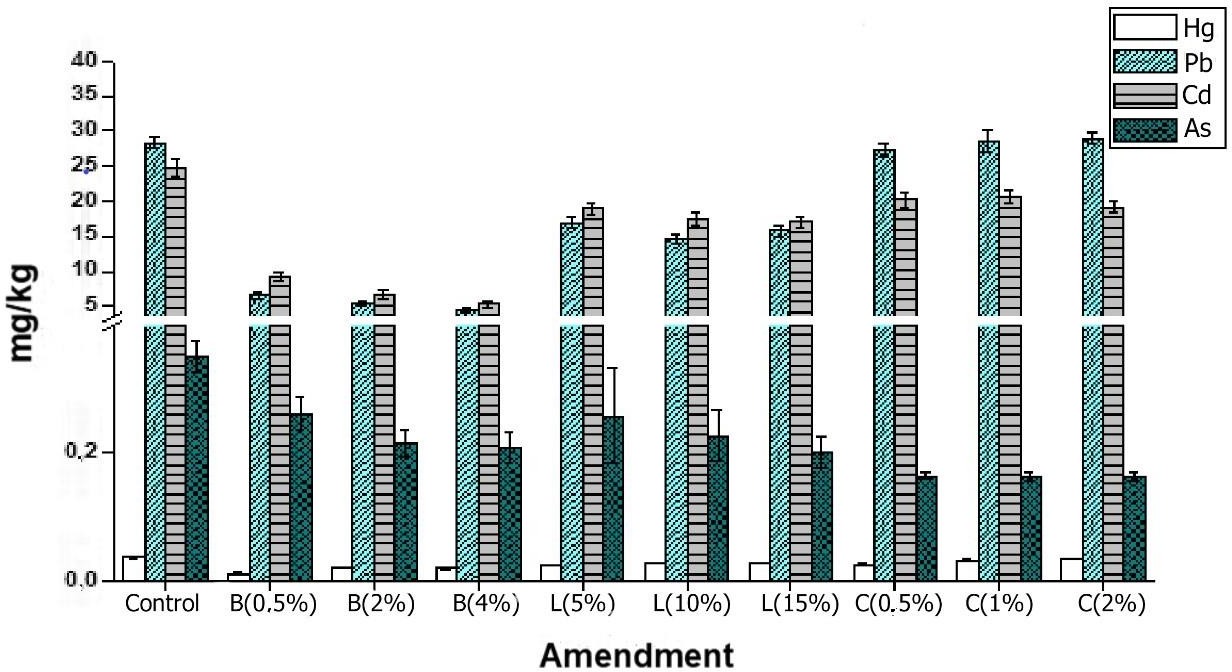
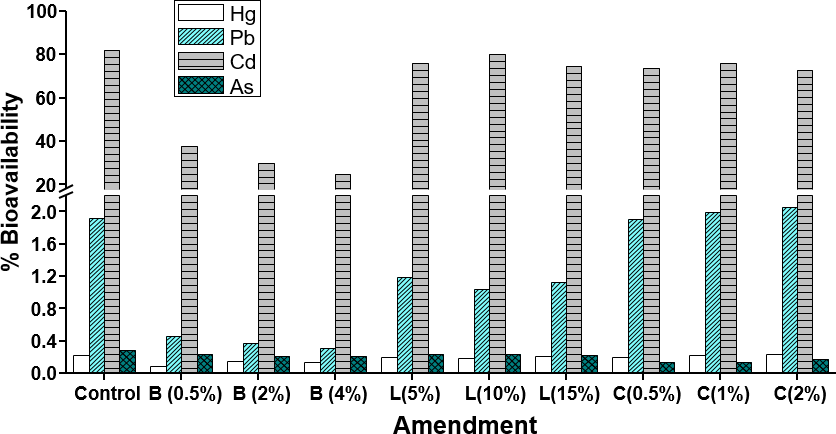
It is well known that the total concentrations of heavy metals do not indicate their mobility and bioavailability in the soil due to the complex distribution of these metals among the various chemical species and soil constituents (Prieto et al., 2009). To measure the bioavailability of heavy metals in the soil, the methodology described by Pueyo et al. (2004), which employs a CaCl2 extractant solution, is used. The bioavailable Pb, Cd, As, and Hg contents and their percentage fraction in the soils subjected to different treatments are shown in figures 2 and 3.
The metals with the highest bioavailable concentrations are Pb (29.04 mg/kg) and Cd (24.72 mg/kg), followed by As (0.35 mg/kg) and Hg (0.037 mg/kg). It should be noted that the metal with the highest percentage of bioavailability is Cd (more than 80 %) for the control soil, followed by Pb, As, and Hg, with percentages lower than 2.1 %. The previous shows that these three metals are not found in their bioavailable forms in these soils, so there is a low probability that the pollutant will move into the environmental compartments and interact with organisms, showing toxicity in these.
Several studies carried out in soils with mining vocation have found that the highest percentage of metals are not bioavailable (60-90%), a fact that is attributed to the association of metals with the mineralogical fraction of the soil (Smith et al., 2012). On the other hand, heavy metals show a strong tendency to bond with organic matter, Fe and Mn oxyhydroxides, and clay minerals (Duarte et al., 2019). However, since these soils show low concentrations of OM, Fe, and Mn, a mineralogical source with high concentrations of heavy metals can be assumed. In fact, this distribution is typical of the soils affected by mining since the parental material extracted from the subsoil is dispersed in the surroundings where this activity takes place (Smith et al., 2012). It should also be noted that the values of bioavailable concentrations are low, and the distribution of the metal in the soil solution could be determined by the contents of OM, CEC, pH, and content of Fe and Mn oxyhydroxides.
Source: Elaborated by the authors
Figure 2. Bioavailability in mg/kg of heavy metals in different phases of the control soil and in those with amendments. B: biochar; L: vermicompost; C: lime.
Source: Elaborated by the authors
Figure 3. Bioavailability percentage of heavy metals in different phases of the control soil and in those with amendments. B: biochar; L: vermicompost; C: lime.
In the case of Cd, its high bioavailability compared to other metals is possibly due to its low affinity for adsorbent forms in the soil, determined by its small atomic size and low electronegativity, limiting its ability to interact with active sites of adsorbent particles and make it easily absorbable and assimilable by plants grown in this type of soil (Seshadri et al., 2015).
The amendment that showed the highest reduction in bioavailability was biochar for Pb, Cd, and Hg, with reductions between 50 and 84 % with respect to the control. In the case of As, the highest reductions were obtained with the lime treatments, possibly because the pH values of the soils treated with this amendment reached values close to 7, favoring the retention of this metalloid by the Fe and Mn oxyhydroxides. For Pb and Hg, there is no significant effect on the bioavailability after the addition of lime compared to the control (p > 0.05), despite the tendency of lime to increase the pH and, therefore, decrease the mobility of heavy metals. In this case, a longer stabilization time is suggested to accentuate the effect of this amendment on the availability of these metals (Seshadri et al., 2015).
With vermicompost, significant reductions in the metals evaluated in relation to the control (p < 0.05) are observed, especially in Pb. It is well known that this metal has a high affinity for OM, given the large CEC provided by the high negative charge of organic particles and the presence of various reactive ligands that carry O, N, and S (Lee et al., 2016). However, in many cases, organo-metallic complexes are formed, facilitating the solubility of the metal, since microorganisms can degrade them and, thus, favor the mobility of metals.
Finally, only a slight decrease in bioavailability is observed when increasing the dose level of the amendment for Cd and Pb with biochar, and for As with lime; however, these differences are not significant (p > 0.05).
Conclusions
In this study, the initial soils presented high heavy metal contents due to their exposure to artisanal gold mining. Cd, Pb, and As are the metals with the highest mobility, even after the addition of organic amendments, such as biochar and vermicompost; Cd is the one with the highest bioavailable fraction with respect to the total concentration.
The metal with the highest bioavailability percentage is Cd (> 80 %) for the control soil, followed by Pb, As, and Hg, with percentages lower than 2.1%. The use of amendments, especially biochar and vermicompost, shows a positive effect on the immobilization of metals in these soils, indicating a low probability that the pollutant will mobilize in the environmental compartments and interact with the organisms showing toxicity in these.
The use of lime only showed positive effects on the immobilization of As and Cd. The amendment that showed the highest reduction in bioavailability was biochar for Pb, Cd, and Hg, with decreases between 50 and 84 % of the bioavailability of these metals in the soils studied.











 text in
text in 



