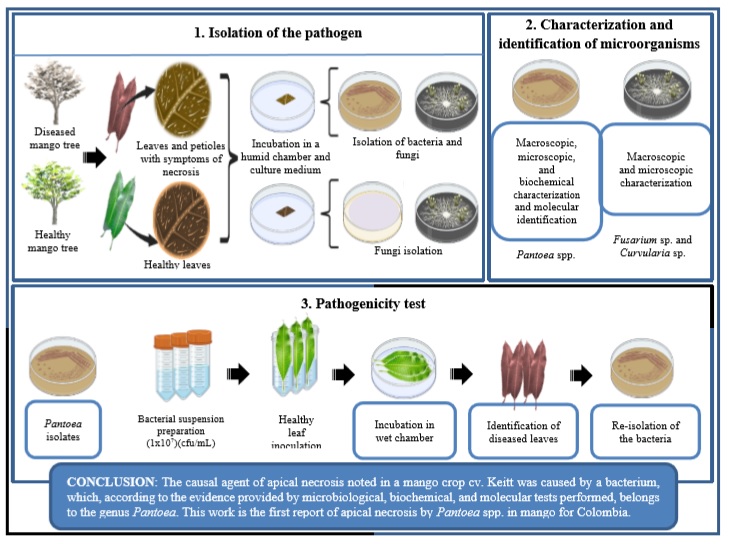
Introduction
Mango, Mangifera indica L. (Anacardiaceae), is a fruit tree species of significant commercial importance due to its nutritional properties, organoleptic value and economic potential (Matheyambath et al., 2016). Due to its adaption and development in different edaphoclimatic conditions and ecological niches, mainly in warmer climates, crops are distributed worldwide and cultivated in most tropical and subtropical regions (Guerra et al., 2018; Rodrigo-Comino et al., 2014). The production for 2017 was estimated at 40 million tons (t) according to the United Nations Food and Agriculture Organization (FAO, 2017), where 74% came from Asia, 15% from Africa, and 11% from Latin America and the Caribbean (Altendorf, 2017).
In Colombia, mango plantations can be found in 20 provinces with a harvested area of about 28,000 hectares (ha) and a production of about 321,000 t, Cundinamarca and Tolima (Central Zone) and Atlántico (North-western Zone) being the most prominent producers (Information and Communication Network of the Agricultural Sector [Agronet], 2018). These provinces contribute 30, 20, and 11% of the nationally produced mangoes (Agronet, 2018). On the other hand, the province of Cesar (North Xone) has a total harvested area of 1,400 ha, with a production of 10,400 t and an average yield of 7.4 t/ha (Agronet, 2018), rising as a potential region to hold this fruit tree crop (Durán et al., 2014).
Due to the growing population, demand for this tropical fruit has increased daily, requiring increased yield and productivity to support such demand (Altendorf, 2017). However, according to the United Nations Conference on Trade and Development (UNCTAD, 2015), producers face multiple challenges to achieving this, including climate variability, variations in precipitation levels, disruption in rainy and drought periods, and the incidence of insecticides pests and diseases. The latter may cause significant disturbances in production and economic losses (Gil- Vallejo et al., 2013).
The major diseases affecting mango foliage and fruit quality are of fungal origin. The ones considered of greater importance in Colombia are anthracnose disease (Colletotrichum gloeosporioides Penz.), drying or regressive death of branches and stems (Lasiodiplodia sp.), and vegetative malformation caused by Fusarium subglutinans [(Wollenw & Reinking) PE Nelson, Toussoun & Marasas] (García et al., 2017). Meanwhile, the bacterial black spot caused by Xanthomonas campestris Pammel (Gil-Vallejo et al., 2013) stands out among bacterial diseases. However, there are other major bacterial diseases such as bacterial apical necrosis caused by Pseudomonas syringae Van Hall and more recently associated with Pantoea spp. (Gutiérrez- Barranquero et al., 2019a, 2019b; Lee & Tzeng, 2006). This disease has been mainly reported in countries such as Spain, Portugal, Italy, Israel, and Australia, where it generates losses of up to 50% of mango crop yield (Gutiérrez-Barranquero et al., 2012).
Pantoea is a bacterium widely distributed in agricultural systems and natural environments (Delétoile et al., 2009), frequently reported as epiphyte and endophyte associated with different plant species and used as a possible biological control agent against fungal phytopathogens and bacteria (Walterson & Stavrinides, 2015). However, reports suggest that Pantoea has naturally transformed into a plant pathogen by acquiring pathogenicity plasmids (Manulis & Barash, 2003), producing substantial impacts on different crops, including fruit trees such as mango (Gutiérrez-Barranquero et al., 2019a, 2019b). The associated symptoms are necrotic lesions on buds and leaves, which extend through the petioles towards the stems. It is also common to see a white exudate in shoots and stems, though not always so evident. The white exudate has not been reported in fruits (Gutiérrez-Barranquero et al., 2019b).
Since apical necrosis in mango has not been reported in Colombia, the present research explores whether symptoms of this disease may be found in a mango cv. Keitt plantation under conditions of the Colombian dry Caribbean. Inferences were supported by microbiological analysis, basic biochemical tests, pathogenicity tests, and molecular identification. The study hypothesis is that Pantoea spp. may be an etiological agent of mango tree disease. This diagnosis is highly relevant since it will ensure a route of solutions and preventions to achieve integrated disease management.
Materials and methods
Location and environmental conditions
Symptoms appeared between September and October 2019 in two batches at the Colombian Corporation for Agricultural Research (AGROSAVIA), Motilonia Research Station, located in the municipality of Agustín Codazzi (Cesar), in an area of approximately 4,000 m.. Eighty-nine two-year-old mangoes (M. indica cv. Keitt) trees were planted at 250 trees/ha and managed under conventional chemical fertilization, drip irrigation, and phytosanitary chemical control scheme. The environmental conditions of the area during these two months were cumulative precipitation of 490 mm, relative humidity (RH) of 76%, an average temperature of 28 °C, and an of five hours sun per day (data provided by the weather station of the Instituto de Hidrología, Meteorología y Estudios Ambientales[IDEAM] located in the Motilonia Research Station).
Symptoms of the disease and management
A total of 40 trees had necrotic spots on the vegetative buds, petioles, ribbed leaves, and secondary branches of the upper third. In some cases, the production of white rubber-like exuded and milky consistency was observed (figure 1). Once the problem was evident, a phytosanitary pruning was carried out, which consisted of removing the affected parts of the plants. Removed tissues were stored in bags and taken from the field. During this process, branch-to-branch tool disinfection was made with poloxamer iodine at a concentration of 2.5 cm3/L and white vinyl mixed with a fungicide with active ingredient (a.i) mancozeb (2.0 g/L) and insecticide with a.i. chlorpyrifos (2.0 g/L). This mixture was used as a healing paste to cover pruning cuts. Subsequently, under the hypothesis that it was a pathogenic agent, chemical inputs were applied at intervals of seven days using fosetyl-aluminum (1.6 cm3/L) in rotation with carbendazim (2.0 g/L), Bordeaux mixture 1% (CuSO 4 + calcium hydroxide), and kasugamycin 7.5 cm3/L to reduce the severity of the attack, reduce the incidence of the pathogen, and prevent its spread. However, the improvement was only observed in eleven trees. The others continued with necrosis symptoms in the buds and petioles, advancing to the leaves through the central rib and leading to a descending death of the plants. The progress of symptoms was rapid and widespread, causing the death of 29 trees 30 days after the onset of the first symptoms.
Laboratory analysis
Sampling
Foliar and petioles samples were taken from affected trees to isolate the causative agent of the disease. The samples were washed with ordinary water for one minute and then with sterile distilled water (SDW) inside a flow cabin (Esco-Streamline ® II). Five 1 cm2 subsamples of the foliar sheet adjacent to the necrotic area were obtained. Cuts of the petioles (0.5 cm long) were also made.
Isolation, characterization, and identification of microorganisms
The foliar subsamples and petioles were superficially disinfected following the methodology proposed by Islam et al. (2013) with some modifications. Samples were submerged in 1.5% v/v sodium hypochlorite for one minute and washed with ADE. Then, they were immersed in 70% v/v ethyl alcohol for one minute and washed again with ADE. They were then deposited in a humid chamber (Petri dish with wet filter paper) and Potato-Dextrose Agar (PDA) growth medium and left for incubation at 25 °C for 72 hours. At the same time, we made observations using a stereoscope (Olympus SZ2, Tokyo, Japan). As a control of the procedure, foliar and petioles samples obtained from visually healthy trees were used and managed under the same experimental conditions. The bacterial and fungal colonies obtained were isolated in Nutrient Agar (NA) and PDA, respectively, for macroscopic and microscopic characterization (Gram staining for bacteria and lactophenol blue for fungi). In the case of bacterial isolations, biochemical characterization was performed by catalase and oxidase testing (Islam et al., 2013) and growth in cetrimide agar.
In addition, molecular identification was made by amplifying and sequencing the gene encoding for the 16S subunit of rRNA using the primers 27F 5’-AGAGTTTGATCCTGGCTCAG-3’ and 1492R 5’-GGTTACCTTGTTACGACTT-3’ (Weisburg et al., 1991). The 16S rRNA gene was amplified by PCR in a final volume of the reaction mixture of 12.5 μL using a final concentration of dNTPs at 0.8 mM, 0.8 μM of each primer, and 5 u/μL of Taq DNA polymerase recombinant Invitrogen. The PCR conditions consisted of an initial denaturation step at 94 °C for 2 min, followed by ten cycles at 94 °C for 15 s, 46 °C for 30 s, and 72 °C for 1 min, 20 cycles at 94 °C for 15 s, 50 °C for 30 s, and 72 °C for 2 min, and a final elongation cycle of 72 °C for 7 min. PCR products were sequenced by Sanger capillary sequencing system at the Molecular Genetics Laboratory, AGROSAVIA. A comparative analysis of the sequences obtained with NCBI sequences was carried out using the BioEdit sequence alignment editor. A distance tree was made from Neighbor-Joining analysis based on the partial sequences of the 16S region of rRNA. The Neighbor-Joining method was implemented with the MEGA 10.2.0 program using 1,000 bootstraps. The Bacillus subtilis IAM 12118 sequence was used as an outgroup.
Pathogenicity test of isolates under in vitro conditions
Bacterial isolations were sown in Petri dishes with NA and incubated at 25 °C for 48 hours. A sample of the colonies was taken and suspended in 1 mL of saline 0.85% w/v. From this concentrated suspension, 5 mL of inoculum was adjusted to a concentration of 107 CFU/mL.
The pathogenicity test was performed based on the methodology used by Sánchez et al. (2003), with some adjustments (disinfection of the plant tissue and cut and inoculation in the petiole of the leaves were carried out) using visually healthy young leaves obtained from mango trees (M. indica cv. Keitt). The leaves were superficially disinfected using 1.5% v/v sodium hypochlorite and 70% v/v ethyl alcohol. As part of the inoculation, three leaves were submerged in each bacterial suspension for 30 minutes, covering the entire petioles. Subsequently, they were left to dry in the flowing cabin for 30 minutes, deposited in the wet chamber (Petri dish with wet filter paper), and incubated at 25 °C for 21 days. A non-inoculated treatment was implemented to control the experiment, submerging the leaves in a saline solution of 0.85% w/v. In order to confirm the presence of inoculated bacteria in plant tissues, a re-isolation was performed from leaves and petioles with symptoms of necrosis. The methodology described above was implemented for this isolation as well.
Results
During a three-day observation period, the growth of hyphae on the tissues was noted. Hyphae were isolated in a PDA medium for further identification based on macro- and microscopic characteristics. Similarly, the presence of exudates on the surface of foliar tissue and colonies from infected petioles (figure 2) were identified, from which samples were taken to be sown in NA.

Source: Elaborated by the authors
Figure 2 Presence of exudates on the surface of foliar tissue (a) and growth of colonies in PDA from petioles of infected plants (b).
As a first result, two fungal morphotypes were isolated from foliar tissue. After five days of growth, the first morphotype was characterized by a woolly growth with a dark brown to black coloration and a slightly white border on the back. At the microscopic level, brown septate hyphae were observed, with straight and septate conidiophores, brown conidia with a curved appearance, rounded at the ends, with three well-defined septa. These characteristics made us infer that this isolate belonged to the genus Curvularia.
The second isolate presented a cottony colony of white color and a slight pink color after five days of growth. At the microscopic level, septate hyphae, short microconidia without septa and ellipsoidal, and elongated macroconidia, slightly curved with up to five transverse septa, were observed. These characteristics correspond to the genus Fusarium.
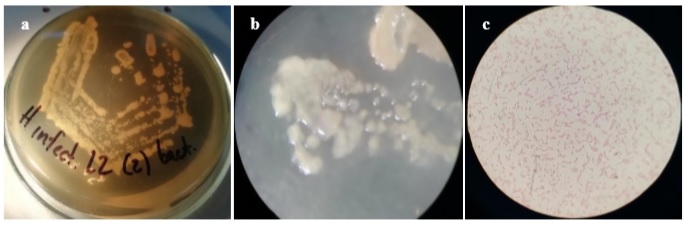
On the other hand, seven bacterial isolations were obtained from leaf exudates and petioles, all characterized by circular colonies with an entire edge, smooth surface, yellow-cream color, and bright. At the microscopic level, short, Gram-negative bacilli were observed without apparent grouping and the presence of spores (figure 3).
Basic tests for preliminary identification showed that the isolates exhibited positive catalase and negative oxidase, while growth in the cetrimide medium was positive, although reduced, with fluorescence under ultraviolet light.
Source: Elaborated by the authors
Figure 3 Macro- and microscopic observation of bacterial isolation obtained from infected tissues. Circular colonies with an entire edge, smooth surface, cream-yellow color (a, b), and short Gram-negative bacilli (c) without spores were observed.
The gene encoding sequences for rRNA subunit 16S showed that all isolations belong to the genus Pantoea with a 99% identity according to the BLAST algorithm of local alignments of the NCBI database (http://www.ncbi.nlm.nih.gov). The comparative analysis of the sequences obtained with NCBI sequences belonging to Pantoea spp. showed a cluster of six isolates with P. dispersa and one isolate with P. septica (figure 4).

Source: Elaborated by the authors
Figure 4 Distance tree performed from Neighbor-Joining analysis based on partial sequences from rRNA region 16S. The NCBI access numbers of the GenBank sequences for analysis are shown in parentheses. The numbers at each node represent the bootstrap obtained after 1,000 replicates. The scale is proportional to the number of nucleotide substitutions per site. • Isolations obtained in this study. The Bacillus subtilis IAM 12118 sequence was used as an outgroup.
In vitro pathogenicity test showed that all isolates generated some degree of necrosis in the petioles and foliar lamina after 21 days of wet chamber incubation (Figure 5), with similar lesions to those observed in the field. From samples of the affected tissue, it was possible to re-isolate inoculated bacteria, which grew around foliar tissue and petioles in the case of inoculated treatments, while control treatment (without inoculation) showed no evidence of bacterial growth (Figure 6). Macroscopic characterization showed that all isolates had circular colonies with an entire edge, smooth surface, yellow-cream color, bright appearance, and a Gram-negative microscopic level. In addition, all isolates were positive catalase and negative oxidase with fluorescence under ultraviolet light in a cetrimide medium.

Source: Elaborated by the authors
Figure 5 In vitro pathogenicity test on mango leaves, using the bacterial isolations. Inoculated leaves with different isolates (a-g); non-inoculated leaves (h). Typical symptoms of necrosis on leaves and petioles marked with a red arrow.

Source: Elaborated by the authors
Figure 6 Observation of plant tissues through the stereoscope. Inoculated leaf with evidence of bacterial growth (marked with a red arrow) around the petiole and foliar tissue (a); uninoculated leaf, with no evidence of bacterial growth in petiole and foliar tissue (b).
Discussion
The isolated fungi correspond to Curvularia and Fusarium according to the macro- and microscopic characteristics observed, which coincide with the reports by Rocha et al. (2018), Manamgoda et al. (2012), and Leslie and Summerell (2006), respectively. Although the genera Curvularia and Fusarium have been reported as plant pathogens, many of their species are part of the epiphytic microbiota of plants, where they behave like saprophytes (Omar et al., 2018; Rajputa & Rao, 2007), so their presence does not indicate that they are the cause of the disease. Additionally, the mango symptoms generated by pathogenic species under field conditions are different from those observed in this study.
Fusarium spp. has been reported as causing mango malformation disease (MMD), resulting in a loss of apical dominance in young plants and vegetative buds and deformed buds (vegetative malformation) with internodes and foliar lamina reduced (Chakrabarti, 2011; Crespo-Palomo, 2014; Kumar et al., 1993). In adult plants, MMD causes malformations in floral tissue, where inflorescences show highly branched, shortened, and thickened primary and secondary axes and an increase in the number of sterile flowers (Ploetz, 2003). As for Curvularia, there are no reports of diseases in mango. However, some authors suggest that in grasses and horticultural crops, the infection can give rise to chlorotic circular foliar spots (Baraona & Sancho, 2007), which were not observed in this case.
We infer that the causal agent of the disease was the bacterial isolates obtained from leaf tissue and petioles, which is supported by the fact that bacterial isolates were not obtained from healthy plants. Besides, the in vitro pathogenicity test on leaves reproduced, the symptoms observed in the field, and the bacteria were re-isolated from the inoculated symptomatic leaves.
Basic biochemical tests and nucleotide sequences of the 16S ribosomal RNA gene and the distance tree confirmed that these bacterial isolates belonged to Pantoea spp. In the last few years, much of the research performed on Pantoea has been based on the genome sequencing and analysis of strains isolated from different environments and with different lifestyles (Weller- Stuart et al., 2017). Nucleotide sequence analysis of the 16S rRNA gene has been used to reveal taxonomic discrepancies in the Pantoea genus (Rezzonico et al., 2009).
This bacterium is well known for its phytopathogenic characteristics and isolated from many ecological niches and hosts (Weller-Stuart et al., 2017). Pantoea has been reported as a common inhabitant of the phyllosphere and buds in mango plants (Lee & Tzeng, 2006) and other fruit crops such as melon (Cucumis melo L.), jackfruit (Artocarpus heterophyllus), and pineapple (Ananas comosus) (Abidin et al., 2021; Coutinho & Venter, 2009; Kido et al., 2008). Its association as a pathogen has also been demonstrated once it enters the tissues of the plant (Bomfeti et al., 2008; Weller-Stuart et al., 2017), mainly through injuries and in some cases through stomata, generating internerval angular necrotic spots, which can fuse and become dark (Gutiérrez-Barranquero et al., 2019). These symptoms were evident in the trees of this study.
Cazorla et al. (1998) suggest that climatic conditions such as low temperatures, low solar radiation, high relative humidity, and increased rains and winds could facilitate the spread of P. syringae, also reported as a causal agent of apical necrosis in mango (Rodrigo-Comino et al., 2014). Authors such as Hattingh et al. (1989) report that some diseases could also be favored when the host plant is under stressful conditions. This information is consistent with the conditions of the present study, where the disease arose in September and October. This period has high rainfall (accumulated of 490 mm), a high RH, an average temperature of 28 °C, and low solar radiation. Additionally, weeks before the first symptoms, the orchard went through a flood stress event for two days, which was quickly corrected by developing drainage channels. Still, this could have favored the spread of the disease caused by Pantoea spp. in the plant tissues, with the consequent onset of symptoms of apical necrosis (Cazorla et al., 1998).
It is also important to mention that the high mortality, in this case, may have been related to a high capacity to spread the disease coupled with a high susceptibility of young individuals (Campos & Calderón, 2015). Unfortunately, there are not sufficiently effective treatments for its control, so it is vital to take preventive measures such as protecting the plantations from the winds, avoiding excess moisture by maintaining good soil drainage, removing affected branches in the shortest possible time, and disinfecting the tools used in the pruning (Campos & Calderón, 2015). Once the diagnostic results are obtained, applications of the Bordeaux mixture are advisable on visually healthy plants to prevent the spread of the disease, as reported by Cazorla et al. (2006) and Gutiérrez-Barranquero et al. (2019), with positive results in terms of unaffected trees.
Although the mango cv. Keitt has been reported as less susceptible to apical necrosis (Cazorla et al., 2006), this study shows that this variety can also be significantly affected by the disease under the agroecological conditions of the Colombian dry Caribbean, consistent with Young (2008), who also reports cases of apical necrosis in this variety in Australia.
Conclusions
We conclude that the causal agent of apical necrosis found in a mango cv. Keitt orchard was caused by a bacterium, which, according to the evidence provided by microbiological, biochemical, and molecular tests performed, belongs to the genus Pantoea. This disease is characterized by the appearance of necrotic spots on buds and shoots that move to the leaves through the petiole, spreading rapidly, stopping the growth of trees, and eventually causing the death of young individuals. We infer that its incidence was favored by some climatic conditions such as high relative humidity and low temperatures and some abiotic stress conditions like flooding. This work is the first report of apical necrosis by Pantoea spp. in mango for Colombia. Knowing the etiology of the disease will allow the implementation of efficient management methods to reduce losses in crop productivity.