Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Medicina Veterinaria
Print version ISSN 0122-9354
Rev. Med. Vet. no.28 Bogotá July/Dec. 2014
Crystallization of Bovine Cervical Mucus at Oestrus:
An Update
Manuel E. Cortés1 / Fernando González2 / Pilar Vigil3
1 Licenciado en Ciencias Biológicas con Certificado Académico en Física Fundamental y doctor en Ciencias de la Agricultura, área Fisiología y Nutrición Animal, Pontificia Universidad Católica de Chile. Licenciado en Educación en Química y Biología y profesor de Estado en Química y Biología, Universidad de Santiago de Chile. Investigador asociado, Programa Teen STAR, Pontificia Universidad Católica de Chile. Investigador postdoctoral, Reproductive Health Research Institute (Chile). Profesor de Fisiología y Fisiopatología, Departamento de Ciencias Químicas y Biológicas, Universidad Bernardo O'Higgins, Santiago, Chile.
manuel.cortes@ubo.cl
2 Licenciado en Ciencias Pecuarias y médico veterinario, Universidad de Chile. Magíster en Producción Animal, Pontificia Universidad Católica de Chile. Profesor asociado, Departamento de Ciencias Animales, Facultad de Agronomía e Ingeniería Forestal, Pontificia Universidad Católica de Chile, Santiago, Chile.
fgonzalm@uc.cl
3 Licenciada en Medicina, médico cirujano, especialista en Obstetricia y Ginecología y doctora en Ciencias Biológicas, mención Ciencias Fisiológicas, Pontificia Universidad Católica de Chile. Estudios postdoctorales en el Texas Institute for Reproductive Medicine & Endocrinology, Estados Unidos, y en el Royal Women's Hospital, Australia. Directora del Programa Teen STAR Internacional. Directora médica del Reproductive Health Research Institute (Chile). Profesora asociada de la Pontificia Universidad Católica de Chile, Santiago, Chile.
pvigil@bio.puc.cl
Received: March 10th, 2014. Accepted: April 27th, 2014
How to cite this article: Cortés ME, González F, Vigil P. Crystallization of Bovine Cervical Mucus at Oestrus: An Update. Rev Med Vet. 2014; (28): 103-16.
Abstract
Bovine cervical mucus changes its biochemical composition and biophysical properties due to the variations in sex steroid levels during the oestrous cycle. As a consequence of oestrogen rise, cervical mucus is produced in larger amounts at oestrusa stage also characterized by an increase in mucus crystallization when observed under light microscopy. The objective of this article is to provide an updated review of the main aspects regarding crystallization of bovine cervical mucus. First, it makes reference to the composition of cervical mucus and the critical functions that this secretion exerts on bovine reproductive physiology, as well as in other species. Then, the article deals with the phenomenon of crystallization observed in cervical mucus, describing the main models used to classify the crystalline patterns observable in mucus at oestrus stage (some of them resembling ferns, palm leaves and stellar patterns, among others). Finally, it addresses the importance of the phenomenon of cervical mucus crystallization for the understanding of bovine reproductive physiology.
Keywords: Bovine physiology, cervical mucus, crystalline pattern, crystallization, oestrus.
Cristalización del moco cervical bovino en el estro:
una actualización
Resumen
El moco cervical bovino cambia su composición bioquímica y sus propiedades biofísicas durante el ciclo estral debido a las variaciones en los niveles de esteroides sexuales, siendo producido en mayor cantidad durante el estro como consecuencia del aumento en el nivel de estrógenos. En dicho estadio también se observa que el moco aumenta su capacidad de cristalizar. El objetivo de este artículo es proporcionar una visión actualizada de los principales aspectos relativos a la cristalización del moco cervical bovino. En primer lugar se hace referencia a la composición del moco y a las importantes funciones ejercidas por esta secreción en la fisiología reproductiva bovina y de otras especies. Más adelante el artículo trata sobre el fenómeno de cristalización observado en el moco cervical en estro, describiéndose los principales modelos utilizados para clasificar los patrones cristalinos observables en él (algunos semejantes a helechos, hojas de palma o figuras estrelladas, entre otros). Finalmente, se discute la importancia del fenómeno de la cristalización del moco cervical para la comprensión de la reproducción bovina tanto en estados fisiológicos así como fisiopatológicos.
Palabras clave: fisiología bovina, cristalización, estro, moco cervical, patrón cristalino.
Cristalização do muco cervical bovino no estro:
uma atualização
Resumo
O muco cervical bovino varia a sua composição bioquímica e suas propriedades biofísicas durante o ciclo estral devido às variações nos níveis de esteróides sexuais, sendo produzido em maior quantidade durante o estro como consequência do aumento no nível de estrogênios. Neste estado também se observa que o muco aumenta a sua capacidade de cristalizar. O objetivo deste artigo é proporcionar uma visão atualizada dos principais aspectos relativos à cristalização do muco cervical bovino. Em primeiro lugar faz-se referência à composição do muco e das importantes funções exercidas por esta secreção na fisiologia reprodutiva bovina e de outras espécies. Mais adiante o artigo trata sobre o fenômeno de cristalização observado no muco cervical em estro, descrevendo-se os principais modelos utilizados para classificar os padrões cristalinos observáveis no mesmo (alguns semelhantes a samambaias, folhas de palmeira ou figuras estreladas, entre outros). Finalmente, se discute a importância do fenômeno da cristalização do muco cervical para a compreensão da reprodução bovina tanto em estados fisiológicos quanto em fisiopatológicos.
Palavras chave: fisiologia bovina, cristalização, estro, muco cervical, padrão cristalino.
INTRODUCTION
From the onset of cow domestication thousands of years ago, man has been able to recognize certain behavioural and physical changes related to the reproductive state of cattle (1,2). Among ruminants, such as bovines, as well as in other species, during the oestrus stage a somewhat translucent and relatively sticky aqueous substance can be seen to copiously come out from the female reproductive tract, one of the signs that has been recognized, from ancient times, as the beginning of sexual receptivity (heat) (1,2). Such secretion, known as cervical mucus, exerts several physiological functions that are critical for the development of the reproductive process in the cow, the same as in other animals in which this fluid is produced.
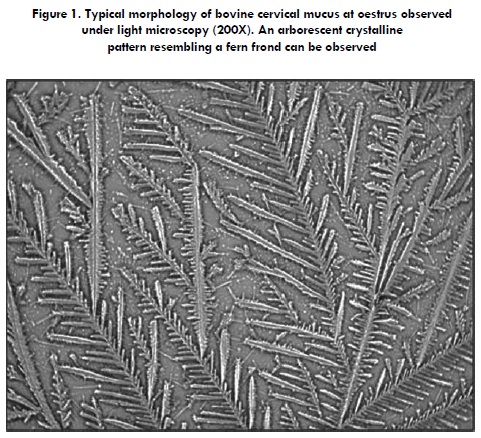
When a drop of cervical mucus collected at oestrus is deposited on a transparent surface and allowed to dry at room temperature, the mucus tends to crystallize in highly arranged geometric patterns, characterized mainly by arborescent morphologies, among other arrangements (1,3-7). Assessment of the properties of cervical mucus throughout the oestrous cycle shows that the extent of the crystallization phenomenon reaches a maximum at oestrus in comparison to any other stage of the cycle (4). This is mainly due to raised levels of oestrogens in this stage which are exerting their effects on the cervical mucosa, and, therefore, changing the mucus features. This explains why the assessment of mucus crystallization has been suggested by some researchers as a useful tool to determine the onset of sexual receptivity in cows (1,8,9) and other animals (10,11).
The objective of this review article is to discuss the main aspects of the phenomenon of mucus crystallization, with special interest in the characteristics of the crystalline patterns observed in bovine cervical secretion.
BOVINE CERVICAL MUCUS
Cervical mucus is produced by mucus-secreting cells that line the grooves and folds resembling 'blind-ended crypts,' present in the cervical epithelium (12). Secreted more copiously at oestrus, mucus volume can reach up to 100 mL. Regarding the chemical composition, cervical mucus is a hydrogel with 92 to 95% of water content (13) and comprises soluble and non-soluble substances (14,15). Among the soluble substances are proteins, such as lactoferrin (16), immunoglobulins, several enzymes, e.g., glucosidases and matrix metalloproteases (17,18), a number of low-molecular-mass compounds, such as carbohydrates (e.g., fructose and glucose) (13,19,20), amino acids, lipids, such as cholesterol (13,20), and inorganic ions (electrolytes), the most important being Na+, K+, Ca2+ and Cl- (21). On the other hand, the non-soluble fraction consists of high-molecular-mass glycoproteins known as mucins (15,22-24), mainly those classified as secreted gel-forming mucins (25). These are highly glycosylated proteins and probably constitute the main factor responsible for the rheological properties of mucus, such as its variable elasticity, viscosity and spinnbarkeit, among others (26).
The cyclical variations in the levels of oestrogens and progesterone have a marked effect on bovine cervical mucus. In general, these hormones exert their influence by directly acting on secreting endocervical cells, mainly via mechanisms mediated by classical steroid receptors (27); as a consequence, the composition, the physicochemical and structural properties, and the rheological attributes of the cervical secretion are changed (28). Regarding this, it has been shown that mucus water content varies along the cycle, increasing at oestrus (29,30), due mainly to the rise in oestradiol levels observed in this stage. Also, mucin types are differentially expressed during the stages of the oestrous cycle (12,24), a change also related to fluctuations in sex steroid hormones.
FUNCTIONS OF BOVINE CERVICAL MUCUS
Bovine cervical mucus has several important functions in the reproductive process, among which are:
The cervical secretion protects the bovine reproductive tract by maintaining the epithelial surfaces moist and lubricated. This is due to the high level of hydration that characterizes this gel (15), since mucins are capable of binding large volumes of water (22,31).
Cervical mucus takes part in sperm selection and transport, being the first medium spermatozoa must go through when ascending to the site of fertilization (15,32,33). During the periovulatory period, mucus secretion increases, becoming less viscous and more hydrated, facilitating the ascent of spermatozoa (15,32,33). Also, in this period, the mucus structure would facilitate the movement of normal spermatozoa and inhibit the ascent of gametes with morphological alterations, acting as a selective filter (33). On the other hand, in the luteal phase, the amount and hydration of the secreted mucus decreases while its viscosity increases, preventing spermatozoa migration. According to Becher et al. (34), this ability of bovine cervical mucus to 'filter' spermatozoa has proven to be useful in the areas of human andrology and gynaecology regarding sperm penetration tests, during which bovine mucus was sometimes used to compensate for the small volume of cervical mucus that can be obtained from a woman.
The cervical secretion constitutes an immune barrier that inhibits the ascent and colonization of microorganisms, since some of the compounds present in mucus can inhibit the penetration and proliferation of microbes (35).
During pregnancy, cervical mucus protects the uterus from environmental noxious agents, since during this period mucus forms a highly viscous barrier known as cervical mucus plug (31,36).
A number of substances have been identified in the fluids of a cow's reproductive tract, among which there are sex steroid hormones (37). These are also present in cervical mucus and probably modulate the acrosome reaction (acrosomal exocytosis), as it has been proposed for human cervical mucus (38-41). In cows, this idea is also supported by evidence obtained by using scanning electron microscopy when studying cervical sections in follicular phase, in which spermatozoa with intact acrosomal membranes in some mucus-filled luminal regions were observed (12). However, further studies are needed to elucidate the role exerted by sex steroid hormones present in bovine cervical mucus on acrosomal exocytosis.
CERVICAL MUCUS CRYSTALLIZATION
In general terms, crystallization can be defined as the process through which a component of a liquid solution changes to its solid phase, tending to separate from the solution and to precipitate in the form of crystals. Crystallization constitutes a means to reach a more stable, lower energy state from a metastable solution by reducing the solute concentration (42). Crystallization is produced by molecular aggregation leading to the formation of crystalline nuclei (nucleation), with the subsequent growth of those nuclei. Therefore, nucleation is the precursor of crystalline growth.
The process of crystallization is not exclusive to cervical secretion; it is present in a number of other biological secretions, for example, human and bovine saliva (43). Secretions able to crystallize are characterized by containing mucoproteins (e.g., mucins) or other organic compounds and electrolytes, especially salts, such as NaCl, KCl and CaCl2 (13,44). In fact, NaCl is the main salt found in cervical mucus, providing the mucus with ionic strength (21). In general, cervical mucus crystallization has been studied by spreading mucus sample drops onto a glass slide so that, after drying at room temperature, a smear or film forms on the slide. This can be observed without staining by using a standard light microscope (1,4,5,45). The first to report on cervical secretion crystallization was Papanicolaou (1946), who focused on the fern-like arrangements observable on women's mucus, suggesting that this phenomenon could be used as a predictor of ovulation (10). As a result of the crystalline shapes that were observed, from then on, cervical mucus crystallization has also been called an 'arborization' or 'ferning phenomenon' (Figure 1). Garm and Skjerven (46) studied crystallization in the cervical mucus of cattle, finding abundant fernlike crystals during the follicular phase, which disappeared during the luteal phase and were non-detectable in the early stages of pregnancy. Later, several researchers determined that the highest arborization occurred at the onset of, or during, oestrus (1,47,48). In this regard, Abusineina (1) stated that the study of cervical mucus in relation to the presence or absence of crystallization, and the type of crystallization observed, is an indicator of the stage of oestrous cycle and the day of ovulation; therefore, it is useful to confirm clinical findings as well as for experimental applications. MacDonald (29) reported that in the periovulatory period, when spermatozoa are able to migrate through mucus, the proportion of water content is over 98%, and the salt content in the dry residue is more than 50%. As the proportion of salts in the dry residue starts to decrease, so does the water content. This change leads to a decrease in the arborizations observed in the dried mucus sample. In the mucus obtained from pregnant cows, the water content is 90% and no arborization can be observed. In relation to this, Noonan et al. (4) noticed an inverse relationship between the extent of arborization and the content of dry matter in cervical mucus samples. The concentration of dry matter in cervical mucus reached a minimum at oestrus and a peak at mid-cycle, while mucus ferning appeared at oestrus to a greater extent than at any other stage of the oestrous cycle.
Also, as for other properties of cervical mucus, variations in the occurrence of crystallization during the oestrous cycle are equally due to the changing levels of sex steroid hormones. In general, oestrogens are considered to promote crystallization, while progesterone decreases it (13). In this regard, it has been proposed that the higher occurrence of arborizations at oestrus depends on oestrogen dominance during the follicular phase (49). Oestrogens would cause an increase in the ferning phenomenon through mechanisms that stimulate the electrolyte metabolism in the cervical epithelium (50,51). On the other hand, in the luteal phase, increased levels of progesterone would counteract the effects of oestrogens on the cervix, explaining the decrease in arborizations (50,52), a fact that is in agreement with the inhibitor effect on crystals previously reported for this hormone (53). The influence exerted by these sex steroids coincides with the observation made by Elstein (54), who states that, among the many attributes of cervical mucus, arborization is, without a doubt, one of the most sensitive to variations in the levels of sex steroids. Nowadays, it is well-known that crystallization constitutes a useful property for studying both cervical mucus and the reproductive cycle (5,55). On the other hand, it is also worth mentioning that the crystallization of cattle saliva undergoes changes during the oestrous cycle, and hence the analysis of such crystallization can help in the diagnosis of early pregnancy (43).
CRYSTALLINE PATTERNS FOUND IN BOVINE CERVICAL MUCUS
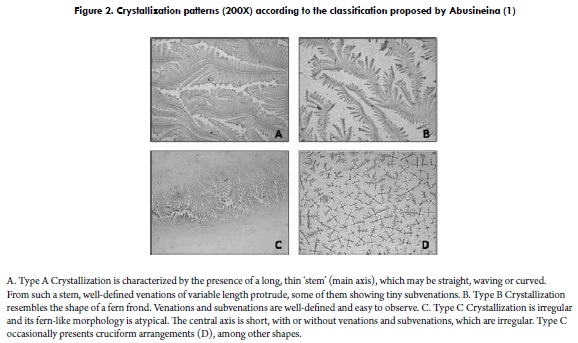
To our knowledge, the first model to classify crystallization patterns of bovine cervical mucus was proposed by Abusineina (1), who divided the observed arrangements into three types. Type A corresponds to the crystallization observed when the mucus is translucent, acellular, elastic and easily obtained from the cervix. Under a light microscope, Type A crystallization is characterized by the presence of a long, thin stem (main axis), which may be straight, waving or curved. From such a stem, well-defined venations of variable length protrude with tiny subvenations (Figure 2A). This type of crystallization would be associated with ovulation and generated as a consequence of high oestrogen levels (1). Type B crystallization corresponds to that observed when mucus is semi-translucent, elastic and easily obtained from the cervix. When observed under a light microscope, this type is nearest in shape to a fern frond (1). Venations and subvenations are well-defined and easy to observe (Figure 2B). Type C corresponds to the crystallization of opaque mucus, evidencing cellularity and being difficult to obtain from the cervix. When observed using light microscopy, Type C crystallization is irregular and its fern-like morphology is atypical. The central axis is short, with or without venations and subvenations, which are irregular (1) (Figure 2C). Some disperse linear crystalline patterns can be found, either cruciform (Figure 2D) or stellate.
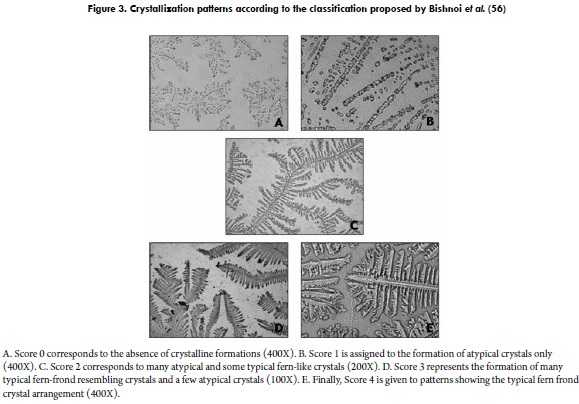
Another model for the classification of bovine cervical mucus at oestrus was proposed by Bishnoi et al. (56) and implemented by Tsiligianni et al. (8), based on an arbitrary scale (ranging from 0 to 4). Score 0 corresponds to the absence of crystalline formations (Figure 3A). Score 1 is assigned to the formation of atypical crystals only (Figure 3B). Score 2 is assigned when many atypical and a few typical fern-like crystals are observed (Figure 3C). Score 3 represents the formation of many typical fern-like crystals and a few atypical crystals (Figure 3D). Finally, score 4 is given to patterns showing the typical fern frond crystal arrangements (Figure 3E).
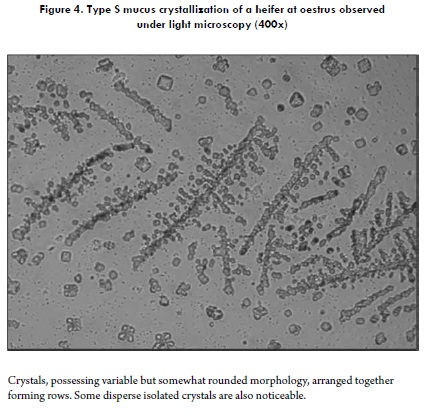
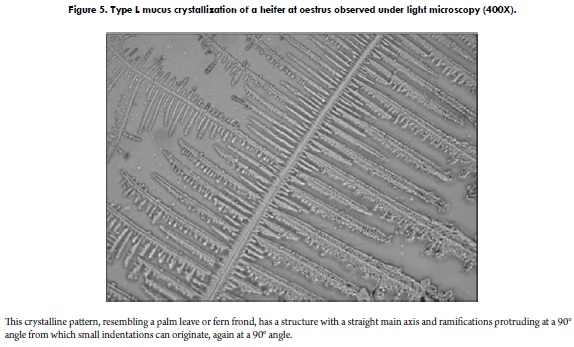
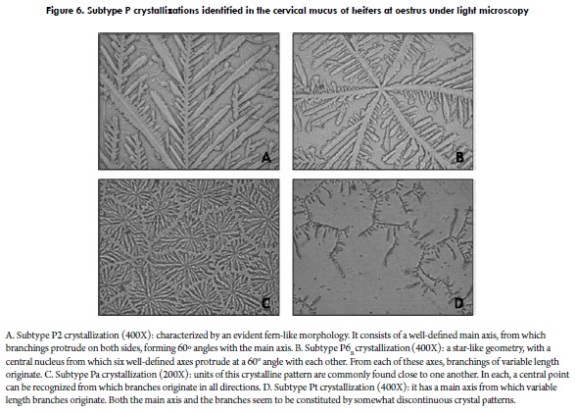
A well-known classification model for the crystallization of cervical mucus of the periovulatory period was reported by Odeblad (57) and later validated by other investigations (58,59). Initially proposed for women's cervical secretion (57), it is interesting and worth mentioning that, when studying bovine cervical mucus according to the classification proposed by Odeblad, geometrical crystallizations very similar to those obtained for human cervical mucus are observed (5,7). The types (and subtypes) proposed by Odeblad, and observable in bovines, are: Type S Crystallization: its morphology resembles straight lines that tend towards a parallel arrangement (Figure 4). Type L Crystallization: it is characterized by a palm leave or fern frond morphology, with a well-defined central axis and 90° branching (Figure 5), similar to the patterns observed in human cervical mucus (57,59,60). Type P Crystallization: grouping several crystalline subtypes, this pattern has 60° angle branchings originating from the main axis. It is divided into five subtypes, but those generally observed in bovine cervical mucus are four: Subtype P2, consisting of a well-defined main stem (axis), from which branchings protrude to both sides, forming 60° angles with the main stem. This crystalline pattern is evidently fern-like (Figure 6A). Subtype P6B has a very attractive geometry, resembling a star, with a central nucleus from which six well-defined axes protrude (7). Each axis forms a 60° angle with the next, and branchings of variable length originate from each axis (Figure 6B). In general, this subtype has been found to form somewhat larger crystalline units than other subtypes of P mucus (58); also, subtype P6B in humans would be linked to the fertility peak (61). Subtype Pa has a crystallization centre from which multiple branchings irradiate in all directions (Figure 6C). Lastly, another subtype corresponds to mucus Pt, which does not have such an orderly arrangement like the aforementioned subtypes; the crystals appear to be more disperse and not always joined (Figure 6D), as observed in women (58,59).
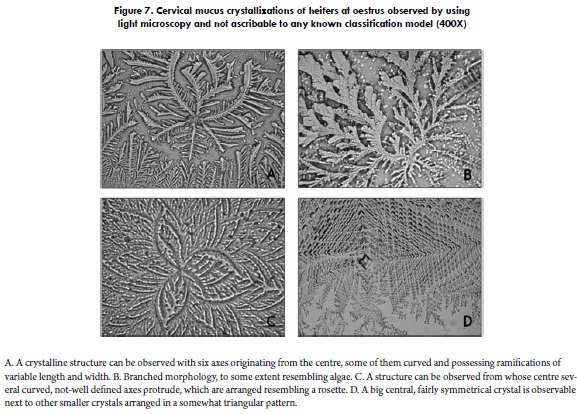
Similarly, it is worth mentioning that, when observed under light microscopy, bovine cervical mucus also shows some crystalline arrangements that cannot be satisfactorily categorized into any of the previously described models (Figure 7). Finally, certain arboriform crystallizations of heifer cervical mucus have been reported to show a fractal-like organization, i.e., crystalline structures are comprised of smaller parts that resemble the whole in a smaller scale (6). These fractal patterns have also been proposed for human mucus (62), even though their biological significance is yet to be explained.
CONCLUDING REMARKS
During the oestrous cycle, and especially at oestrus, it is possible to identify several geometric arrangements for the crystalline patterns of bovine cervical mucus; this fact has made it possible to propose the previously described classifications. The reason for the existence of such types of crystallization has not been completely elucidated, but considering that bovine cervical epithelium could be comprised of different secretory regions (12,63), those crystalline patterns would be result of the differential influence exerted by raised levels of oestrogens at oestrus on such regions. Thus, cervical mucus is probably a heterogeneous entity formed by the admixture of several subtypes of secretion (59,64,65), with proportions varying in the periovulatory period and, to a lesser extent, during other stages of the cycle. This fact would explain the existence of different morphological types (and subtypes) of mucus crystallization; other possible underlying causes are variations in salt content (as a result of modifications in electrolyte metabolism at the level of the cervix) and water content, as well as the arrangement and type of mucin present in cervical mucus due to changes in levels of sex steroid hormones.
The study of the crystallizations present in bovine cervical mucus at oestrus and other stages could lead to a deeper understanding of bovine reproductive physiology, both in physiological and pathophysiological conditions. Being able to relate one specific type of cervical mucus crystallization with a cow's fertility peak could be especially relevant in reproductive management. In addition, associating a certain pattern of crystallization with oestrogens and progesterone levels could be of great importance in the field of veterinary medicine. In this regard, it is known that alterations in cervical mucus ultrastructure are linked to fertility problems (lack of pregnancy) in cows (66,67), as it has also been reported in women (60). Among women with fertility problems due to endocrine-metabolic disorder known as polycystic ovary syndrome (PCOS), an alteration in cervical mucus ultrastructure has been observed together with modifications in the patterns of mucus crystallization (60); in addition, altered rheological properties (e.g., changes in elasticity) have also been reported in cervico-vaginal secretions of PCOS women (68). Considering the foregoing, it is possible that changes in mucus ultrastructure and rheology are also accompanied by changes in crystallization patterns in some cows with fertility problems secondary to endocrine disturbances.
Finally, further research in this area should focus on identifying the physiological meaning of the different crystalline patterns of bovine cervical mucus at oestrus, as well as on identifying the biochemical mechanisms triggering changes in electrolyte metabolism, in mucus hydration, and in mucin expression at the cervix, all of which could explain the observed variations in crystallization patterns. It is also of importance to elucidate the mechanism through which changes in sex steroid levels influence the types of crystallizations observed among healthy cows and in those suffering from reproductive disorders.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest in publishing this review article.
ACKNOWLEDGEMENTS
M.E. Cortés thanks Conicyt (Chile) for the scholarship (# 21080589) granted for PhD studies. The authors also wish to thank Dr. Jenness Fulton and Dr. Leonard F. Blackwell (Massey University, Palmerston North, New Zealand) for their useful comments about this manuscript.
References
1. Abusineina ME. A study of the fern-like crystalline patterns of the cervical and vaginal mucus of cattle. Vet Rec. 1962;74:619-21. [ Links ]
2. Pommerenke WT. Some biochemical aspects of the cervical secretions. Ann N Y Acad Sci. 1962;97:581-90. [ Links ]
3. Lamond DR, Shanahan AG. Chemical changes in cervical mucus from normal and ovariectomized cows treated with hormones. Biol Reprod. 1969;1(4):335-43. [ Links ]
4. Noonan JJ, Schultze AB, Ellington EF. Changes in bovine cervical and vaginal mucus during the estrous cycle and early pregnancy. J Anim Sci. 1975;41(4):1084-9. [ Links ]
5. Cortés ME. Morphological and ultrastructural characterization of different types of bovine cervical mucus using light and scanning electron microscopy [tesis doctoral]. [Santiago de Chile]: Pontificia Universidad Católica de Chile; 2012. [ Links ]
6. Cortés ME, Hauyón R, Vigil P, González F. Evidence of fractality in a pattern of crystallization of bovine cervical mucus obtained at oestrus. Int J Morphol. 2012;30(4):1461-5. [ Links ]
7. Cortés ME, González F, Hauyón R, Vigil P. Highly symmetrical crystallization in six rectilinear and well-defined axes found in bovine cervical mucus obtained at oestrus: a finding. Rev Fac Med Vet Zoot. 2014;61(2):164-70. [ Links ]
8. Tsiligianni T, Karagiannidis A, Brikas P, Saratsis P. Relationship between certain physical properties of cervical mucus and fertility in cows. Deutsche Tierárztliche Wochenschrift. 2000;107:28-31. [ Links ]
9. Tsiligianni T, Amiridis GS, Dovolou E, Menegatos I, Chadio S, Rizos D, Gutiérrez-Adán A. Association between physical properties of cervical mucus and ovulation rate in superovulated cows. Can J Vet Res. 2011;75(4):248-53. [ Links ]
10. Papanicolaou GN. A general survey of the vaginal smear and its use in research and diagnosis. Am J Obstet Gynecol. 1946;51:316-28. [ Links ]
11. McDonald MF, Raeside JI. Use of the cervical mucus smear in assessing ovarian activity in the ewe. Nature. 1956;178(4548):1472-3. [ Links ]
12. Mullins JK, Saacke RG. Study of the functional anatomy of bovine cervical mucosa with special reference to mucus secretion and sperm transport. Anat Rec. 1989;225(2):106-17. [ Links ]
13. Tsiligianni T, Karagiannidis A, Brikas P, Saratsis P. Chemical properties of bovine cervical mucus during normal estrus and estrus induced by progesterone and/or PGF2a. Theriogenology. 2001;56(1):41-50. [ Links ]
14. Schumacher GFB. Soluble proteins in cervical mucus. En: Blandau RJ, Moghissi KS (eds). The biology of the cervix. Chicago: University of Chicago Press; 1973. p. 201-33. [ Links ]
15. Rutllant J, López-Béjar M, López-Gatius F. Ultrastructural and rheological properties of bovine vaginal fluid and its relation to sperm motility and fertilization: a review. Reprod Domest Anim. 2005;40(2):79-86. [ Links ]
16. Rao KSPB, Roberts TK, Masson PL, Heremans JF. Lactoferrin, a major soluble protein of bovine oestrous cervical mucus. J Reprod Fertil. 1973;32(1):89-92. [ Links ]
17. Tsiligianni T, Karagiannidis A, Saratsis P, Brikas P. Enzyme activity in bovine cervical mucus during spontaneous and induced estrus. Can J Vet Res. 2003;67(3):189-93. [ Links ]
18. Kim SH, Baek JS, Lee HJ, Min KS, Lee DH, Yoon JT. Detection of matrix metalloprotease-9 and analysis of protein patterns in bovine vaginal mucus during estrus and pregnancy. J Embr Transfer. 2012;27:93-100. [ Links ]
19. el-Naggar MA, Baksai-Horváth E. The sugar content of the cervico-vaginal mucus of cattle during the sexual cycle, with special reference to fructose. Acta Vet Acad Sci Hung. 1971;21(1):15-20. [ Links ]
20. Zaaijer D, van der Horst CJG. Cyclic changes in hormones, carbohydrates and indole metabolism in cervical mucus of normal, fertilizing cows and the relationship with non-fertility. Cytobios. 1983;37(146):113-27. [ Links ]
21. Sato M, Nihei A, Ohta M, Masaki J. Changes in sodium, potassium and chloride concentrations of bovine cervical mucus during the time of estrus induced by prostaglandin F2a analogue. Tohoku J Agricult Res. 1981;32(4):40-9. [ Links ]
22. Gibbons RA. Chemical properties of two mucoids from bovine cervical mucin. Biochem J. 1959;73(2):209-17. [ Links ]
23. Gibbons RA, Glover FA. The physicochemical properties of two mucoids from bovine cervical mucin. Biochem J. 1959;73(2):217-25. [ Links ]
24. Pluta K, Irwin JA, Dolphin C, Richardson L, Fitzpatrick E, Gallagher ME, et al. Glycoproteins and glycosidases of the cervix during the periestrous period in cattle. J Anim Sci. 2011;89(12):4032-42. [ Links ]
25. Gipson IK. Human endocervical mucins. Ernst Schering Research Foundation Workshop. 2005; 52:219-44. [ Links ]
26. Wolf DP, Sokoloski J, Khan MA, Litt M. Human cervical mucus. III. Isolation and characterization of rheologically active mucin. Fertil Steril. 1977;28(1):53-8. [ Links ]
27. Nicosia SV. Physiology of cervical mucus production. Semin Reprod Med. 1986;4(4):313-21. [ Links ]
28. Sharma V, Prasad S, Gupta HP. Studies on physical and rheological properties of cervico-vaginal mucus during early pregnancy in buffaloes (Bubalus bubalis). Vet World. 2013;6(8):508-11. [ Links ]
29. MacDonald RR. Cyclic changes in cervical mucus. 2. The role of saline. J Obstet Gynaecol Br Commonw. 1969;76(12):1094-9. [ Links ]
30. Merilan CP. Evaluation of bovine cervical mucus during estrous cycle by nuclear magnetic resonance. J Dairy Sci. 1983;66(5):1184-8. [ Links ]
31. Becher N, Waldorf KA, Hein M, Uldbjerg N. The cervical mucus plug: structured review of the literature. Acta Obstet Gynecol Scand. 2009;88(5):502-13. [ Links ]
32. Barros C, Vigil P, Herrera E, Pérez A, Guadarrama A, Bustos-Obregón E. In vitro interaction between human spermatozoa and human cervical mucus. Microsc Electrón Biol Cel. 1983;7:13-8. [ Links ]
33. Barros C, Vigil P, Herrera E, Argüello B, Walker R. Selection of morphologically abnormal sperm by human cervical mucus. Arch Androl.1984;12 Suppl:95-107. [ Links ]
34. Becher AC, Failing K, Kauffold J, Wehrend A. Establishment of a practical sperm penetration test for bovine semen. Tierárztliche Praxis Grofitiere. 2013;41(5):297-303. [ Links ]
35. Brownlie J, Hibbitt KG. Antimicrobial proteins isolated from bovine cervical mucus. J Reprod Fertil. 1972;29(3):337-47. [ Links ]
36. Marshall FHA, Hammond J. Fertility and animal breeding. Bull Ministry of Agriculture and Fisheries. 1937;39:1-52. [ Links ]
37. Short RV. Steroid concentrations in normal follicular fluid and ovarian cyst fluid from cows. J Reprod Fertil. 1962;4:27-45. [ Links ]
38. Vigil P, Toro A, Godoy A. Physiological action of oestradiol on the acrosome reaction in human spermatozoa. Andrologia. 2008;40(3):146-51. [ Links ]
39. Vigil P, Orellana RF, Cortés ME. Modulation of spermatozoon acrosome reaction. Biolog Res. 2011;44(2):151-9. [ Links ]
40. Vigil P, Cortés ME. El misterio del inicio de la vida humana. Diálogos. 2011;1:22-5. [ Links ]
41. Vigil P. La fertilidad de la pareja humana. 4ta ed. Santiago de Chile: Ediciones Universidad Católica; 2013. p. 76-77. [ Links ]
42. Weber PC. Physical principles of protein crystallization. Adv Protein Chem. 1991;41:1-36. [ Links ]
43. Skalova I, Fedorova T, Brandlova K. Saliva crystallization in cattle: new possibility for early pregnancy diagnosis? Agric Tropica et Subtrop. 2013;46(3):102-4. [ Links ]
44. Rydberg E. Observation on the crystallization of the cervical mucus. Acta Obstet Gynecol Scand. 1948;28(2):172-87. [ Links ]
45. Odeblad E. The spread out technique. Advantages, pitfalls and biological interpretation. Actas IV Symposium Internacional de Métodos Naturales. Barcelona; 1995. p. 295-303. [ Links ]
46. Garm O, Skjerven O. Undersokelse av cervikalslim for diagnose av tidlig drektighet og endokrint betingede forstyrrelser av seksualcyklus hos husdyr. Nordisk Veterinrmedicin. 1952;4:1098-103. [ Links ]
47. Bone JF. Crystallization patterns in vaginal and cervical mucus smears as related to bovine ovarian activity and pregnancy. Am J Vet Res. 1954;15(57):542-7. [ Links ]
48. Alliston CW, Patterson TB, Ulberg LC. Crystallization patterns of cervical mucus as related to estrus in beef cattle. J Animal Sci. 1958;17(2):322-5. [ Links ]
49. Ghannam SA, Sorensen AM. Early pregnancy diagnosis in the bovine. J Dairy Sci. 1967;50(4):562-7. [ Links ]
50. Roland M. A simple test for the determination of ovulation, estrogen activity, and early pregnancy using the cervical mucus secretion. Am J Obstet Gynecol. 1952;63(1):81-9. [ Links ]
51. Zondek B. Some problems related to ovarian function and to pregnancy. Rec Prog Hormone Res. 1954;10:395-423. [ Links ]
52. Forman I. Cervical mucus arborization; aid in ovulation timing. Obstet Gynecol. 1956;8(3):287-92. [ Links ]
53. Campos da Paz A. The crystallization test as a guide to the treatment of cervical hostility. Fertil Steril. 1953;4(2):137-48. [ Links ]
54. Elstein M. Cervical mucus: Its physiological role and clinical significance. Br Med Bull. 1978;34:83-8. [ Links ]
55. Silaban NL, Setiatin dan Sutopo ET. Tipologi ferning sapi jawa brebes betina berdasarkan periode berahi. Animal Agriculture J. 2012;1(1):777-88. [ Links ]
56. Bishnoi BL, Vyas KK, Dwaraknath PK. Note on spinnbarkeit and crystallization pattern of bovine cervical mucus during oestrus. Indian J Anim Sci. 1982;52(6):438-40. [ Links ]
57. Odeblad E. The discovery of different types of cervical mucus and the Billings ovulation method. Bulletin of the Natural Family Planning Council of Victoria. 1994;21:1-34. [ Links ]
58. Menárguez M. Caracterización morfológica de diversos tipos de moco cervical humano mediante microscopía de luz y microscopía electrónica de barrido [tesis doctoral]. [Murcia]: Universidad de Murcia; 2012. [ Links ]
59. Menárguez M, Pastor LM, Odeblad E. Morphological characterization of different human cervical mucus types using light and scanning electron microscopy. Hum Reprod. 2003;18(9):1782-9. [ Links ]
60. Vigil P, Cortés ME, Zúñiga A, Riquelme J, Ceric F. Scanning electron and light microscopy study of the cervical mucus in women with polycystic ovary syndrome. J Electron Microsc. 2009;58(1):21-7. [ Links ]
61. Odeblad E, Ingelman-Sundberg A, Menárguez M, Temprano H, Pouyanmehr S, Vigil P, et al. Types of cervical secretion. Actas VIII Symposium Internacional sobre Regulación Natural de la Fertilidad: Aplicaciones a la Salud Reproductiva. Leioa, España; 2006;1-15. [ Links ]
62. Yang G, Yang Z, Zhou H, Huang T. Fractal analysis of the fernleaf crystallization of cervical mucus. J Yunnan Univ National. 2011;20(1):75-8. [ Links ]
63. Heydon RA, Adams NR. Comparative morphology and mucus histochemistry of the ruminant cervix: differences between crypt and surface epithelium. Biol Reprod. 1979;21(3):557-62. [ Links ]
64. Odeblad E. The functional structure of human cervical mucus. Acta Obstet Gynecol Scand. 1968;47(S1):57-79. [ Links ]
65. Richardson L, Hanrahan JP, O'Hara L, Donovan A, Fair S, O'Sullivan M, et al. Ewe breed differences in fertility after cervical AI with frozen-thawed semen and associated differences in sperm penetration and physicochemical properties of cervical mucus. Anim Reprod Sci. 2011;129(1-2):37-43. [ Links ]
66. López-Gatius F, Labérnia J, Santolaria P, Rutllant J, López-Béjar M. The relationship of the rheological behavior of the vaginal fluid at the time of insemination to the pregnancy rate in dairy cows. Theriogenology. 1997;48(5):865-71. [ Links ]
67. Rutllant J, López-Béjar M, Santolaria P, Yániz J, López-Gatius F. Rheological and ultrastructural properties of bovine vaginal fluid obtained at oestrus. J Anat. 2002;201(1):53-60. [ Links ]
68. Shamim N, Usala SJ, Biggs WC, McKenna GB. The elasticity of cervical-vaginal secretions is abnormal in polycystic ovary syndrome: Case report of five PCOS women. Indian J Endocrinol Metab. 2012;16(6):1019-21. [ Links ]