INTRODUCTION
Intravenous anesthesia advantages include rapid onset of action independent of ventilation status, reduction of adverse effects of other anesthetic drugs if used in balanced anesthesia protocols, allowing for provision of continuous analgesia if needed, smoother recovery from anesthesia, low costs (considering that the minimum requirement is a needle and a syringe), and reduction of the hazards of occupational health and atmospheric pollution1 . Surgical procedures in small ruminants can be performed under intravenous anesthesia. Xylazine and ketamine combinations have been popular for anesthetizing small ruminants since 1970. Although rapid onset and short duration of action are the advantages of a single bolus injection ofxylazine and ketamine, repeated dosing or continuous infusion often results in a prolonged recovery 2 . Injectable general anesthesia using drug combinations such as ketamine and xylazine with or without guaifenesin are common in certain surgical procedures 3.
Xylazine induces dose-dependent sedation and central nervous depression in sheep 4. Alpha-2 adrenoreceptor agonists are potent sedative drugs that provide muscle relaxation and analgesia. A range of xylazine doses alters respiratory mechanics and gas exchange, causing tachypnea, increased airway pressures and respiratory resistance, decreased lung compliance, pulmonary edema, and hypoxemia with or without hypercapnia 5, produced by vasoconstriction, which follows peripheral α2B-receptor stimulation caused by high plasma α2-agonist concentrations (6. In sheep, it has a short elimination half-life and it is rapidly cleared from plasma after intramuscular (IM) and intravenous (IV) administration 7. The effects of xylazine on the digestive tract are variable and include reduced rumen motility and ruminal tympany 8. In adult sheep, recumbency occurred 15 minutes after the administration of 0.3 mg/kg BW of IM xylazine and lasted 55 minutes in adult rams 9. In contrast, IV injection of xylazine (0.3 mg/kg BW) in adult ewes caused recumbency within 3 minutes, which lasted for 54 minutes 10. After the administration of 0.4 mg/kg BW IV xylazine, adult sheep were recumbent for 41 minutes, followed by 34 minutes of head drooping 11.
The α2-adrenergic agonists are often combined with ketamine for anesthesia induction in pregnant sheep 12. Ketamine is commonly used in small ruminants for induction and maintenance of anesthesia. It is a dissociative anesthetic with analgesic effects, which provides mild cardiovascular stimulation and largely maintains the swallowing and cough reflexes 13. Apnea is not uncommon, especially after rapid IV injection, and significant salivation can also be observed 14. When used alone, ketamine provides poor muscle relaxation and the peripheral reflexes are maintained. Therefore, it is strongly recommended to administer ketamine in conjunction with a α2-adrenoceptor agonist to improve muscle relaxation and sedation 15. The combination of ketamine and α2 adrenergic agonists enhances the degree of analgesia and prolongs anesthesia, but respiratory depression may be severe and the recovery delayed when large doses are used 16. Ketamine possesses most of the characteristics required for suitability for continuous intravenous anesthesia, lending itself well for field anesthesia 17.
Guaifenesin is not an anesthetic but a muscle relaxant that disrupts nerve impulse transmission at the level of the internuncial neurons of the spinal cord, brain stem, and subcortical areas of the brain, with no effect on diaphragmatic function, and mild, if any, analgesic and sedative effects 18. Cardiovascular and respiratory depression is minimal. Dilution in 5 % solutions avoids potential hemolysis and, in case of perivascular administration, tissue necrosis 19. It is not often used alone in small ruminants, but it can be used in combination with xylazine or ketamine 20 to induce and maintain short-term anesthesia. It can also be used as a constantrate infusion, being part of a solution ofxylazine (50 mg), ketamine (500 mg), and guaifenesin (500 mL of a 5 % solution) 21, often referred to as "triple drip". Anesthesia is induced in 5 to 10 minutes by the rapid administration of 0.5 to 2 mL/kg BW of the above-mentioned mixture and maintained by infusion at 2 to 2.6 mL/kg/hour. A constant level of anesthesia is produced, and recovery is smooth but not rapid 22.
Therefore, the objective of this study was to evaluate the clinical usefulness and anesthetic effect of xylazine-ketamine (XK) and to compare these effects with those of xylazine-ketamine-guaifenesin (XKG) in order to perform exploratory laparotomy and allow the flushing of the uterine horns in ewes.
MATERIALS AND METHODS
Animals
Sixteen healthy, adult Colombian creole ewes aged 95.06 ± 9.94 months and weighing 26 ± 7 kg were used in this study. All animals were subjected to anthelmintic prophylaxis two months before surgery. Prior to the study, the health of the animals was evaluated using a complete blood count, liver and renal biochemical profile, and fecal parasitologic examination. The ewes were housed in groups (eight animals) per pen in a facility at the Veterinary Clinic of the University of Nariño with free access to food and water. Before the study, food and water were withheld for 24 hours and the hair over the right jugular vein was clipped. This study was reviewed and approved by the Animal Ethics Committee of the Veterinary program of the Animal Science Faculty at University of Nariño.
Anesthesia Protocols
A sheep was removed from its place, weighed and taken to the preparation room. Jugular groove was cleaned and treated with 1 % cetrimide. A teflon intravenous catheter was placed into the jugular vein after infiltration of the skin with 1 mL lidocaine via a 25 gauge needle. The catheter was secured in place and flushed with 5 mL of heparinized saline (10 IU/mL). Anesthesia was induced in animals of group XK (n=8) using 0.2 mg/kg BW xylazine intramuscularly (IM) and, after 5-7 minutes, 10 mg/kg BW ketamine was administered intravenously (IV). Ewes of the XKG group were anesthetized with a constant-rate infusion of a solution of xylazine (50 mg), ketamine (500 mg), and guaifenesin (500 ml of a 5 % solution) and the maintenance with an infusion at 2.2 ml/kg/hour intravenously.
Physiological Parameters
Cardiopulmonary data were collected in the following order: Heart rate (HR, bpm) was measured using transthoracic auscultation with a stethoscope in the region of the fourth left intercostal space for 1 minute; respiratory rate (ƒR rpm) was assessed by observing the movements of the thorax for 1 minute; rectal temperature (RT, °C) was measured with a clinical mercury-in-glass thermometer inserted into the rectum for 1 minute; variables were measured before drug administration (baseline, T0) and 15 minutes after drug administration in four time points: T15, T30, T60, and T120 minutes.
Anesthesia Evaluation
Clinical signs relating to the quality of induction including muscle twitching of the face, neck and whole body along with the presence or absence of nystagmus, leg movement and paddling were recorded. Anesthesia was evaluated recording time, jaw relax, skin sensitivity (response to pin prinks) and reflexes (palpebral and gag).
The quality of recovery was scored on a visual analogue scale for each anesthetic agent used. The scale ranged from zero, indicating an excitable recovery with numerous unsuccessful attempts to stand and obvious distress, to 10, indicating a fast, smooth recovery with rapid return to normal behavior.
Surgery
Animals were solid fasting for twenty-four hours before surgery, and liquid fasting for 12 hours. The surgery technique for uterine flushing and embryo recovery was the same used for the two groups. The ewes were placed in spinal recumbence, head downwards on an inclined stretcher. The surgical area was shaved and disinfected. A midline laparotomy of 5-7 cm and 3 cm in front of the udder was carried out.
Analysis of Data
All data are presented as mean (x) and standard error (SE). Sedation scores were compared by analysis of variance (ANOVA). Changes over time and differences between groups were explored by ANOVA for repeated measures for the time points followed by Bonferroniadjusted t-tests for effects over time where appropriate. Significance level was set at p<0.05. The results were analyzed using descriptive statistics with the Statistical Package for Social Sciences (SPSS) 19.0 23.
RESULTS
The combinations produced satisfactory anaesthesia and no major adverse events were seen during any phase of the anesthetic induction or recovery. Table 1 shows the time lapse for recumbency, surgery and recovery of ewes receiving multimodal anesthesia xylazine (50 mg), ketamine (500 mg) and guaifenesin via IV by infusion at a rate of 2.2 ml/kg/hour and the combination xylazine 0.2 mg/kg BW IM and ketamine 10 mg/kg BW IV Ewes of the xylazine-ketamine group showed a slight regurgitation, expressed ataxia with head drooping and a sternal recumbency after administration of xylazine 0.2 mg/kg BW IM. Following the intravenous application of ketamine, sheep were in lateral recumbency in 9.06 ± 0.73 min. The animals had diminished response of pinpricks. We did not observe any muscle twitching of the face and neck, nystagmus or paddling.
Table 1 Time lapse expressed by average ± standard (SD) deviation for recumbency, surgery and standing ewes after administration of two injectable anesthesia combinations
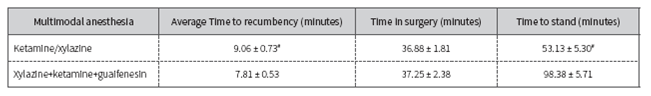
# Significantly different from that of sheep receiving xylazine-ketamine-guaifenesin (p<0.05).
After a rapid administration (0.8 ml/kg) of the XKG solution via IV by infusion at a rate of 2.2 ml/kg/hour, ewes showed salivation and a poor response of pinpricks. Time to recumbency was significantly (p<0.05) faster (7.81 ± 0.53 min) compared to xylazine-ketamine.
The ewes were anesthetized during surgery for 37.06 ± 2.04 min. A midline laparotomy of 5-7 cm and 3 cm in front of the udder was carried out for the exteriorization of the uterine horns. Recovery embryos consisted in injecting a liquid medium that produces a flushing current through the uterine horns (Figure 1).
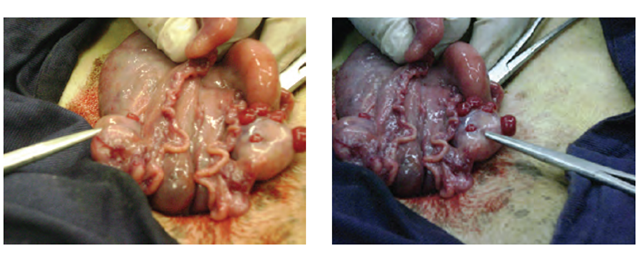
Figure 1 Midline laparotomy and exteriorization of the uterine horns for flushing and embryo recovery
After surgery, the sheep in the xylazine-ketamine group took a sternal recumbency and were standing up in 53.13 ± 5.3 min. Meanwhile, at the end ofthe laparotomy, the administration of the XKG solution was suspended and recovery to standing occurred in 98.38 ± 5.71 min (Table 1). The recovery of XK group was significantly (p<0.05) faster compared with xylazine-ketamine-guaifenesin.
The effects before, during and after surgery under anesthesia with xylazine/ketamine and xylazine-ketamine-guaifenesin combinations on the clinical parameters (heart rate, respiration rate, body temperature, and rumen motility) of ewes are presented in Table 2.
Table 2 Mean clinical parameters of Colombian creole ewes (n=16) during laparotomy for embryo recovery under two injectable anesthesia procedures, XK (n=8) and XKG (n=8)
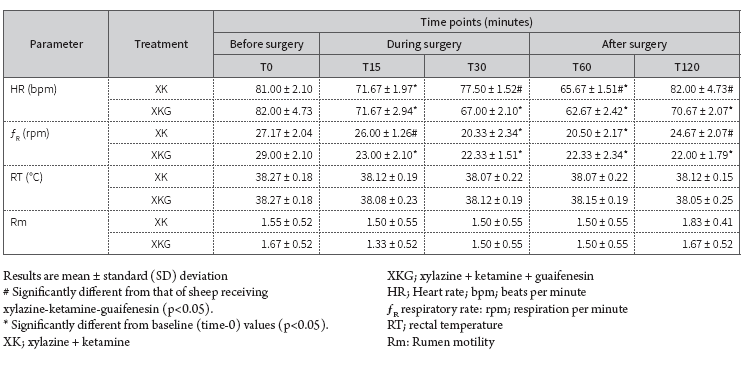
Both groups presented heart and respiratory rates that were within the physiological limits during the surgical procedure. Throughout the procedures, there were no significant differences in corporal temperature and rumen motility between the groups and among the treatments. These parameters remained within the normal limits for each group.
Heart rate decreased at all time points in every treatment compared to the baseline. There was a significant difference in heart rates between the groups (p<0.05) at T30, T60 and T120. In XK group assessments, there was a statistically significant difference in heart rate at T15 (77.5 ± 1.52 bpm) and at T60 (65.67 ± 1. 51 bpm) (p<0.05) compared to the baseline values (81 ± 2.1 bpm). Ewes receiving the XKG combination presented statistically significant differences in heart rate at four time points-T15 (71.67 ± 2.94 bpm), T30 (67 ± 2.1 bpm), T60 (62.67 ± 2.42 bpm), and T120 (70.67 ± 2.07 bpm)-compared to baseline values (82 ± 4.73 bpm). Heart rate increased during the exteriorization and manipulation of the uterine horns for flushing and embryo recovery.
As to respiratory rate, there was a statistically significant difference between the XK and XKG groups (p<0.05) at T15 and T120. Compared to baseline values (27.17 ± 2.04 rpm) in the xylazine-ketamine group, we observed a significant difference (p<0.05) in the respiratory rate at T30 (20.33 ± 2.34 rpm) and T60 (20.5 ± 2.17 rpm), while in the xylazine-ketamine-guaifenesin group, the respiratory rate decreased significantly at T15 (23 ± 2.1 rpm), T30 (22.33 ± 1.51 rpm), T60 (22.33 ± 2.34 rpm), and T120 (22 ± 1.79 rpm), compared to the baseline rate (29 ± 2.1 rpm).
DISCUSSION
Xylazine, ketamine and guaifenesin were chosen for anesthesia in ewes because they are inexpensive and available drugs in Colombia. Although injectable anesthetics are available and commonly used, their safe use by veterinarians and scientists require experience and accurate calculation of the dose in order to prevent or minimize undesirable effects 24. For surgical embryo transfer, the practitioner must choose the appropriate anesthetic agents or a combination of agents to be safe and fast during the surgical procedure in sheep.
Between the two multimodal protocols, respiratory rate showed a significant difference at T15 and T120. In the XKG group, there was a significant depression in the respiration rate in the induction at T15, T30, T60 and T120, while the difference was at T15 and T60 in XK group. After T60, however, respiration rates mildly increased. This may have occurred partly because ketamine induces a mild respiratory inhibition, which usually appears at an increased rate (25. Ketamine has desired effects such as maintenance and stimulation of respiration, bronchodilation, maintenance of functional residual capacity, and achievement of equivalent minute ventilation rates both in spontaneously breathing individuals and in those that are wide awake 26. Hobbs et al. 27 reported this pattern of breathing in other small and laboratory animals. Sedation with XK and XKG treatment initially resulted in lateral recumbency, which may have limited lung expansion and resulted in decreased ventilation 28.
Both protocols presented a significant decrease (p<0.05) of 15 % in heart rate (11 ± 1 bpm) at T15, which are values similar to those reported by Hughan et al. 29. Between T30 and T60, heart rate showed a significant difference in the XK group compared to the XKG group. This may be caused by xylazine administration, which causes dose-dependent cardiovascular depression and bradycardia by enhancing vagal tone and baroreceptor reflexes 30. The Reports 31 of intravenous administration of xylazine at 0.4 mg/kg BW, decreases heart rate by 30 % and a bolus of 0.5 mg/kg BW causes an immediate decrease in heart rate by 25 % and in cardiac output by 37 %, followed by a sustained bradycardia for 60 minutes 32. In contrast, an intramuscular administration of xylazine at a dose of 0.05 mg kg induces only minor cardiovascular changes 33. Xylazine possesses hypotensive and hypoxic effects by depressing cardiovascular and respiratory activities. In contrast to most anesthetic drugs, ketamine has been shown to have incremental effects on heart rate, blood pressure and respiratory rate, due to the increase in sympathetic activation 34. Lin et al. 35) reported a transient bradycardia followed by a period of stabilization during administration of ketamine. In this study, heart rate increased in both anesthetics protocols during manipulation of the uterus and the flushing in surgery. Singh et al 36 described the autonomic response, such as tachycardia and hypertension, due to the manipulation of organs during surgical procedures.
Although cyclical contractions of the reticulum and rumen are inhibited by administration of xylazine due to sympathetic blockade and reduction in norepinephrine release 37, no significant differences between rumen motility measured before and after anesthesia with xylazine-ketamine or xylazine-ketamine-guaifenesin were observed.
Regarding body temperature, there were no significant differences between rectal temperatures measured before and after anesthesia. In the present study, the decrease in rectal temperature of sheep identified after xylazine administration was not clinically significant. The decrease in body temperature caused by depression of the thermoregulatory center 38 and reduced muscular activity 39 may be the result of administering α2-agonists. Nevertheless, Zuhair et al. 40 reported a significant decrease in the body temperature of sheep after 60 minutes of anesthesia with a combination of xylazine-ketamine. The results indicated a stable body temperature in all the ewes.
In this study, induction with xylazine-ketamine was significantly higher in time and smoother compared to the xilazine-ketamine-guaifenesin protocol. There were no time significant differences between the two groups during surgery, although recovery was significantly faster in the xylazine-ketamine group than the xylazine-ketamineguaifenesin group. After standing, the return of muscle strength appeared to be more complete, as the sheep in this group appeared to be less ataxic and they were able to walk sooner than those anesthetized with xylazine-ketamine-guaifenesin. A rapid recovery is generally considered desirable, if it is accompanied by a shorter period of anesthesia. The longer recovery time in XKG ewes may be attributed to slower drug elimination from the plasma and to the guaifenesin muscle relaxant effect. Guaifenesin's mechanism of action is to disrupt nerve impulse transmission at the level of the internuncial neurons of the spinal cord, brain stem, and subcortical areas of the brain 41.
When compared to xylazine-ketamine anesthesia, xylazine-ketamine-guaifenesin induced comparable anesthetic effects in healthy sheep. Changes in the cardiopulmonary system were similar in both anesthetic regimens and these changes were within acceptable clinical ranges. The time it took for the sheep to stand was significantly shorter for xylazine-ketamine than the combination of xylazine-ketamine-guaifenesin. We conclude that, in short surgical interventions involving midline laparotomy, xylazine-ketamine anesthesia is preferred over xylazine-ketamine-guaifenesin, as it achieves a rapid anesthetic induction, maintenance of physiological parameters within optimal limits and a faster recovery.














