Introduction
Fasciola hepatica (also known as “the Liver Fluke”), is the main trematode responsible of fasciolosis, which affects bovines and goat livestock populations worldwide (1). The infection is not just a problem for cattle, but also a public health issue, considered as an increasingly relevant zoonosis in many countries (2). One of the most important aspects of fasciolosis infection is that the pathology and disease take place as early as three or four weeks after infection. During this time, the diagnosis can only be confirmed by coprology at two months post infection in cattle (3). This parasite can infect animals and last for years inside the definitive host without causing death. The fact that this parasite can persist for long periods of time suggests that the feces of infected animals can contaminate pastures and intermediate hosts, allowing it to extend in time the cycle of the parasite (4).
The epidemiology of fasciolosis is strongly related to the ecology of the snails that serve as intermediate hosts of the parasite. Lymnaeid snails are responsible for F. hepatica transmission worldwide (5). In Cuba, there are only two types of lymnaeid snails, Galba cubensis and Pseudosuccinea columella, whose susceptibility to F. hepatica infection has been proved (6, 7, 8), and their settlement in subtropical areas is favored by the high moisture associated with frequent rainfall and moderate temperatures. Other malacological studies (7,9,10,11) have been carried out in Cuba, which made it possible to understand the biology and population dynamics of lymnaeid snails.
On the other hand, between 1944 and 2007, at least seven major human outbreaks have been reported in Cuba, three of which occurred in the Pinar del Río province, involving more than 1000 people (12). This province is one of the most used for cattle breeding, but it is not frequent to find reports regarding the prevalence of fasciolosis in bovines. Nevertheless, it is well known that local endemic animal fasciolosis is commonly associated with human infection (13) and the reservoir role of domestic animals is related with transmission of human fasciolosis (14).
Taking into account the severe impact on Cuban bovine livestock farming due to fasciolosis (15,16) and the effect of this disease on the human population (12), the aim of this work was to determine the prevalence of Fasciola hepatica in cattle and its association with the intermediate hosts inhabiting in a rural area of the Pinar del Río province.
Material and Methods
The study was carried out in a livestock farming area (including 14 farms with 1528 bovines) located in the Consolación del Sur municipality, Pinar del Río province, Cuba, from February 2011 to March 2012. The entire area surrounding the rural community of “El Canal” is inhabited by more than one thousand people, whose main source of employment is stockbreeding.
Ten farms were randomly selected. The infection of F. hepatica in cattle was determined in each farm as well as in the collection of snails (Figure 1).
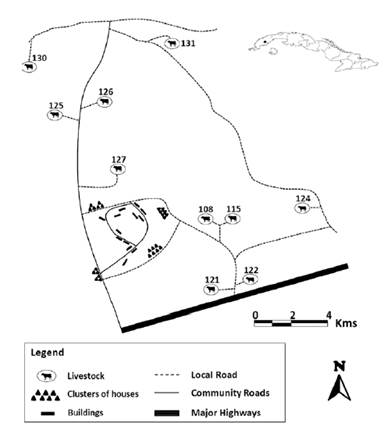
Figure 1 Map of the “El Canal” community, Consolación del Sur municipality, Pinar del Rio province, Cuba. Source (17).
Study animals: A single stool sample from 455 dairy cows was studied. The animals were randomly selected, and the samples were kept at -20oC until the infection was determined. The totality of cattle was from dairy farms that grazed in open field pastures of mixed grasses (Panicum maximum, Brachiaria brizantha, Cynodon nlemfuensis) and legumes (Leucaena leucocephala and Pueraria phaseoloides). The cattle were also given harvested forages (Saccharum officinarum, Pennisetum purpureum).
Detection of Fasciola hepatica infection: It was carried out using a non-commercial sandwich-ELISA called FasciDIG®, which was developed for diagnosis in humans and later validated in animals (18,19).
Collection of snails: A total of 50 snails were collected from each bovine livestock farm using forceps and sieves (11). The mollusks were kept in moist conditions and taken to the Malacology Laboratory at the Institute of Tropical Medicine where they were subsequently dissected to identify larval stages of F. hepatica.
Results
F. hepatica infection in cattle
Considering the ten farms studied, 146 of a total of 455 collected faecal samples tested positive for F. hepatica (32.09%). Every farm was found to be infected with F. hepatica, which varied in prevalence with ranges from 9.5% to 84% among farms (Table 1).
It was also possible to detect the presence of the two species of intermediate hosts of F. hepatica in Cuba, Galba cubensis and Pseudosuccinea columella, with the former as the only one infected with F. hepatica (table 1).
Table 1 Fasciola hepatica infection in cattle and presence of the parasite in intermediate snails collected in the livestock
| Livestock | Cattle | Intermediate snails of F. hepatica | |||
| Total | Infected by F. hepatica | ||||
| No. | (%) | G. cubensis | P. columella | ||
| 108 | 80 | 26 | (32.5) | - | - |
| 115 | 63 | 6 | (9.5) | - | 0 |
| 121 | 39 | 12 | (30.8) | - | 0 |
| 122 | 35 | 16 | (45.7) | + | 0 |
| 124 | 25 | 21 | (84.0) | - | 0 |
| 125 | 87 | 15 | (17.2) | - | - |
| 126 | 20 | 5 | (25.0) | - | 0 |
| 127 | 56 | 17 | (30.4) | + | 0 |
| 130 | 25 | 17 | (68.0) | - | 0 |
| 131 | 25 | 11 | (44.0) | + | 0 |
| Total | 455 | 146 | (32.09) | ||
(0) snail species absence, (-) negative for F. Hepatica, (+) positive for F. hepatica
Freshwater snail associated to the livestock farms
Eight species of freshwater snails (six pulmonates and two caenogastropods) were found in the entire area. Detailed information about snail species and the type of ecosystem found in each farm is summarized in Table 2.
G. cubensis was found in every farm, while P. columella occurred only in two of them. The savanna habitat and the low species richness predominated, with the exception of the pond located nearby farm 125, where the highest number of snail species was found.
Table 2 Presence/absence of freshwater snails in the studied farms
| Livestock | Habitat | Families of fresh-water snails | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lymnaeidae | Physidae | Planorbidae | Thiaridae | Ampullariidae | |||||
| G.cub | P. col | P. acu | D.ana | D.luc | B.hel | T. gra | P. poe | ||
| 108 | Savanna | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 115 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 121 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 122 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| 124 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 125 | Pond | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| 126 | Savanna | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 127 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| 130 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 131 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| (0) Absence (1) Presence | G.cub= Galba cubensis; P. col= Pseudosuccinea columella; P.acu= Physa acuta; | D.ana= Drepanotrema anatinum; D.luc= Drepanotrema lucidum; B.hel= Biomphalaria helophila; T.gra= Tarebia granifera; P. poe= Pomacea poeyana | |||||||
Discussion
Fasciolosis causes significant economic losses worldwide in animal husbandry, mainly ruminants, estimated at US$ 3 billion per annum, due to reduction in meat and milk production (20). The Cuban bovine cattle is a product of a prolonged process of natural selection, of the descendants of the cattle Bos taurus introduced by the Spanish conquerors and of animal coming from Africa, mainly Bos indicus. This crossbreeding has allowed great adaptability to tropical climates and more resistance to the infectious diseases (21). However, and in spite of this, cattle losses due to F. hepatica infection are still reported every year (16,22).
In the present study the number of cattle infected found in the whole area can be due to a number of factors such as: (I) every farm is located in a suitable ecosystem for lymnaeid snails settlement; (II) bad management of livestock allows the encounter of both intermediate and definitive hosts; and (III) grazing animals usually drink contaminated water with metacercariae in the field. During the dry season, ponds and streams experience a significant shrinkage and snail populations are forced to concentrate in the remaining waters, which are at the same time the only one available for cattle. Thus, infection may increase severely under these conditions.
The levels of cattle infection (32.09% in the whole area) are a reflection of this problem. Since every infected animal is an excellent disseminator of the parasite (rates of excretion of F. hepatica eggs can reach thousands/day; see (4) for review), these numbers may actually be considered as very high. The prevalence found in this study also agrees with the values found in other countries (23,24,25,26), and similar results of infection rates have been found in Chile (30.1% of infected cattle) (24) and Zimbabwe with the Fasciola gigantica species (31.7% of infected cattle) (27). A higher prevalence of 46.58% has been reported in Ethiopia (28), and the level of infection in a study carried out in five districts of Vietnam varied from 60 to 76% (13).
Fasciolosis is considered the vector-borne disease that has the widest geographical distribution (1,29), placing it as the most prevalent helminthic infection in cattle in the tropics with a variable frequency from 30 to 90% (30). In Cuba, this disease is one of the most important parasitic diseases in livestock animals (12,31) and differences in prevalence of fasciolosis in different areas may be attributed to variations in ecological, climatic and animal husbandry practices (32).
The diagnosis by FasciDIG® allowed the quick detection of F. hepatica, especially because this technique only needs a single sample to detect coproantigens. It also provides positive results four weeks before egg excretion starts, preventing economic losses (15,19,33). Also, the use of feces rather than serum to perform the test has better practical and ethical advantages. This method has been validated in human and animals using stool samples (18,19,34) and has been successfully used in fasciolosis-endemic areas of South America like Colombia, Argentina, Peru, Uruguay, and Venezuela, leading to more accurate diagnosis of this disease (23,35,36).
Intermediate snail host found
Most of the snail species found (P. poeyana, P. cubensis, T. granifera, D. lucidum, G. cubensis and P. columella) were also identified by Cañete, Yong, Sánchez, et al. (37) and Gutiérrez, Hernández, and Sánchez (11) in studies conducted in similar ecosystems in the province of Pinar del Río. Diversity in freshwater snail communities may affect the distribution and abundance of several species, especially those acting as intermediate host of parasites (38). Galba cubensis have been found as a dominant species in low diversity habitats, whereas in highly diverse ecosystems it may occur with low abundance (39). Interestingly, G. cubensis populations were found in all dairies studied, while P. columella was found in two livestock, which enhances the idea of the former being the main agent responsible for fasciolosis in Cuba (6,7,11,40).
The higher ecological diversity observed in the pond belonging to farm 125 may be due to its ecological features (abundant aquatic vegetation, depth, area). The results obtained in the distribution of G. cubensis in the whole area agrees with the general pattern observed in Cuba in this species (mainly in areas of low diversity and high human activity) while on the contrary, P. columella has more affinity to sites with less human activity (7). Although P. columella has been found only in two sites, it does not diminish its importance as a transmitter of F. hepatica. Its role in the transmission of fasciolosis has been referred in laboratory conditions (6,11,41) and recently in nature for the first time in Cuba and the Caribbean (8). Natural infection of this species has also been reported in other countries like Brazil, Australia, and Argentina (42,43), and it was introduced in France with the possibility of transmission capacity (44).
The rates of infection found in every farm indicate that the F. hepatica life cycle is running perfectly in the whole study area. This fact may jump to the idea that, although the sampling of non-infected snail may occur in a particular area where cattle are infected, it does not necessarily mean that they do not exist. The transmission of any parasite is a probabilistic event that ultimately depends on the distribution of its hosts (45). Hence, sampling size is a key factor to detect infected snails in the field but it is not a determinant fact to prove transmission.
The epidemiology of any snail-borne disease is closely related to the ecology of the snails. To date there are approximately 100 known species of lymnaeids, some of which have been identified in the Americas (G. cubensis, P. columella, Galba truncatula, Lymnaea humilis, G. viatrix, among others) (5). As a result of a presumed endemism of fasciolosis in Cuba and that there are favorable conditions (high humidity and rainfall) for the settlement of lymnaeids, several studies have been developed to investigate the ecology and population dynamics of these snails (7,11,41,46).
These investigations have helped to understand more about the epidemiology of this disease, but there are still some problems, such as lack of medicines to treat animals and poor husbandry practices that undermine the control of fasciolosis (12). Main attention must be given to the bovine prevalence found in this study to prevent future outbreaks of this trematodosis, as has occurred over the last few years in this geographical area.














