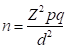Introduction
Nigeria with its estimated 180,000,000 population, is the most populous country in the African continent. It has a great proportion of the world's poor livestock keepers and provides a focal point for neglected zoonosis (1). Brucellosis is a devastating zoonotic disease causing huge economic losses for livestock farms around the globe (2, 3). It also poses serious human health hazards worldwide (4, 5, 6).
The genus Brucella comprises various species with both veterinary and human medical importance and contains a group of closely related species (7). This group includes Brucella melitensis affecting primarily small ruminants while B. abortus affects primarily cattle, B. suis affects pigs, B. ovis affects sheep, B. canis affects dogs while the other members include, B. neotomae, B.microti, B. ceti and B. pinnidepialis (8, 9, 10,11).
Brucella spp. infect not only their specific hosts but also other domestic and wild animal species, which in turn can serve as disease reservoirs for other animal species and humans (2). Brucellosis, a major neglected zoonotic disease, is transmitted by direct contact with infected animals, their secretions, or by ingesting their dairy products, which requires intensive attention in many communities around the globe (2, 12). It could also be transmitted by consumption of contaminated food of animal origin, and through aerosol (13). Infection can also result through contact with infected aborted materials such as aborted foetuses, placenta membranes or fluids and other vaginal exudates (2,11).
A report of brucellosis outbreak was firstly reported in a government cattle farm located in Zaria in 1934. Serum plate agglutination test showed positive reaction in about 15% of the total animals in the farm (14).
Brucellosis is a reportable disease in Nigeria, but its incidence, prevalence and distribution is difficult to determine as the system of disease surveillance and reporting is incomplete and inept (15,16). Serological prevalence rates between 0.20% and 79.70% have been reported in animals and humans in various parts of the country (17, 18, 19, 20, 21, 22) emphasis is laid mostly on cattle, sheep and goat; but the same cannot be said of dogs.
Canine brucellosis can lead to reproductive losses both in dog and human infections due to contact with infected urine or other genital secretions (23). Brucella canis, the aetiology of Canine brucellosis, is considered a rare cause of human brucellosis but the clinical importance of this infection may have been underrated so far because of frustrations and complications associated with making a diagnosis (24).
The rise in pet ownership in Nigeria, especially dogs, is linked with some risk factors that render the Nigerian human population vulnerable to this disease. It is worth mentioning that many imported foreign dog breeds are not screened before entering into our country (25), thus providing a source of infection. In turn, dogs from Nigeria can be found elsewhere around the world.
As a zoonosis, it is also regarded as a significant public health issue, thereby making susceptible some persons in occupational risk like butchers, abattoir workers, veterinarians and livestock owners as well as other humans (26, 27, 6).
This study will provide information on the current seroprevalence of Brucella antibodies in dogs in Enugu and Anambra States, Nigeria as well as the prevalent species. It will also provide information on sex, breed, and age distribution as well as some risk factors that may influence Brucella infection in dogs, thus contributing to the epidemiology of Canine Brucellosis in Enugu State and Anambra State, Nigeria.
Materials and Methods
Study design
The study was conducted as a cross-sectional survey using purposive sampling technique to screen dogs for Brucella antibodies.
Study Area
Enugu State is located between latitude 5o55”N and 7055”N and longitude 6053”E and 7055”E while Anambra State is located between latitude 6o20”N and longitude 7o00”E. Enugu state covers a total land area about 802,295 Km2 and has a population of 2,500,000 with a population density of 240 persons per square kilometers (28). On the other hand, Anambra State covers a total land area about 4,844 Km2 and according to the National Population Commission (2006), it has a population about 3,902,051 with a population density of about 840 persons per square kilometre (28). Dogs are kept as part of the people’s culture in these 2 States for breeding, hunting and protecting their homes. Dog meat is eaten by some parts of the population.
Study Population
The study purposively targeted 3 groups: (a) Dogs taken to veterinary clinics around the states; (b) Household dogs with history of infertility or abortion; and (c) Dogs at slaughter points in the markets as shown in Figure 1.
Sample Size Determination
The required sample size was determined using the following formula: (29)
Where n = Desired sample size, Z2 = 1.96 (normal distribution) from table, p = Prevalence rate from the average of previous studies, d = Desired absolute precision of ± 5% with 95% CI, q = 1 - p. In this study, according to Adedoyin (30), a prevalence rate of 7.94% was used for sample size determination. Using the formula above, a sample size of 112 was calculated. However, a sample size of 123 dogs was screened in this study.
Sampling Technique
Five major veterinary clinics, 2 in each Enugu and Onitsha metropolis, one in Nsukka Urban; 2 major slaughter points in each state; and households with dogs having history of infertility or abortion were selected by purposive sampling method. Visits were made to the purposively selected veterinary clinics, households, and slaughter points, once every other week for 6 months. A total of 123 dogs made up of 65 Clinic dogs, 34 Slaughter dogs and 24 Household dogs were screened. Profiles of the dogs taken to the clinics and household dogs were also collected. Gathered data include: sex, age, and breed, history of infertility / abortion in female dogs, and some possible risk factors / management practices to Brucella infection in dogs.
Collection of Blood Samples
Five mililiters of blood were aseptically collected from the cephalic vein of each animal, after proper restraint. The blood was kept in a slanted position for about 30 minutes to allow for proper clotting. It was then centrifuged at 3,000 rpm for 5 minutes. The sera was then harvested one after the other using separate syringes into well labelled bijoux bottles and stored at - 20oC until they were analysed.
Serological Sample Analysis
For B. abortus identification, 2 tests were used, namely, Rose Bengal Plate Test (RBPT) and Serum Agglutination Test (SAT). The Antigen was procured from Central Veterinary Lab., New Haw, Weybridge Surrey, England. Both of the tests were done as described by Alton et al., (31) and Morgan et al (32). Titres of 1:40 (50 IU/ml) and above were taken as diagnostic for B. abortus as determined by Morgan et al., (32) and Sati, (33).
Identification of B. canis was done using ImmunoComb® Canine Brucellosis Antibody Test Kit specific for B. canis antigen (Biogal Galed Labo., Kibbatz Galed 19240, Israel) and this was done as previously described by Muhairwa et al. (34). Furthermore, Blood bacterial culture was used for confirmation of the seropositive sampled dogs. The bacteria were cultured aerobically and the identity confirmed by standard gram staining, microscopic and biochemical tests characteristics as described by Chessbrough, (35).
Ethical Considerations
Ethical considerations guiding the use and behavior in experiments on animals were strictly observed and the experimental protocol was approved by the University of Nigeria Nsukka Senate Committee on Medical Research Ethics. Proper permit and consent were obtained from the Veterinary Clinics before obtaining the data for this experiment.
Statistical Analysis
Using Statistical Package for Social Sciences (SPSS) 17.0, Chi-square (χ2) statistic, odds ratio and 95% confidence interval were used to determine whether there were significant association between Brucella antibody prevalence and sex, age, breed, history of infertility/abortion in female dogs, and some possible risk factors/management practices to Brucella infection in dogs.
Results
Distribution of Dogs based on States and Sources
Over the study term, a total of 123 dogs were screened. Table 1 summarizes the sources/categories of the dogs screened. Enugu State accounted for 68 of the total dogs while Anambra accounted for 55 of the total dogs screened. Veterinary clinics accounted for 65 of the total dogs screened while Slaughter points and households for 34 and 24 dogs screened, respectively.
Prevalence of Brucella abortus Antibodies Based on the Sources of the Dogs
Out of the 123 sera samples screened for Brucella abortus antibodies, none (0%) was positive using both the Rose Bengal Plate Test (RBPT) and the Serum Agglutination Test (SAT) (Table 2).
Table 2 Prevalence of Brucella abortus Antibodies Based on the Sources of the Dogs
| Source | Number screened | Number positive | |
|---|---|---|---|
| RBPT | SAT | ||
|
Vet. Clinics (Clinical cases) Slaughter points Households |
65 34 24 |
0 (0.00) 0 (0.00) 0 (0.00) |
0 (0.00) 0 (0.00) 0 (0.00) |
| Total | 123 | 0 (0.00) | 0 (0.00) |
Legend: RBPT=Rose Bengal Plate Test SAT= Serum Agglutination Test.
Source: own work
Prevalence of Brucella canis Antibodies Based on the Sources of the Dogs
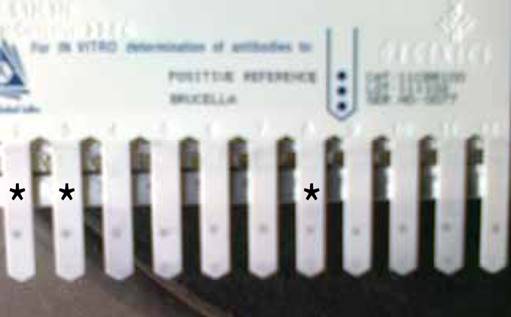
Out of the total 123 dogs from the different sources screened, 34 were positive for B. canis antibody using the Immunocomb® Canine Brucella Antibody Test Kit as shown in Figure 2. This gave an overall seroprevalence rate of 27.7%. Out of 65 dogs screened in Veterinary Clinics (clinic dogs) in Anambra and Enugu States, 22 or 18% were positive for B. canis (Table 3). Four (3.3%) out of 34. slaughter dogs screened were positive. Out of the 24 household dogs screened, 8 or 6.5% were positive. There was no association between infection rate and sources/categories of the dogs screened (χ2 = 5.925, P>0.05).
Source: own work
Figure 2 Immuno-Comb showing positive serum samples * represents the positive serum samples.
Table 3 Prevalence of Brucella canis Antibodies Based on the Sources of the Dogs
| Source | Number screened | Number positive Immunocomb® B. canis Antibody Test Kit |
|---|---|---|
|
Vet. Clinics (Clinical dogs) Slaughter points Households |
65 34 34 |
22 (18) 4 (3.3) 8 (6.5) |
| Total | 123 | 34 (27.7) |
(χ2 = 5.925, P>0.05)
Source: own work
Antibody Titre Levels of B. canis Positive Dogs Screened in Anambra and Enugu States
Out of the 34 B. canis positive dogs as shown in Figure 1, 16 (47.1%) had a titre level of 1:200 (IFA Titre); 10 (29.4%) had titre level of 1:400 (IFA Titre), 4 (11.8%) had a titre level of 1:600 (IFA Titre); while the remaining 4 (11.8%) had a titre level of 1:800 (IFA Titre) (Table 4).
Table 4 Antibody Titre Levels of Brucella canis Positive Dogs Based on the Sources of Dogs
| Titre level | Total | Clinical dogs (%) | Slaughter Dogs (%) |
Household Dogs (%) |
|---|---|---|---|---|
| 1 : 200 | 16 | 10 (29.4) | 4 (11.8) | 2 (5.9) |
| 1 : 400 | 10 | 7 (20.6) | 3 (8.8) | 0 (0) |
| 1 : 600 | 4 | 3 (8.8) | 0 (0) | 1 (2.9) |
| 1 : 800 | 4 | 2 (5.9) | 1 (2.9) | 1 (2.9) |
| Total | 34 | 22 (64.7) | 8 (23.5) | 4 (11.8) |
(χ2 = 3.767; p>0.05)
Source: own work
Antibody Titre Levels of Brucella canis Positive Dogs Based on the Sources of Sogs
The antibody titre levels of B. canis positive dogs based on sources/categories of screened dogs are shown in Table 4. Ten (29.4%) of 16 positive dogs with antibody titre 1:200 (IFA Titre) are clinic dogs; 4 (11.8%) are slaughter dogs and 2 (5.9%) are household dogs. Seven (20.6%) of 10 positive dogs with titre 1:400 (IFA Titre) are clinical dogs; 3 (8.8%) are slaughter dogs and none from the households. Five (14.7%) of 8 dogs with titre 1:600 (IFA Titre) and above are clinic dogs; 2 (5.8%) were household dogs and only one (2.9%) was a slaughter dog. Chi- Square analysis showed no association between antibody titre levels and sources of the dogs (χ2 = 3.767; p>0.05). Two household dogs and 2 of the 5 clinic dogs with titre 1:600 (IFA Titre) and above had history of recent abortion or infertility.
Sex Distribution of Brucella canis Antibody Positive Dogs
Female dogs had a seroprevalence of 22% (Table 6) while male dogs had a seroprevalence of 6%. There was a strong association (p<0.05) between the infection of Brucella canis and sex of the dogs screened (Table 5).
Antibody Titre Levels of B. canis Positive Dogs according to Sex
Out of the 27 positive female dogs, 13 (38.2%), 8 (23.5%), 3 (8.8%) and another 3 (8.8%) had antibody titre levels of 1:200 (IFA Titre), 1:400 (IFA Titre), 1:600 (IFA Titre) and 1:800 (IFA Titre), respectively. In males out of the 7 positive dogs, 3 (8.8%), 2(5.9%), 1 (2.9%) and 1 (2.9%) had titre levels of 1:200 (IFA Titre), 1:400 (IFA Titre), 1:600 (IFA Titre) and 1:800 (IFA Titre), respectively. Chi-square analysis showed that there is no association (P>0.05) between titre level and sex (Table 6).
Age Distribution of Brucella canis Antibody Positive Dogs (Clinic and Household Dogs)
Dogs below 1 year old had a seroprevalence of 3.4%, dogs 1-<3 years of age had seroprevalence of 10.1%, while those 3-<5years and 5 years and above, had seroprevalence of 15.7% and 4.5%, respectively. Thus, 23 out of 66 dogs aged between 1 year and 5 years were positive, having a seroprevalence of 34%. There was no association (p>0.05) between Brucella canis infection and age in the dogs screened (Table 7).
Breed Distribution of Brucella canis Antibody Positive Dogs
Twenty-six out of 76 exotic breeds of dog were positive for B. canis antibody, which provides a seroprevalence of 21.1% (Table 8). Mixed and local breeds each had seroprevalence of 3.3%. Chi-square analysis revealed that there was a strong association (P<0.05) between infection and the breeds of dogs, being the infection higher in exotic breeds of dogs.
Table 8 Breed Distribution of Brucella canis Antibody Positive Dogs
| Breed | Number sampled | Number Positive (%) |
|---|---|---|
| Exotic* | 76 | 26 (21.1)a |
| Mixed | 13 | 4 (3.3)b |
| Mongrel | 34 | 4 (3.3)b |
| Total | 123 | 34 (27.6) |
(Χ2 = 0.04; p<0.05)
*Total no of dogs sampled= Rottweiler 28 (36.8%); Mastiff 20 (26.3%); Caucasian 13 (17.1%); Alsatian 13 (17.1%); Boar bull 1 (1.3%); Persian 1 (1.3%).
Source: own work
Table 9 shows the risk factors associated with the presence of Brucella canis antibodies in dogs sampled in Enugu and Anambra States of Southeast Nigeria. Female dogs, exotic breeds and freely roaming are at a higher risk of Brucella infection in the study area.
Table 9 Risk Factors associated with the presence of Brucella canis antibodies in dogs presented at the clinics and household dogs sampled in Enugu and Anambra States
| Parameter | Total no sample | P. value |
|---|---|---|
| SEX Male Female |
45 78 |
0.023* - |
| AGE Less than 1 year | 11 | 0.682 |
| 1 - < 3years | 32 | |
| 3 - < 5years | 34 | |
| 5 and above | 12 | |
| Breed Exotic | 76 | 0.050* |
| Mixed Mongrel | 13 | |
| Local | 34 | |
| Other animals in the household Sheep | ||
| Sheep | 1 | 0.072 |
| Others | 6 | |
| None | 82 | |
| Animal move freely in the neighbourhood | ||
| Yes | 35 | 0.000* |
| No | 54 | |
| Feeding habits of dog | ||
| Scavenging all the time | - | - |
| Both scavenging and household | 27 | 0.354 |
| Household food only | 62 | |
| Purpose of keeping dogs | ||
| Companion for children/security | - | - |
| Breeding | 87 | 0.308 |
| Hunting | 2 | |
| Dog sharing rooms with the household | ||
| Yes | 22 | 0.462 |
| No | 67 | |
| History of infertility/abortion in females | ||
| Yes | 46 | 0.898 |
| No | 22 | |
| Male dog used for mating | ||
| Yes | 16 | 0.627 |
| No | 5 |
Source: own work
Discussion
Brucellosis is endemic in Nigeria and risk factors enhancing its transmission are prevalent (36, 16). Brucella abortus antibody was not detected in any of the 123 dogs screened. The zero prevalence suggests that B. abortus, though important in livestock ruminants, is not significant in the epidemiology of canine brucellosis in the study area. The apparent absence of B. abortus antibody may be attributed to the type of management system practiced in Southeast Nigeria. Dogs are either housed or caged and though they may roam freely in the neighbourhood, they rarely come in contact with livestock ruminants. The dogs medical profiles also showed that most clients feed their dogs with cooked household foods only. Therefore, the likelihood of being infected with B. abortus is rare. The findings of this study on B. abortus is in contrast to the works of Cadmus et al., (25) and Adedoyin et al., (30) who reported seroprevalence of 5.46% and 2.48%, respectively, in household dogs in Ibadan, Southwest Nigeria.
The result of the present study suggests a high seroprevalence (27.7%) of B. canis in dogs taken to veterinary clinics, apparently healthy slaughter dogs and those in households which were screened. None of the dogs screened in the study were vaccinated, as vaccination of dogs against brucellosis is not routinely carried out in Nigeria because there is no information of any vaccine against B.canis (37). Therefore, appearance of antibodies to Brucella in dogs in the study area is suggestive of natural exposure to the organism. It also indicates the lack of brucellosis control programme. The seroprevalence of B. canis in this study was relatively high when compared to other studies in Nigeria. This may be attributed to the higher sensitivity of the diagnostic technique (Biogals Immunocomb® Canine Brucella Antibody Test Kit) used. Cadmus et al., (25) and Adedoyin et al., (30) reported a seroprevalence of 0.27% in household dogs and 3.11% in household and hunting dogs, respectively, using B. canis Rapid Side Agglutination Test (RSAT). However, this result was lower than the prevalence of 59.43% reported by Cadmus et al., (25) in dogs used for hunting and a seroprevalence of (29.2%) which was reported by Momoh et al., (38). The hunting dogs may have higher exposure probability to Brucella spp according to the studies conducted by Cadmus et al., (25).
Female dogs had a higher seroprevalence percentage (21.3%) than male dogs (6.0%). A major contributing factor to higher rates in females could be that a single male dog, if infected, is used in mating different females, it can transmit the infection through infected semen (25). Also it may be due to the fact that most dog owners in our study area preferred to keep more female dogs than males for the purpose of additional income through the sale of their puppies. This increases the chances of more females getting infected during mating. However, Radostits et al., (39) have shown that erythritol, a polyhydric acid found in higher concentration in the placentas and foetal fluids of females than in seminal vesicles and testis of males, can be responsible for females being more susceptible than males. This result was in agreement with the findings by Cadmus et al. (25) who reported a prevalence of 6.17% in females and 4.9% in males and Momoh et al., (38) who reported a prevalence of 29.3% in female dogs and 28.6% in male dogs. However, it disagrees with findings in a previous study where a slightly higher rate in males (29.6%) than in females (26.7%) was recorded by Adesiyun et al., (40).
The decrease in the positive samples as titre level increases may be attributed to the disposing or selling off of non-producing dogs by humans as the infection may have entered into a chronic phase, thereby making these infected dogs not to be productive while those with lower titre levels are more in number because the infection may not have started manifesting its clinical signs.
Dogs taken to the clinics had the highest numbers of positive samples in the different titre levels and this may be attributed to the fact that more samples were gotten from clinical cases more than the other categories of dogs sampled.
The different titre levels were also higher in females more than in males and this may be attributed to the fact that female dogs where sampled more in the study. In addition, due to the asymptomatic nature of B. canis in dogs, most male dogs used for breeding are unscreened, and carry the infection for a long period of time, shedding it in the environment through urine and semen; this may result in bitches being mated many times by the same infected male dog as a result of repeated unsuccessful breeding attempt, thereby increasing the infection load in females.
Prevalence was lower among the young animals screened as compared to the older ones. Usually young animals are protected by maternal immunity and thus they are less susceptible to infections. This shows that the infection increases with age. The high prevalence seen in older animals shows the chronic nature of brucellosis as it has been shown to increase with age, and most affected animals carry the infection throughout their lives (39). The reason for the increase in prevalence as the animal age increases may be due to the fact that the bacteria localizes mainly in the reproductive tracts, especially in gravid animals. There is also evidence that the mammary gland may be even a more probable area of localization than the reproductive tract (41). Age-wise prevalence studied by Aulakh et al. (42), Abubakar et al., (43) and Momoh et al.(38), showed that the incidence is higher in sexually mature animals. Therefore, the increase in age, increases probability of exposure to infection in dogs. However, the results in this study do not agree with previous study by Cadmus et al. (25), as they reported more prevalence in dogs below one year old than in adult dogs.
There is a strong association between the infection rate and breeds of dogs screened; with infection occurring more in the exotic breeds than the mixed and local (mongrel) breeds. This may be related to the fact that exotic breeds are the dogs of choice among owners in both states and, therefore, they were the prevalent breeds (61.8%) in the population sampled (76/123). This way the probabilities of occurrence is higher in these breeds. Behzadi and Mogheiseh (26), argued that the detection of canine brucellosis in exotic dogs may indicates a new source of infection from abroad as these dogs may be imported from countries and regions where the disease is endemic. The higher prevalence among the exotic breeds is in agreement with the findings by Behzadi and Mogheseh (26); they recorded a prevalence of 19.35% in exotic breeds. It is also in agreement with the findings of Cadmus et al., (25) who got 50.55% in Alsatian breeds of dogs.
There is a strong association (P<0.05) between B. canis infection and some risk factors such as sex, breed and dogs moving freely in the neighbourhood; This was further supported by the multivariate logistic regression. Notwithstanding, some of the other risk factors such as age, dogs being used for breeding, dogs sharing rooms with humans, female dogs with history of infertility and abortion, and male dogs used for mating, all provided a considerable higher positive numbers and increased the chances of Brucella infection in dogs in the study area, even though the numbers are not significant.
Clinical diagnosis of canine brucellosis just based on the clinical signs is not sensitive enough and a negative blood culture cannot rule out the disease (44; 45). Apart from the use of clinical signs and microbiological cultures, serological tests such as Rapid Slide Agglutination Test (RSAT), 2-mercaptoethanol Rapid Slide Agglutination Test (2ME-RSAT), Agar Gel Immunodiffusion (AGID), Indirect Enzyme Linked Immunoassay (IELISA) and Polymerase Chain Reaction (PCR) can be used for routine diagnosis (46, 44).
Based on the findings of this study, we deduced that the zero seroprevalence of B. abortus antibodies in dogs in this study rules out the possibilities of mixed infections but does not imply the absolute absence of the disease in the studied localities. However, it may be inferred that B. abortus in dogs does rarely occur in this region, except when the dogs are in close contact with infected farm animals, as proven by previous researchers.
This study has also shown a Brucella canis antibodies seroprevalence of 27.6 % in the study area. It means that the general population is at risk and it calls for serious interventions considering the zoonotic implications of the disease as infected dogs can be a source of infection not only to animals but also to humans, especially in close contacts with these animals due to their occupation. Some factors such as sex, breed, age, dogs moving freely in the neighbourhood, dogs being used for breeding and male dogs used for mating all of them increase the chances of Brucella infection in dogs in the study area and, therefore, should be considered in the epidemiology of canine brucellosis in the study area.
The Immunocomb® Canine Brucellosis Antibody Test Kit used for this study has proven to be a reliable test method (99% sensitive and specific) in the diagnosis of Brucella canis in dogs, which agrees with the findings by Muhairwa et al., (34) and Chinyoka et al., (47).
We therefore make the following recommendations: free roaming of dogs in dog care management practices is a risk factor for transmission of Brucella canis infection in the study area; thus, dogs should be restricted within cages in a fenced home. Feeding dogs with fresh carcasses from abattoirs is associated with higher seropositive of Brucella canis infection; then this habit should be avoided, unless carcasses are cooked. Exotic breeds of dogs imported into Nigeria should be properly screened for Brucellosis. Control measures should also be enforced in the study area to remove or eradicate the infections among dogs and avoid the spread to other uninfected dogs as well as the possible transmission to humans. We also recommend that appropriate hygienic measures such as proper disposal of aborted fetuses, placenta and other contaminated materials and disinfection of kennels, premises should be strictly observed by the dog owners, especially those having dogs as pets and allow them into their homes. Finally, Butchers, animal health workers, meat handlers and laboratory workers, including veterinarians, should wear protective garments to avoid direct contact and inhalation of contaminated aerosols in the clinics and kennels. State and federal health agencies should create a veterinary public health unit that will be handling zoonotic diseases as in the developed countries, for the prevention, surveillance and control of zoonosis in general and brucellosis in particular.