Introduction
Poultry production in both developed and developing countries such as Nigeria is being hampered by the expensive cost of feed and drugs used in the therapeutic treatment of diseases (1). These reasons forced researchers to divert their attention to non-conventional feed and drug sources. In Enugu State, Southeast, Nigeria, cashew stem is being used as a chewing stick for the treatment of cough and for clearing the congested throat.
Medicinal plants are known to be a significant source of new chemical substances with potential therapeutic effects; therefore, interest in medicinal plants has become increasingly stronger in recent times with great emphasis on their potential therapeutic effects both on humans and animals (2,3). Evaluation of harmlessness or danger caused by substances such as pharmaceuticals and natural products prior to their use is very important and must be undertaken (2). Laboratory studies on blood profile are an important device that aid in detecting any abnormal change (4), especially when a new substance is introduced into the body system.
The medicinal use of Anacardium occidentale (AO) plant has been observed worldwide. Anacardium occidentale belongs to the family Anacardiaceae. It is popularly known as cashew tree and originated from Brazil. The Anacardiaceae family consists of several plants with a lot of pharmacological activities. Out of these plants, AO has been reported to have great pharmacological and therapeutic activities (5). Anacardium occidentale tree has been known as a multipurpose tree whose leaf, stem and bark extracts are broadly used for treatment of different diseases (6,7,8). Early researchers have shown that AO possesses Phyto-constituents such as saponins, tannins, among others, which have been known to have antioxidant activities (6,9). It has been established that AO can be used in treatment of many ailments such as tumours, venereal diseases, warts, and nasal congestion (7,10).
Hematologic and serum biochemistry parameters are then used to evaluate the systemic relationship and physiological changes in the body of animals exposed to toxicants and stresses due to environmental factors (11,12). The integrity of the hepatocellular membrane, skeletal, smooth, and cardiac muscle fibres when compromised, will lead to discharges of the marker enzymes into the blood (13,14).
In our earlier work, Anacardium occidentale stem extract has been established to be relatively safe in in-vitro and in-vivo models (15); however, its effects in hematologic and serum biochemistry have not been investigated. Hence, the present study evaluates the hematologic and serum biochemical changes in cockerels administered with graded acute oral doses of stem extract from AO.
Materials and methods
Collection and identification of Anacardium occidentale
The AO stem was collected from a Nguru cashew plantation in the Nsukka Local Government Area, Enugu State of Southeast Nigeria, in January 2018. It was identified and authenticated by a plant taxonomist at the Department of Plant Science and Biotechnology, University of Nigeria Nsukka and the voucher sample was provided/kept (UNN|H.AO|2018.1).
Preparation and extraction of the plant
The AO stem was dried in the shade in World Bank Assisted STEP B Drug Discovery laboratory, at the Department of Veterinary Physiology and Pharmacology, UNN. Dried specimens were pulverized using a hammer mill at the Department of Crop Science, UNN. The pulverized material was extracted using cold maceration by soaking 500 grams in 3 litres of 70 % hydro-methanol for 48 hours. The extract was filtered using Whatmann filter paper n.° 1 and concentrated with hot air oven (Gallenkamp) at 40 °C. The crude extract was weighed to determine the percentage yield. The hydro-methanol stem extract from AO was stored in a refrigerator at 4 °C before its use. Reconstitution of the extract was done using distilled water. The brine shrimp lethality and acute toxicity test of stem extract was done using standard procedures as described by Omeke et al. (15).
Experimental Birds
Forty-day-old cockerels were procured from Zartech Hatchery Ibadan, Oyo State, Southwest Nigeria for this experiment and were bred for two weeks keeping the biosecurity measures. Feed (Top Starter and Grower Feed®, Premier Feeds Company Ltd, Ibadan, Nigeria) and water were supplied ad libitum.
Experimental Design
At eight weeks of age, the birds were randomly assigned into four groups (1, 2, 3 and 4) with 10 birds each. The extract was administered orally in graded doses of 3000, 1500 and 500 mg/kg body weight (bw) to groups 1, 2 and 3, respectively. Group4 (control) received drinking water (10 mg/kg). They were monitored for 48 h post-treatment. The birds were weighed before starting the experiment (before treatment) and 48 h post-treatment, and a week post-administration (PA).
Haematology
Collection of Blood Samples
Blood samples (1 ml) were collected from five birds through wing vein prior to treatment (day 0). Forty-eight hours after administering the extract, one millilitre of blood sample was randomly collected from five birds for haematology into sterile sample bottles containing ethylenediaminetetraacetic acid (EDTA). Packed cell volume (PCV) was determined by the microhematocrit method (16,17). The haemoglobin concentration (HbC) was determined by the cyanmethemoglobin procedure (18,19) with an automated blood biochemistry analyser (Wuxi Hiwell Diatek Instruments Co. Ltd, Wuxi, China). Red blood cell (RBC) and total white blood cell (TWBC) counts were done by the haemocytometer method (20,21,22). Differential leucocytes count (DLC) was determined using the Leishman technique (21,22).
Serum Biochemistry
Two millilitres of blood were randomly collected from five birds into plain sample bottles for serum biochemistry. At each collection, the blood samples could clot for 45 minutes and centrifuged at 3000 revolutions per minute. The serum supernatant was carefully aspirated and transferred to Eppendorf tubes (1.5 ml capacity) and used immediately for the biochemical analysis.
The serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were assayed based on the Reitman-Frankel colorimetric method (23,24), using commercially available ALT and AST test kit sourced from Química Clínica Aplicada (QCA, Spain). The serum alkaline phosphatase (ALP) activity was determined by the phenolphthalein monophosphate method (24, 25), using the ALP test kit (QCA, Spain). Cholesterol was determined by enzymatic colorimetric test (Chop-Pap) procedure (26). Serum total protein levels were assayed by direct Biuret method (31), while the serum albumin level was determined based on the bromocresol green method (27,28), using total protein and albumin test kits (QCA, Spain), respectively. Serum globulin was also assayed (27). The serum calcium levels were determined by the ortho-cresol phthalein method (29), while the serum phosphorus determination was done following Fiske-Subba Row procedure (30,31), using the calcium and phosphorus test kits (QCA, Spain), respectively. Serum uric acid levels were also assayed (32), using the uric acid test kit (Dialab, Austria), while the serum creatinine was determined by the modified Jaffe method (33).
Gross Pathology and Histopathology
At the termination of the experiment, three birds from a group were humanely sacrificed through cervical dislocation for gross and histopathological examination. Spleen, liver, and Bursa of Fabricius samples were collected. They were specifically examined for atrophy, enlargement, or presence of ulceration. They were fixed in 10 % buffered formalin for not less than 24 hours. The histopathology was done as described by Drury and Wallington (34). The slides were stained with haematoxylin and eosin stains. Photo-microscopic pictures were taken using 3.2 moticam.
Results
Plant extraction and acute toxicity of MSEAO: The extraction yielded 5.30 % w/w solid material. The birds in all the groups did not show any clinical signs of abnormality post-administration of the extract. There was no significant (P>0.05) variation between the mean body weight of the extract-treated groups and the birds that received distilled water (control). See Table 1.
Table 1 Mean body weight of birds (g) administered with graded doses of hydro-methanol stem extract from Anacardium occidentale (MSEAO)
| Days weighed | Mean body weight ± Standard error of the mean | |||
|---|---|---|---|---|
| Group A (3000 mg/kg) | Group B (1500 mg/kg) | Group C (500 mg /kg) | Group D (control) | |
| Day 0 | 454.00 ± 36.47 | 394.00 ± 71.97 | 428.00 ± 84.68 | 520.00± 135.09 |
| 48 h PA | 710.00 ± 89.44 | 610.00 ± 96.18 | 640.00± 138.74 | 622.00± 119.87 |
| One Week PA | 744.00± 100.90 | 690.00 ± 74.16 | 740.00± 143.18 | 620.00± 130.58 |
There was no significant difference in body weight among the groups.
PA= Post-administration
Source: own work
Effect of MSEAO on vital organs. Gross and histopathological examinations showed no significant (P > 0.05) lesions in the vital tissues (Bursa of Fabricius and spleen) examined as compared to the control group. See Figures 1 and 2.
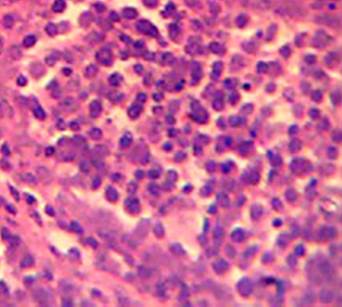
Source: own work
Figure 1 Bursa of Fabricius showing no obvious lesions 48 h post-administration. H&E X 400.
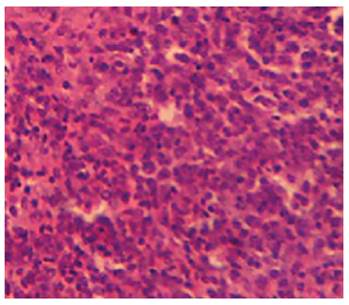
Source: own work
Figure 2 Spleen of chicken in group 1 showing no obvious lesions 48 h post-administration. H&E x 200.
Effect of MSEAO on haematology. There is no significant difference in PCV, RBC, HBC, and total WBC in all three treated groups as compared to group 4 (control). This is shown in Table 2. The heterophil count was significantly (P < 0.05) higher in chickens in groups 1 and 3 than those in group 2. The results of monocyte and eosinophil counts showed no significant (P > 0.05) variation across the groups.
Table 2 Haematological values of chicken administered with varied doses of Anacardium occidentale stem extract
| Mean values, with standard error of the mean in brackets | ||||
|---|---|---|---|---|
| Haematological Parameters | Group 1 (3000 mg/kg) |
Group 2 (1500 mg/kg) |
Group 3 (500 mg/kg) |
Group 4 (control) |
| Packed Cell Volume (%) | 29.00 (0.58) |
30.00 (1.32) |
29.17 (0.93) |
28.69 (1.21) |
| Haemoglobin concentration(g/dl) | 8.74 (0.17) |
8.93 (0.22) |
8.70 (0.20) |
8.69 (0.29) |
| Red Blood Cell count (106/µl) | 3.89 (0.18) |
4.02 (0.16) |
3.87 (0.18) |
3.68 (0.27) |
| Total White Blood Cell (103/µl) | 102.83 (14.62) |
108.33 (7.65) |
120.50 (13.19) |
105.64 (10.48) |
| Lymphocyte counts (103/µl) | 45.72 (7.17) |
66.99 (4.17) |
56.11 (9.53) |
53.21 (7.02) |
| Heterophil counts (103/µl) | 42.56 a (8.26) |
20.09 b (5.76) |
40.16 a (4.80) |
27.42ab (2.98) |
| Monocyte counts (103/µl) | 12.50 (2.51) |
19.77 (3.00) |
18.04 (5.32) |
18.01 (4.96) |
| Eosinophil counts (103/µl) | 2.06 (0.35) |
1.09 (0.08) |
3.61 (2.53) |
2.68 (0.29) |
a b c Alphabetical superscripts in a row indicate significant differences between the means of the groups, P < 0.05.
Source: own work
Effect of MSEAO on serum biochemistry. Serum AST activity did not vary significantly (P > 0.05) among all the groups, while the serum ALT activity was significantly (P < 0.05) lower in chickens in groups 1 and 2 as compare to those in groups 3 and 4. This is shown in Table 3. The effect on serum total protein, albumin, and globulin showed no significant (P > 0.05) variations among all the groups. Serum total cholesterol and triglyceride level were lower in all the groups administered the extract when compared to the control group. The serum uric acid and creatinine levels were significantly (P < 0.05) lower across all the treatment groups as compared to the control group (Table 3). Serum calcium level was significantly (P < 0.05) lower in groups 2 and 3 as compared to groups 1 and 4. There was a significant (p<0.05) decrease in the levels of serum uric acid and creatinine in all the treated groups.
Table 3 Changes in serum biochemistry parameters in chickens administered with graded doses of hydro-methanol stem extract from Anarcadia occidentale (MSEAO)
| Mean values, with standard error of the mean in brackets | ||||
|---|---|---|---|---|
| Serum Biochemistry Parameters | Group 1 (3000 mg/kg) |
Group 2 (1500 mg/kg) |
Group 3 (500 mg/kg) |
Group 4 (control) |
| Aspartate aminotransferase (IU/L) | 87.27 (1.96) |
86.90 (3.24) |
87.16 (1.82) |
91.89 (2.14) |
| Alanine aminotransferase (IU/L) | 7.71 a (0.39) |
7.22 a (0.25) |
10.92 b (1.24) |
9.81 ab (0.79) |
| Alkaline phosphatase (IU/L) | 544.41 a (1.02) |
535.44 b (2.46) |
551.25 c (1.54) |
540.04ab (1.81) |
| Total proteins (g/dl) | 3.94 (0.08) |
4.00 (0.04) |
4.12 (0.12) |
4.01 (0.09) |
| Albumin (g/dl) | 2.32 (0.04) |
2.38 (0.05) |
2.40 (0.02) |
2.30 (0.07) |
| Globulin (g/dl) | 1.63 (0.11) |
1.62 (0.08) |
1.70 (0.14) |
1.72 (0.13) |
| Total Cholesterol (mg/dl) | 118.76 (7.68) |
114.57 (8.33) |
117.52 (4.83) |
124.54 (3.41) |
| Triglyceride (mg/dl) | 87.99 (6.45) |
95.92 (3.97) |
90.30 (4.22) |
99.68 (4.83) |
| Uric acid (mg/dl) | 5.86 a (0.78) |
6.02 a (0.30) |
6.70ab (0.08) |
8.31 b (0.68) |
| Creatinine (mg/dl) | 0.30 a (0.009) |
0.29 a (0.12) |
0.28 a (0.152) |
0.36 b (0.019) |
| Calcium (mg/dl) | 9.75ab (0.24) |
9.10 b (0.23) |
9.25 b (0.20) |
10.08 a (0.29) |
| Phosphorus (mg/dl) | 4.40 (0.14) |
4.30 (0.12) |
4.64 (0.16) |
4.24 (0.16) |
a b Alphabetical superscripts in a row indicate significant differences between the means of the groups, P < 0.05.
Source: own work
Discussion
The effect of hydro-methanol stem extract from A. occidentalis on haematology and serum biochemistry in cockerels was investigated using standard procedures. No obvious gross and histopathological findings herein disagree with the results by Tédonget al. (35) and Omorodion et al. (3), who reported histopathological lesions in the liver.
Heterophils equivalents of mammalian neutrophils are critical to the immune defence of birds. Since MSEAO enhanced the level of heterophils in this study, it could boost the body defence system of birds. The significantly higher heterophil in chickens in groups 1 and 3 imply that the extract administered at these doses may be useful in the treatment and management of bacterial infections. This observation agrees with the reports by Baptiste et al. (36). ALT is a good parameter used in assessing the liver status in animals; the result of low ALT levels in the chickens in two groups that received the lower doses shows that the extract at these doses had a hepatocyte membrane-stabilizing effect and thus can be hepatoprotective in disorders and diseases associated with breaches in hepatocyte integrity (37,38). The stem extract from A. occidentale did not caused a significant effect on serum total protein, albumin and globulin in all treated groups, an indication that the extract had no effect on protein synthesis in chickens.
Serum total cholesterol and triglyceride level were lower across all the groups administered with MSEAO. These results suggest that the extract had a hypolipidemic effect. It also implies that the extract can be used to treat ailments associated with derangement in serum lipid profile. These observations support the findings by Olatunji et al. (39). Serum uric acid and creatinine levels are parameters used in the determination of the status and the functional integrity of the kidneys (40).
Elevated levels of these parameters indicate kidney damage and disease, and a decrease suggests protection. Since MSEAO significantly lowers the levels of serum uric acid and creatinine, it suggests that the stem extract may have a kidney protective effect. Renal diseases have been shown to be common in avian species (41); therefore, the significant lower levels of uric acid and creatinine in all three treated groups implies that the stem extract from AO may be important in the management of diseases that affect the kidney function. Calcium and phosphorus are important nutrients in poultry diet formulations, especially for bone formation and as enzyme cofactors (42, 43). In addition, calcium is essential to blood coagulation, eggshell formation, and muscle and nerve functions (44). Calcium and phosphorus homeostasis are largely under endocrine control -parathormone and hormonal form of Vitamin D3. In this study, MSEAO (500 and 1500 mg/kg) depleted serum calcium levels and enhanced phosphorus levels, which may adversely affect the functions.
In conclusion, the hydro-methanol stem extract from A. occidentale exhibited elevation of heterophils (improved immune system), reduced liver marker enzyme (hepatoprotection), hypolipidemic and kidney protective effects. However, the extract may affect the roles of calcium and phosphorus in chickens.















