INTRODUCTION
Toxoplasmosis is a parasitic disease, whose etiological agent is Toxoplasma gondii, an obligatory intracellular parasite of worldwide distribution, which infects almost all animal species (birds and mammals), including man. The definitive hosts are domestic and wild cats, all those non-feline hosts are intermediaries (1). Humans and other animals can become infected by ingesting tissue cysts from undercooked meat or food, or drink contaminated with oocysts shed in cat feces. It is a disease of difficult parasitological diagnosis since it is not easy to demonstrate the etiological agent and to establish the relationship between the infection and the disease; for this reason, the use of serological tests as indirect indicators of the infection is indispensable to make the diagnosis of the etiological agent, based on the presence of antibodies type immunoglobulin G or M (IgG or IgM), equivalent to chronic or acute infections, respectively.
The indirect hemagglutination test (IHT) is used as a routine clinical test in veterinary hospitals because of its level of sensitivity and ease of use (2). The test is based on the property of anti-T. gondii immunoglobulins to produce agglutination in the presence of cytoplasmic antigen-sensitized red blood cells and the parasite’s membrane. It is considered a reliable method for the determination of specific immunoglobulins with values of sensitivity 89.80% to 92.85%, specificity 96.60% to 100%, and efficiency 94.80% (1, 3,4,5).
In Venezuela, previous studies of exposure to T. gondii in captive neotropical wild felid species there are not known, and reports in domestic or stray animals are scarce. The aim of the present study was to determine the occurrence of anti-T. gondii in a) wild felids that live in confinement in two zoological institutions, and b) stray cats from a private animal shelter using the technique of indirect hemagglutination.
Materials & methods
Animal sample size
In both the zoos and the animal shelter, the total number of animals that could be sampled was chosen and defined by those directly responsible for each facility. This study does not evaluate in any way the management or housing protocols of the animals sampled. Although no information of feeding protocols available was observable on site, it was noted that all animals were fed with a variety of uncooked meat of different species (cattle, horse, sheep, pork, chicken, pigeon, fish, mice, rat, guinea pigs, rabbit), and commercial food.
Sample collection
Blood samples were collected by venipuncture from jaguars (Panthera onca Linnaeus, 1758), ocelots (Leopardus pardalis Linnaeus, 1758), and pumas (Puma concolor Linnaeus, 1771) kept in the zoos Metropolitano del Zulia, Zulia State, and Paraguaná, Falcón State, in northwestern Venezuela. As well as from a population of stray cats (Felis catus Linnaeus, 1758) kept in an animal shelter located in La Vela de Coro, Falcón State. A volume of 3 ml of blood was obtained from each animal. The blood samples were transported, within 2 h of collection, to the research laboratory and centrifuged for 10 min at 1500 g. The separated sera were stored frozen at (−20°C) until analysis.
Serological test
The presence of T. gondii antibodies was detected by an indirect hemagglutination test (Toxotest-HAI® Wiener Lab), which recognize antibodies (IgG, IgM) against T. gondii. The results are interpreted as negative when button-shaped sediment or regular edge ring is present, and positive with the formation of a film or mantle covering 50% or more of the bottom of the wells, equivalent to values ≥ 1:16 (cut-off point suggested by the manufacturer). It was taken as cut-off titer ≥ 1:64 modifying the cut-off value. To determine the presence of both heterophile antibodies and IgM, the positive sera were subjected to 2-mercaptoethanol (MO) titration, so it could be indicated if we are facing an acute or chronic infection.
Statistical analysis
Since this is a descriptive study, it was estimated the relative frequency as the percentage by analyzing the number of animals of each species reacting to toxoplasma. To determine whether significant differences existed between species, the Kruskal-Wallis test was applied, with a significance p ≤ 0.05.
Results
A total of 35 animals (4 jaguars, 3 pumas, 6 ocelots, and 22 stray cats) were evaluated by IHT. Any serum above the cut-off point was considered positive. The results revealed 80% of seropositivity for T. gondii. Jaguars (75.00%), ocelots (66.67%), and stray cats (95.45%). The pumas were all negatives (see Table 1).
Table 1 Data of species, age, and sexes of animals included in the study
| Subfamily | Males | Females | ||||
|---|---|---|---|---|---|---|
| Species | n | Age(yr) | + | - | + | - |
| Felinae | ||||||
| Felis catus Linnaeus, 1758 | 22 | >2 <6 | 10 | 0 | 11 | 1 |
| Leopardus pardalis Linnaeus, 1758 | 6 | >5 <9 | 2 | 1 | 2 | 1 |
| Puma concolor Linnaeus, 1771 | 3 | >5 <9 | 0 | 1 | 0 | 2 |
| Pantherinae | ||||||
| Panthera onca Linnaeus, 1758 | 4 | >5 <9 | 2 | 0 | 1 | 1 |
| Total | 35 | 14 | 2 | 14 | 5 | |
n= sample size, += positives, - = negatives
Source: own work
Of the total seven positive sera of wild cats, in jaguars, one showed titer 1:512, and two titers 1:2048, while in ocelots one showed titers of 1:64, one titer 1:256, one titer 1:1024, and one with titers 1:2048. Of the total of 21 positives sera from stray cats, 4 showed titers of 1:64, 4 titers of 1:128, 1 titer of 1:256, 1 titer of 1:512, 3 titers of 1:1024, and 8 titers of 1:2048 (Figure 1).
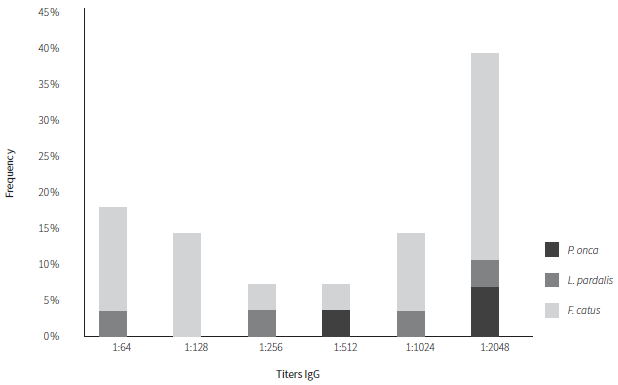
Source: own work
Figure 1 Anti-Toxoplasma gondii IgG titration. The bars represent the frequency of positive cases
High titers of 1:1024 or higher do not always correspond with acute infection. In sera from animals treated with 2-mercaptoethanol, the titers decreased by at least two dilutions, corresponding to the elimination of IgM; consequently, a total of eleven animals showed chronic infection (two jaguars, one ocelot, and eight stray cats). The Kruskal-Wallis test showed a statistically significant difference between the three species (H = 8.413, p = 0.015). However, between jaguars and ocelot, the seropositivity showed no differences (H = 0.508, p = 0.476).
Discussion
According to the author’s knowledge, this study reports for the first time the detection of antibodies against T. gondii in jaguars and ocelots in Venezuela. Similar studies have been conducted for both captive and free-living jaguars in French Guiana (6) and Brazil (7,8,9). The overall seropositivity for T. gondii in jaguars in the present study was lower than those other studies. For ocelots, the overall seropositivity (66.67%) was slightly different from the previous report in Bolivia (10), Brazil (11, 12), and Mexico (13). On the other hand, anti-T. gondii antibodies for T. gondii in stray cats in this study were significantly higher than those reported in stray cats (45.20%) in Bogota, Colombia (14), and in pet cats (48.30%) in San Carlos, Chile (15). However, it might not be possible to compare results because of the sample size and the serologic test used.
For South America, the global seropositivity observed for wild felids ranges from 41% up to 91% (16). Whereas for jaguars’ ranges from 63.5% up to 84%, ocelots from 58.1% up to 73.8% (17). For domestic cats, it has been observed from 29% up to 45% (16) and from 34% up to 49.6% (17).
It is important to note that both in the two zoos and the shelter tested wild birds (pigeons, vultures), dogs and stray cats, rodents (rats and mice), and insects (flies and cockroaches), can enter and leave without restriction. The above-mentioned insects are known to be defined as transport hosts for T. gondii, as they can spread the oocysts present in contaminated fecal material (18). Likewise, birds and rodents are described as intermediate hosts of great importance, which constitute the favorite living prey of cats (19). Additionally, all animals receive raw meat in their diet, which also provides an opportunity for infection with T. gondii. This can explain the high seroprevalence stated in this study among the tested cats (95.45%).
The difference in titers depends on the antigen profiles presented in each test for antibody detection. Under normal conditions, Ig is produced after infection and a cell-mediated response occurs, and those can be considered as markers of the acute or chronic phase of the infection (20). Classically, IgM is considered as a marker of the acute phase of a disease because it is the first to appear. However, it is known that IgM titers can remain detectable for many months or even years after a first infection. The absence of IgM, therefore, rules out recent infections. A cat with active toxoplasmosis will have a high IgM titer. The second marker is IgG. Its presence implies that the animal has been in contact with the parasite at some point in its life. Specific IgG appears at three weeks and a very high titer persists for a long time, especially in the case of cats that can remain up to five years. Therefore, this fact indicates that when only one IgG titer is observed, active toxoplasmosis cannot be suspected. This only indicates the presence of the antigen in the patient and not the disease.
In the current study, 50% of jaguars, 16.67% of ocelots, and 63.64% of stray cats were IgM positive, which may indicate a recent infection. Because IgM is occasionally also detected in the serum of animals with chronic infection, or as a false-positive IgM caused by autoimmune diseases (20), it is likely that not all tested animals with anti-T. gondii IgM had been recently infected.
The toxoplasma indirect hemagglutination test, reported in this study, can be used as a rapid screening test for toxoplasmosis because it has high sensitivity and specificity, is not very laborious, and is free of contamination risks.
Ethical statement
To carry out the study, approval was obtained through an informed consent read and signed by the administrative representatives of each facility. All protocols followed good practices and animal welfare principles set forth in the Law on the Practice of Veterinary Medicine Official Gazette No. 28.737 dated 24 September 1968 and the Law on the Protection of Wild Fauna Official Gazette No. 28.289 dated 11 August of 1970 and approved by Animal Research Ethical Committee.















