Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.24 no.1 Santa Marta Jan./Dec. 1995
BENTHIC COMMUNITIES AND GEOMORPHOLOGY OF THE TESORO ISLAND CORAL REEF, COLOMBIAN CARIBBEAN
Juan Armando Sánchez M.
Instituto de Investigaciones Marinas y Costeras-INVEMAR, AA. 1016, Santa Marta, Colombia.
ABSTRACT
The benthic communities distribution in Tesoro Island (Colombian Caribbean) coral reef was determined by cartography of reef morphology and functional groups from aerial photographs, theodolite triangularon, and bottom transects over depths ranging from 0 to 30 m. Tesoro Island is a sand cay reef developed over an emerged reef platform whose basal cone possibly originated by mud diapirism on the continental shelf. The benthic communities are distributed as subzones of the geomorphologic units. The reef crest lies along the breaker zone, formed by Millepora spp. buttresses covered on their tops by Palythoa spp. and seawardly by a turf of Dictyota spp. The rear reef (varying from 0.5-1.5 m in depth) is composed of pavement and grooves with live Porites porites and P. astreoides. The fore-reef terrace (2 to 9 m in depth) shows a low relief spur and grooves, composed of Acropora palmata skeletons on the windward side and A. cervicornis on the leeward side; sandy accumulations with dense and tall colonies of the gorgonaceans Pseudopterogorgia spp. are also appreciated. In the sandy reef platform there are monospecific patches of Montastraea annularis, P. porites, M. faveolata, dead Acropora cervicornis and wide sandy zones where patches of Halimeda monile are found. The slope edge, (depths ranging between 7 to >30m) shows mixed corals and gorgonaceans, at the drop-off, laminar corals, especially Montastraea franksi and Agaricia spp., jointly with other deep water organisms such as ellisellid gorgonaceans and antipatharians are found. The benthic communities distribution is influenced by reef geomorphology, wave energy diffraction and the past mass mortality of Acropora.
RESUMEN
Se determinó la distribución de las comunidades bentónicas de los arrecifes coralinos de Isla Tesoro (Caribe colombiano) cartografiando su morfología arrecifal y grupos funcionales mediante el uso de fotografías aéreas, triangulación con teodolito y transectos de fondo entre 0 y 30 m de profundidad. Isla Tesoro es un arrecife de cayo arenoso desarrollado sobre una plataforma arrecifal emergida cuyo cono basal posiblemente fue originado por diapirismo de lodo en la plataforma continental. La distribución de las comunidades bentónicas coincide con subzonas de las unidades geomorfológicas. La cresta arrecifal se dispone a lo largo de la rompiente, formada por contrafuertes de Millepora spp. cubiertos en la parte superior por Palythoa spp. y hacia el mar por un tapete de Dictyota spp. El arrecife trasero (0.5-1.5 m) esta compuesto por pavimento y canales, dominando Porites porites y P. astreoides. La terraza prearrecifal (2-9 m) con espolones y canales de bajo relieve, esta compuesta por esqueletos de Acropora palmata hacia barlovento y de A. cervicornis hacia sotavento, son también abundantes algunas acumulaciones arenosas con agregaciones de enormes colonias de Pseudopterogorgia spp. En la plataforma arrecifal arenosa se encuentran parches monoespecíficos de Montastraea annularis, Porites porites, M. faveolata, Acropora cervicornis muerto y extensas zonas arenosas con Halimeda monile. El talud (7 a más de 30 m) presenta hacia el borde, corales y gorgonáceos mixtos, y cuando aumenta la pendiente dominan los corales laminares, en especial Montastrea franksi y Agaricia spp., junto a otros organismos de aguas profundas como antipatarios y gorgonáceos elliséllidos. La distribución de las comunidades bentónicas está influenciada por la geomorfología arrecifal, la dispersión de la energía del oleaje y la pasada mortalidad de Acropora.
INTRODUCTION
Due to the complex distribution of benthic communities on coral reefs, it is necessary to develop studies in order to recollect detailed geographical and geomorphological information. This will be useful for following researchers in the selection of study areas. There are few published works for the Colombian Caribbean, which results in lack of detailed cartography of coral reefs, as compared with the information available from studies done elsewhere in the Caribbean (e.g. Saint John: Kumpf and Randal, 1961; Saint Croix: Adey, 1975; Dry Tortugas: Davis, 1982; Belize: Riitzler and Macintyre, 1982; Bonaire and Curasao: Van Duyl, 1985; among others). However, in Colombia we can mention the works of Geister (1975) at San Andrés Island, and Robertson and Cano (1987), Márquez (1987) and Geister (1992) at Providencia and Sta. Catalina islands and recently Díaz et al. (in press) at Courtown and Albuquerque atolls. At Tesoro Island, previous coral reef studies are restricted to general revisions of the composition and abundance of corals populations in the whole Rosario Archipelago (Pfaff, 1969; Werding and Sánchez, 1979; Sarmiento et al, 1989). Quintero et al (1993), in a preliminary work, a bioecological map of the island was achieved; however its geomorphology and benthic communities were not defined.
The origin of a coral reef is usually related to such classic types as fringing reefs, barrier reefs, atolls or banks. Their basic morphology changes according to their position in relation to the continent, waves or recent sea level changes among others (Geister, 1983). Tesoro Island is located on the continental shelf in a place where several geological processes were active in the Holocene, such as mud diapirism, recent tectonism and a continuous reef formation (Erffa and Geister, 1976; Vernette, 1986), making its origin difficult to categorize in the classical reef model. Its early evolution appears to have been a complex sequence of events. The purpose of this study is to give a detailed map of the actual geomorphology and of the benthic communities found in Tesoro Island reef and also to present an explanation about its origin.
STUDY AREA
Tesoro Island (Treasure Island) is a small island located in the Rosario Archipelago of the Caribbean coast of Colombia (northern South America, 10°14'13" N - 75°44'36" W) (Fig. 1). This archipelago is a National Natural Park since 1988 and Tesoro Island lodges a permanent military post for the Colombian Army. Actually, the island is not influenced by tourism. Tesoro is lying in the path of the Intertropical Convergence Zone under trade winds dominion, which alternate from northwest to southeast. The average annual sea-water temperature is 27.5°C and the average annual precipitation of 1013.1 mm, typical of dry tropical climate. The main current has the same direction as the northern winds which are stronger between December and April during the dry season. In the rain season, May through November, the Colombian current comes from the south, and it has an temporary influence on the archipelago area by bringing continental runoff from the Bahía de Barbacoas (Fig. 1). In 1992, I observed waves breaking throughout the year at the northern side of the island. The average salinity in the area is 35 mg/l (Martinez and Vernette, 1981; Sanchez, 1989). Additional information about the study area is available from Sanchez et al (1992), Quintero et al. (1993), and Sanchez and Ramírez (1994).
MATERIALS AND METHODS
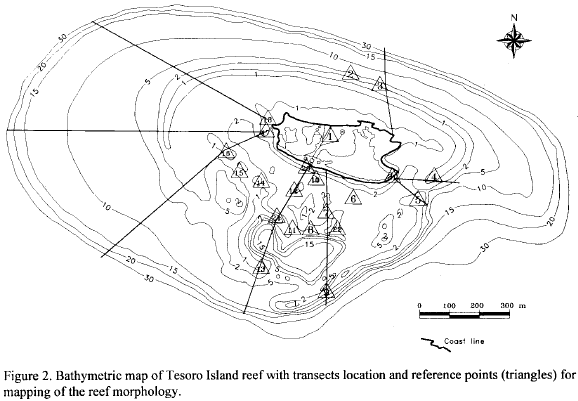
Preliminary cartography of Tesoro Island coral reefs was drawn by the interpretation of black and white aerial photographs (scale: 1:40000) and oblique color photographs taken during a low altitude flight (altitudes ranging from 100 to 300 m) above the island; from these, an approximate map scale 1:15000 was composed. The delineation of reef morphology was based on sandy zones, dense reef and reef slope patterns, as recommended by Hopley (1978). The position calibration of the distinguished reef features was made during 1992 in two phases. The first, the land phase, was accomplished by the implementation of the theodolite triangulation method (Stoddart, 1978) measuring the horizontal angles of 23 projecting reef points (Fig. 2) from two previously selected and fixed points on the island. The triangulation of these points improved the position of the reef features on the map and permitted to correct calculated distance erros. The second phase was achieved by skin and SCUBA diving tasks, in this phase 8 transects perpendicular to the island shore that crossed previously defined reference points already located (Fig. 2).
In each transect the bathymetry was recorded from the island shore down to 30 m of water depth; the substrate types (mud, sand, rubble and coral rock) and the most important functional groups in the sessile macrobenthic communities (hermatypic corals, sea grasses, turf algae, fleshy macro algae, calcareous macro algae, crustose coralline algae, sponges, gorgonaceans and antipatharians) were registered. These components were ranked in situ every two meters with an observation field approximately 5 m in width, in both sides of the line transect, and using a subjective frequency scale (+++= very common, ++= common, += occasional, -= rare), modified from Rutzler and Macintyre (1982). Also, a manta board tows survey was practiced in order to complete the observations.
For the zonation and reef geomorphology the terminology of Geister (1983), Battistini et al. (1976), Burke (1982) and Rutzler and Macintyre (1982) was applied. The most frequent taxonomic groups were identified in the field. The hermatypic corals were identified following Wells (1973), Cairns (1982) and Fenner (1993). The species of the Montastraea complex recently reviewed by Weil and Knowlton (1994) were identified from video records taken in 1992. For the conspicuous algae the field guide of Littler et al (1989) was employed. Gorgonaceans octocorals were collected, dried and identified according to Bayer (1961).
RESULTS
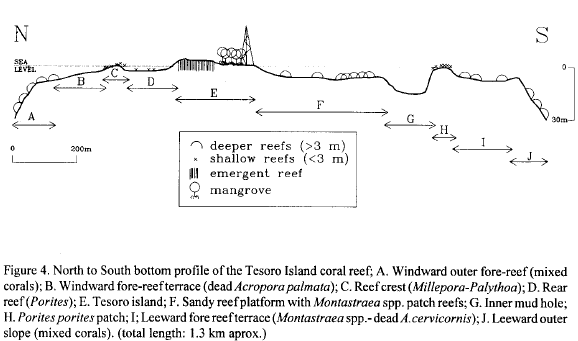
The Tesoro Island reef-complex is approximately 2.5 km in length and 1.5 km in width, displaying an ovoid shape (Fig. 3). Five main geomorphologic zones are evident (Figs. 3 and 4).
Sandy reef platform: an extensive sand plain of lagoonal environment, 0-7 m deep, on the leeward side of the island; in it there are scattered large Montastraea annularis and M. faveolata patches (Fig. 5d), stands of Acropora cervicornis skeletons colonized by algae, and shallow patches of Porites porites; in this zone there is a mud hole with 15 m in depth (Fig. 3).
Rear reef: is a narrow flat located behind the crest platform, ranging from 0.5 to 1.5 m in depth, which levels towards the sandy platform (Fig. 5b); it is formed by pavement with shallow grooves and presents scattered growth of Porites porites and P. astreoides corals.
Reef crest: lies at the breaker zone (Figs. 5a, b), with depths varying between 0 and 2 m. It is the most pronounced relief of the reef (Fig. 4) and it is composed by buttresses of the hydrocorals Millepora complanata and unidentified Millepora spp. covered on the top by the zoanthids Palythoa spp.
Fore-reef terrace: also refered as inner fore-reef is located seaward in front of the crest, with depths varying from 2 and 9 m (Fig. 5b); it is the largest zone of the reef, nearly forming a fully circular shape. It is characterized by a low relief spur and groove system. Its basal substratum on the windward side is composed of dead Acropora palmata stands, which are replaced gradually by A. cervicornis at the leeward side.
Outer slope: or outer fore-reef is between 7 and 40 m in depth, the live coral growth reaching more than 30 m in depth on the windward side. The edge begins with a mixed corals and gorgonaceans zone at the windward side that gradually becomes a low ridge of A. cervicornis skeletons at the leeward side. The drop-off (40-70° of inclination angle) is colonized by platy corals, especially Montastraea franksi and Agaricia spp.
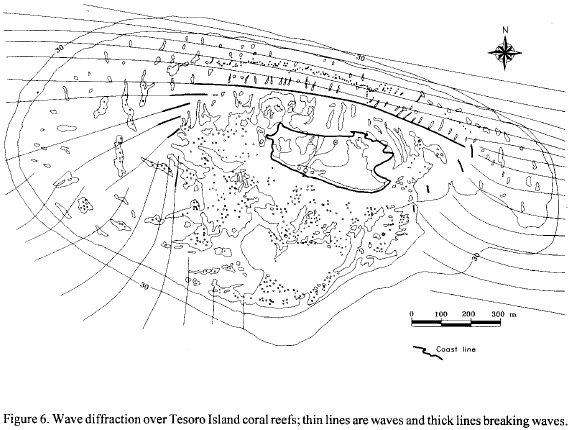
The morphology of the zones is clearly influenced by diffraction of waves over the reef, which decrease in potency towards the eastern and western sides until the harmonic movement is lost in the south (Fig. 6). It is also influenced by the ancient morphology of a bank reef platform.
Benthic communities
Twelve properly defined communities or multispecies assemblages, were recognized and mapped (Fig. 7) according to the abundance of benthic components (about 80 species of sessile organisms). Reef building corals are represented by 40 species and 10 forms (see appendix for systematic list).
Patches of Montastraea annularis and M. faveolata: the sandy reef platform, 2 to 7 m in depth, positioned nearby the cay, shows numerous and extensive patches of M. annularis. Seaward, these patches are a mixture of M. annularis with M. faveolata, and occasionally found with M. franksi in deep parts of the calm turbid waters of the mud hole. In some places it is possible to find the three species together.
Their colonies exhibit massive growth, reaching areas ranging from 1 and 7 m in diameter. On the sides and undersides they support diverse benthic assemblages composed of turf algae, crustose coralline algae, frondose algae such as Galaxaura oblongata, Amphiroa tribulus, Halimeda opuntia and H. tuna, the anemone Condylactis gigantea, the gorgonians Briareum asbestinum and Plexaura homomalla, the small hydrocoral Stylaster roseus, many crustose sponges, and few other corals such as Isophyllastrea rigida, Agaricia tenuifolia, Siderastrea siderea and Diploria spp.
Stands of Porites porites: in some areas of the rear reef and the sandy reef platform, in conditions of moderate wave energy and shallow depth (0.05-1.5 m), there are dense monospecific stands of Porites porites, which in some cases cover an uninterrupted surface areas lager than 50 m2. The inferior parts of the branches are densely colonized by frondose algae such as Halimeda opuntia, Amphiroa tribulus, Anadyomene sp. and Glacilaria sp.; also by many epibionts like the foraminifera Homotrema rubrum and Peyssonnelia sp. and other crustose coralline algae. Sometimes colonies of Porites astreoides and the hydrocorals Millepora alcicornis and M. complanata are found. These flats of P. porites contribute with a large amounts of calcareous rubble to the reef sediments.
Stands of Porites astreoides: in the rear reef behind the reef crest there are large areas composed of P. astreoides crusts of 5 to 40 cm in diameter, in depths from 1 to 2 m. Generally, it is the only organism found there, growing over a substratum composed basically of pavement, rubble and sand. Close to the crest this stand mixes with other species such as Agaricia tenuifolia, P. porites, Millepora complanata, Palythoa spp., some crustose algae, Halimeda opuntia, Dictyopteris delicatula and extensive beds of filamentous turf algae. In the rear reef mixed stands of P. porites and P. astreoides occasionally conform a particular assemblage with abundant algae such as H. opuntia, Amphiroa tribulus, Laurencia sp. and Dictyota spp.
Buttresses of Millepora complanata and Palythoa spp.: in conditions of very high wave energy in the breaker zone, it is possible to find the association between the hydrocorals M. complanata and Millepora spp. with the zoanthids Palythoa spp., which occupy nearly 100% of the reef flat substratum. The hydrocoral builds buttresses (0-2 m in depth) transversed by admission grooves, and the zoanthid covers the superior surface. The sides of these buttresses have many attached organisms such as Agaricia tenuifolia, Porites porites, P. astreoides, Plexaura flexuosa, Dictyopteris delicatula, Halimeda opuntia and several species of crustose coralline algae (i.e. Lythophyllum, Porolithon etc.).
Seagrasses: toward the southern coast of the island, in the leeward region beyond the beach, there are a few beds of Halodule found in depths ranging from 0.5 to 1 m, being the only significant sea-grass meadows in these reefs. On this sea-grass beds, some algae such as Padina sp., Caulerpa cupressoides, C. sertulariodes, Dictyota spp. and Sargassum polyceratum, exist among others. In the west side of the island near the coast and nearby the small fossil reef rocks, there is a small meadow of the algae Avranvillea digitata with turtle grass Thalassia testudinum. They are rooted in thick sand substratum with some rubble.
Stands of Halimeda monile: on the sandy reef platform and in the sandy channels of the fore reef, there are numerous H. monile algae rooted in the sand, in depths varying from 2 to 6 m. Usually, it is one of the few epibenthic colonizers. It is also possible to find it with Dictyota sp. and, in some places where there is rubble accumulation, with Udotea spp. and Penicillus pyriformis. At the inner slope of the mud hole, it is possible to find it with Lobophora variegata and occasionally with H. incrassata and also with branching sponges such as Aplysina spp.
Dead Acropora palmata: on the windward fore reef terrace there is an extensive monospecific stand of dead skeletons of A. palmata and some areas of bare calcareous bottom with scattered corals. These Acropora skeletons are almost completely collapsed. Only a few sites present live colonies or skeletons in life-position. Growing over the dead corals there are many species of crustose coralline algae, especially Lithophyllum sp., and of filamentous turf algae, brown sponge Cliona aprica, and occasionally some corals such as Diploria strigosa, Siderastrea siderea and Montastraea faveolata, the zoanthid Palythoa caribaeorum, and the hydrocoral Millepora alcicornis. The gorgonacean Pterogorgia citrina is generally found.
Dead Acropora cervicornis: on the leeward fore-reef terrace there are large monospecific stands of A. cervicornis found in depths from 2 to 9 m. Actually in full death, but still mostly in live-position properly cemented by crustose coralline algae and Peyssonelia spp. The dead coral is covered by an algal community composed of Galaxaura oblongata, Amphiroa tribulus and Dictyota sp. In depths of 5 m and beyond the dead coral is covered by Lobophora variegata. It is also possible to find some corals, especially Millepora alcicornis, Colpophyllia natans, Eusmilia fastigiata, Mussa cubensis and Porites porites. Recolonization foci by live A. cervicornis are very rare and often invaded by filamentous algae and damselfish territories.
Mixed corals and gorgonaceans: a the windward edge of the slope, just above the drop-off, in depths from 7 to 15 m, there is a community of organisms with the greatest number of coral and gorgonacean species in the whole reef-complex. It consist of the scleractinian corals Montastraea annularis, M. faveolata, M. franksi, Colpophyllia natans, Siderastrea siderea, Madracis decactis, M. miriabilis, Diploria strigosa, D. labyrinthiformis, Mycetophyllia ferox, M. danaana, Eusmilia fastigiata, Porites astreoides, P. porites, Isophyllia sinuosa, Leptoseris cucullata, Mussa angulosa, M. cubensis and Meandrina meandrites among others, and the gorgonaceans Pseudopterogorgia spp., Plexaura spp., Pterogorgia citrina, P. homomalla, Plexaurella spp., Muriceopsis flavida, Eunicea spp., Muricea spp. and Gorgonia ventalina, just to mention some.
Pseudopterogorgia zone: in the windward fore-reef terrace over large sand accumulation in depths ranging from 5 to 10 m, there are many gorgonacean colonies, until 2 m in height, of Pseudopterogorgia americana, P. rigida and P. acerosa, which form a dense forest.
Dictyota spp. turf: in the seaward margin of the reef crest the hard substratum, in depths ranging approximately from 1.5 to 3 m, is colonized by several species of Dictyota. The species with greater coverage is the creeping and iridescent Dictyota cervicornis and D. pfaffi, while D. jamaicensis and D. ciliolata are fixed to the substratum with the thalli constantly moving due to the wave motion. It is also possible to find Stypopodium zonale, Laurencia sp. and Amphiroa hancockii jointly with other reef-crest species such as Millepora alcicornis, Palythoa spp. and the gorgonacean Plexaura flexuosa.
Outer slope: in the deep slope zone, in depths varying from 15 to 60 m, there is a community formed by corals with laminar or platy shape, especially Montastraea franksi, M. cavernosa, Diploria labyrinthiformis, D. strigosa, Porites astreoides, Leptoseris cucullata, Colpophyllia natans, Mycetophyllia aliciae, M. lamarckiana, M. reesi, Madracis decactis, M. miriabilis, M. formosa, Agaricia agaricites (several forms), A. lamarcki, A. undata and A. fragilis. There is also a large cover of the alga Lobophora variegata, crustose coralline algae and Peyssonelia sp. and some tubular sponges of different sizes. There are also black corals (Antipatharia), these are generally found beyond 15 m depth, under or in the sheltered sides of platy corals; the wire coral Sticopathes spp. is the most common. The majority of antipatharians occur in large reef buttresses where very dense aggregates of Antipathes gracilis, A. atlantica, A. pennacea and Antipathes sp. are found jointly with large colonies of the azoxanthellated gorgonacean Iciligorgia schrammi. Among these, Ctenocella schmitti is the most numerous, forming dense aggregations under laminar corals. In this zone the sea whip C. barbadensis can reach 2 m in height. Some zooxanthellated gorgonaceans such as Pseudopterogorgia bipinnata, P. americana, Pseudoplexaura spp., Eunicea spp. and Muricea laxa among others, tend to disappear with increasing water depth between about 30 and 60 m.
DISCUSSION
Tesoro Island is a sand cay reef formed over an emergent Holocene coral reef; the recent reef complex has a platform composed by large amounts of sand and rubble on the leeward, but it has neither a developed lagoonal terrace, nor an extensive seagrass flat (Battistini et al., 1975); this makes it different from other conventional reef types. The geomorphological zonation of the sand cay reef of Tesoro Island reflects both its geological origin and wave energy diffraction. Its size is the same of the subrecent development of the coral bank platform, when it emerged was exposed to a very regular direction of waves at the windward side. Its benthic communities, as others in the Caribbean, change in composition according to the decrease of wave energy (Geister, 1977; 1983). However, Tesoro Island case is slightly different to the standard example of Caribbean reef (Grauss and Macintyre, 1989). It is important to note the absence of an outer Acropora cervicornis zone below A. palmata on the windward side, as known from other Caribbean coral reefs (Goreau, 1959; Riitzler and Macintyre, 1982) as well as from Isla Grande, the main island of the Rosario archipelago (Coral and Caycedo, 1983). The seasonal effect of the 'Northers', the main perturbation of the West Indian trade wind circulation, on exposed reefs at Tesoro Island, may cause abrasion on the windward fore-reef terrace, as well as breakage and death to both A. cervicornis and A. palmata, forming an active abrasional platform or hardground (Geister, 1992). Tesoro Island don't lie in a hurricane belt, however, 'Joan' in 1988 had slightly affected these area, unafortunately post-hurricane records or observations at Tesoro reefs are not available. A comparable reef to Tesoro Island is found at Lobos Island, off the east coast of Mexico (Rigby and Maclntire, 1966). But there are morphologic differences between both reefs, possibly due to their different origins.
The benthic communities of Tesoro island are composed of a group of species adapted to determined environmental conditions related to water depth, wave exposure and the basic type of substratum. This view agrees with that of many authors who consider that the reef geomorphology or the physical environment are responsible for community organization (Liddell and Olhorst, 1987; Jaap et al., 1989, among others).
Possible origin of Tesoro Island reef
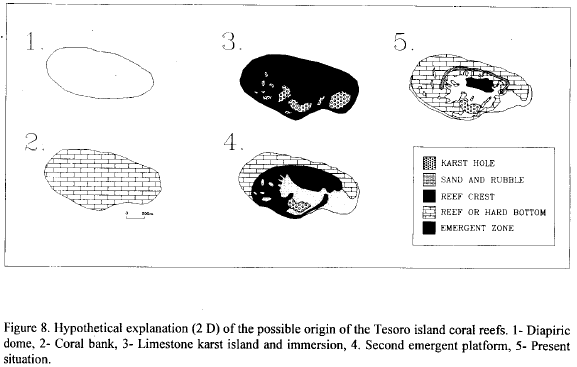
As shown by seismic data, the Rosario Archipelago originated by progressive coral colonization on banks of diapiric origin formed at the continental shelf (Vernette, 1986). The regional geomorphology, the actual Tesoro Island reef, subrecent corals preserved in the cay, as well as the presence of emerged terraces in the other archipelago islands, suggest clues for the development of a hypothesis to explain the origin of Tesoro Island reef and also to better understand some aspects of its evolution. In the proposed hypothesis (Fig. 8) the exact time and sequence of events of reef formation are unknown; thus, these stages must remain speculative until new and conclusive evidence by further research.
- Diapiric dome: as shown by seismic data, mud diapirism at the inner continental shelf was originated from movements of unconsolidated material forming muddly layers that crossed the overlying beds to form a dome-like topography. The diapirs change the morphology of the shelf, with a series of domes oriented 30°N, according to the structure of the Sinu belt (Vernette, 1986; Vernette, 1989). The Tesoro island dome may have been smaller than the present reef. The age of its formation is uncertain, but it possibly dates back to the Plio-Pleistocene, similar to tectonic that took place at nearby Tierra Bomba and Baru Islands, and at Tortuga Bank (Vernette, 1986).
- Coral bank: Apparently, when the diapiric dome reached the photic zone, it was colonized by hermatypic corals with the subsequent topography flattening as the reef developed (Vernette, 1986). The formation of the coral bank began with growth of massive and foliaceous corals on top of the diapiric dome. The same species (i.e. Montastraea spp.) could take platy shapes on the reef slopes. This first step for the reef development, usually takes place in calm or deep waters (Geister, 1983).
- Limestone karst island: During the last glacial stages the sea level was 60 to 100 m lower than the actual and the coast line was close to the northern side of Tesoro island (Vernette, 1986). Before the last transgression, the Tesoro bank reef emerged and formed a large island, with erosion processes producing karst holes in the platform, like the one that become the actual inner "mud hole". It is possible that a stillstand during the transgression subsequently molded the fore-reef terrace.
- Tectonic elevation: on the subrecent plain of the cay an extensive reef emerged composed principally by the scleractinian coral Agaricia tenuifolia followed in abundance by Acropora cervicornis, Montastraea faveolata, Colpophyllia natans and Siderastrea siderea is preserved. Taking these species as sea level and environmental indicators (Geister, 1984), the top of this platform possibly reaches to 5 to 12 m of water depth. The corals of the emergent reef of Tesoro Island date from 2780 ± 120 years according to dates by C14 (Vernette, 1986). This age agrees with the more than 10 m in height tectonic elevation, found in the reef terraces of Tierra Bomba and Bani islands, few kilometers away, which took place no more than 3000 years ago (Erffa and Geister, 1976; Geister, in. litt.) creating Tierra Bomba, Bani, Grande and Rosario islands as well as the reef platforms, such as found in Tesoro Island (Vernette, 1989). This catastrophic event is the most important for the origin of Tesoro island sand cay reef, due to it, the reef platform emerged, producing great erosion in the reef and also sand accumulation on the leeward region. Wave action formed an enormous sandy platform and the "mud hole" is gradually filled with sediments.
- Actual sand cay reef of Tesoro Island: as the sea level rose to the present level, heavy reef platform erosion took place. On the new substrata reef organizing facies of began to develop, eventually reaching superficial waters. Because of the actual stability of sea level structures, the reef crest was formed at the same place where it is today. The reef crest protects a limited area of the subrecent plain were the cay was formed. During this stage, important reef facies were formed such as monospecific stands of Acropora palmata on the windward side, and A. cervicornis and reef patches of Montastraea spp. and Porites porites in the leeward side. At this stage the reef started to develop, reaching a structural climax in the windward side where in front of the crest there are large areas of A. palmata and A. cervicornis, and mixed corals towards the slope (Grauss et al., 1987), the low relief spur and groove system developed in the A. palmata zone. Acropora species easily colonized sand and hard substrata, in sites of low and high wave energy respectively (Geister, 1983; Multer and Zankl, 1985).
Present situation
The sand cay reef of Tesoro Island is marked by large areas of substratum covered by Acropora palmata and A. cervicornis skeletons, whose mass mortality occurred approximately twelve years ago. Werding and Sánchez (1979) described these zones when they were alive, and some years later Coral and Caycedo (1983), Ramírez (1986), Sarmiento et al. (1989) and Garzón-Ferreira and Kielman (1993) when they were dead. This mortality also took place in many areas of the Caribbean (Jordán, 1992) sponsoring changes in the geomorphology of the reefs. The presence of A. palmata stands in the inner fore-reef terrace is record of water depth levels in the development of a reef framework (Macintyre and Glynn, 1976). Since, this is one of the rapid growth species, it helps to build structures against wave abrasion, originating the spur and grooves systems which formed by growing coral colonies and by erosion channels which admit wave energy (Shinn et al, 1981), the latter being appropriate for coral-octocorals communities (Riitzler and Macintyre, 1982). However, with the death of Acropora, the structures of high and moderate relief that were beginning to form spurs were flattened by bioerosion. As the colonization of this species at 2 to 5 m depth is slower than the rate of abrasion by waves (Jordán, 1992), a gap is created due to the appearance of new low relief substrata where the abrasion of unconsolidated sediments does not permit the settlement of coral larvae of species unadapted to abrasive environments (Jordán, 1989). The fore-reef terrace of Tesoro Island, where the stand of Acropora palmata once existed, originally exhibited a moderate relief, which became a low relief abrasion terrace. On the leeward side where A. cervicornis dominated, the recolonization process is slower, possibly due to the large quantity of algae covering the substratum, thereby, making the recolonization by corals very difficult. There is also strong grazing pressure on the recruits and surviving colonies of this species, which after mass mortalities, may have carried populations to a collapse (Knowlton et al, 1990).
On the other hand, it is also important to note that numerous axial skeletons of Gorgonia ventolina in the shallow fore-reef near to the crest are possibly an indicator that a past mass mortality occurred in this zone (Garzón-Ferreira and Zea, 1992). However, gorgonaceans of the genus Pseudopterogorgia and possibly Pterogorgia are capable of colonizing sand accumulations or abrasive areas, such as dead Acropora palmata stands. Their larvae are able to bury in sand until they find a hard substratum. In addition, the sun light reflection over the sand greatly benefits those species associated with zooxanthellae (Kinzie, 1973). Also Briareum asbestinum, thanks to its successfully stolon-vegetative propagation (Lasker, 1983), colonizes hard substrata in low energy environments as dead A. cervicornis stands.
At the present time, the largest scleractinian coral development is on the slope edge and on the sandy reef platform with low wave energy, where the most important species are Montastraea annularis, M. faveolata and M. franksi These species are sedimentation resistant (Macintyre and Grauss, 1982) and indicate the lowest wave energy level areas in the reef (Geister, 1977; Grauss and Macintyre, 1989). However, on heterogeneous environments they seem to have a bathymetric separation, M. franksi being the deepest and M. faveolata the shallowmost species (Van Veghel, 1994). Recently, their taxonomical status has been discussed (Van Veghel and Bak, 1993; Weil and Knowlton, 1994). Although the slope is an area of low energy area and appropriate for coral development, the coral cover is not as high as it should be, perhaps due to the presence of the brown unpalatable algae Lobophora variegata, which inhibits coral larvae recruitment (Kinzie, 1973; Jordán, 1989). The reason for its abundance could be the Caribbean-wide mass mortality of the sea urchin Diadema antillarum, which occurred some years ago, which was one of the few consumers of this alga (Lessios et al, 1984).
Particular to Tesoro Island reef are the small beds of Thalassia testudinum and Avrainvillea digitata, being this the only locality where the latter alga is found in all the Rosario Archipelago (Díaz-Pulido per. com.). Sea-grasses are poorly developed at Tesoro Island reef possibly due to the absence of a 'boat channel', which would offer a more suitable place for sea-grass growth. Other algae such as Dictyota jamaicensis and Stypopodium zonale among others collected in Tesoro Island were first records for the Rosario Islands (Bula et al., 1993). The slope with laminar corals offers to black corals sites sheltered from light, ideal places for their larval settlement (Ortiz and Sánchez, 1992). On the slope there are also numerous ellisellids, azooxanthellated gorgonaceans showing a characteristic red color, that seem to share the same habitat of the antipatharians (Sánchez, 1992).
Finally, it is necessary to note that the method used to prepare the basic map is considered by Stoddart (1978) as very expensive because it is necessary to have additional people and some special conditions in the reef, such as fixed marks, usually placed in varying depths with the tips showing clearly above the sea level. However, in Tesoro Island many marks were found on the reefs that were ideal for the mapping process. It is possible to suggest as a conclusion that the employment of joint aerial photographs, transects and triangulation, is one of the best methods for reef mapping and results in a clear structures definition.
ACKNOWLEDGMENTS
I wish to express my sincere acknowledgment to Dario and Nohora de Sánchez for their support and assistance. To Alvaro Ramírez for his valuable collaboration. To Jörn Geister (U. Bern, Switzerland) and Sven Zea (U. Nacional-INVEMAR, Colombia) for their valuable comments and criticisms. The present work is part of a B.Sc. thesis for the Marine Biology program of University of Bogotá Jorge Tadeo Lozano (UJTL), in collaboration with the Centro de Investigaciones Oceanógraficas e Hidrográficas de la Armada Nacional (CIOH), Cartagena. Thanks to Ricardo Quintero and Orlando Herrera from CIOH. To Pierre-Ives Genet from the Technical French Mission (1992). To Juan Manuel Diaz and the project "Evaluación bioecológica y ambiental de áreas arrecifales del Caribe colombiano" from the Instituto de Investigaciones Marinas y Costeras-INVEMAR (Programa de Ecosistemas Marinos) where this paper was written and to Monica Puyana for her kind collaboration.
LITERATURE CITED
1 Adey, W. H. 1975. The algal ridges and coral reefs of St. Croix: their structure and Holocene development. Atoll. Res. Bull., 187: 1-67. [ Links ]
2 Bayer, F. M. 1961. The shallow water Octocorallia of the West Indian region. Studies of the fauna of Curacao and other Caribbean islands, XII. 373 p. [ Links ]
3 Battistini, R., F. et al. (23 authors more). 1975. Elementes de terminologie récifale indopacifique. Téthys, 7(1):1-111. [ Links ]
4 Bula, M.G., G. Díaz and A. Celis. 1993. Adiciones a las macroalgas de los arrecifes coralinos de las islas del Rosario, con nuevos registros para el Caribe colombiano y el Atlántico. An. Inst. Invest. Mar. Punta Betín, 22: 21-29. [ Links ]
5 Burke, R.B. 1982. Reconnaissance study of the geomorphology and benthic communities of the outer barrier reef platform, Belize, p. 509-526. In K. Rützler and I. Macintyre (eds) The Atlantic barrier reef ecosystem at Carrie Bow Cay, Belize. I. Smithsonian Inst. Press, Washington. [ Links ]
6 Cairns, S.D. 1982. Stony corals (Cnidaria: Hidrozoa, Scleractinia) of Carrie Bow Cay, Belize: 271-302. In K. Rützler and I. Macintyre (eds): The Atlantic barrier reef ecosystem at Carrie Bow cay Belize. I. Smithsonian. Contr. Mar. Sci., 12. [ Links ]
7 Coral, D. A. and A. Caycedo. 1983. Descripción de la formación arrecifal de isla Grande (Islas del Rosario) con anotaciones ecológicas. B.Sc. Thesis, Univ. Jorge Tadeo Lozano, Bogotá. 110 p. [ Links ]
8 Davies, G. 1982. A century of natural change in coral distribution at the Dry Tortugas: a comparison of reef maps from 1881-1976. Bull. Mar. Sci., 32: 608-623. [ Links ]
9 Díaz, J.M, J.A, Sánchez, S. Zea and J. Garzón-Ferreira. Morphology and marine habitats of two Southwestern Caribbean atolls: Albuquerque and Courtown. Atoll Res. Bull, (in press). [ Links ]
10 Erffa, A. Von and J. Geister. 1976. Über ein holozänes Korallen und Mangrovenvorkommen nahe Santa Marta, Kolumbien. Mitt. Inst, colombo-alemán Invest, cient., 8: 165-186. [ Links ]
11 Fenner, D. 1993. Species distinctions among several Caribbean stony corals. Bull. Mar. Sci., 53 (3): 1099-1116. [ Links ]
12 Garzón-Ferreira, J. and M. Kielman. 1993. Extensive mortality of corals in the Colombian Caribbean during the last two decades. Proceedings of the Colloquium Global aspects of coral reefs: Health, Hazards and History. Univ. Miami: 247-253. [ Links ]
13 Garzón-Ferreira, J., and S. Zea. 1992. A mass mortality of Gorgonia ventalina (Cnidaria:Gorgoniidae) the Santa Marta area, Caribbean coast of Colombia. Bull. Mar. Sci., 50: 523-526. [ Links ]
14 Geister, J. 1975. Riffbau und geologische Entwicklungsgeschichte der Insel San Andrés (westliches Karibisches Meer, Kolumbien). Stuttgarter Beiträge zur Naturkunde, 15: 1-203. [ Links ]
15 Geister, J. 1977. The influence of wave exposure on the ecological zonation of Caribbean reefs: 23-29 In D. Taylor (ed): Proceedings of the Third International Coral Reef Symposium Miami Univ. Press. [ Links ]
16 Geister, J. 1983. Holozäne westindische Korallenriffe: Geomorphologie, Ökologie und Fazies. Facies, 9: 173-284. [ Links ]
17 Geister, J. 1984. Die paleobathymetrische Verwerbarkeit der scleractinen Korallen: 46-95. In H.P. Luterbacher: Paläobathymetrie Paläontologische Gesellschaft, München. [ Links ]
18 Geister, J. 1992. Modern reef Development and Cenozoic Evolution of an Oceanic Island/Reef Complex: Isla de Providencia (Western Caribbean Sea, Colombia). Facies, 27: 1-70. [ Links ]
19 Goreau, T. 1959. The ecology of Jamaican coral reefs. I. Species composition and zonation. Ecology, 40: 67-90. [ Links ]
20 Graus, R.R. and I.I. Macintyre E. 1989. The zonation patterns of Caribbean coral reef as controlled by wave and light energy input, bathymetric setting and reef morphology: computed simulation experiments. Coral Reefs, 8: 9-18. [ Links ]
21 Graus, R.R., I.I. Macintyre E. and B.E. Herchenroder. 1984. Computer simulation of the reef zonation at Discovery Bay, Jamaica: Hurricane disruption and long-term physical oceanographic controls. Coral Reefs, 3: 59-68. [ Links ]
22 Hopley, D. 1978. Aerial photography and other remote sensing techniques 23-43. In D.R. Stoddart and R.E. Johannes (eds): Coral reefs: research methods. UNESCO Monograph on Oceanographic Methodology 5. Page Brothers, London. [ Links ]
23 Jaap, W.C., W.G. Lyons, P. Dustan and J.C. Halas. 1989. Stony coral (scleractinia and milleporina) community structure at bird key reef, ft Jefferson.National Monument, Dry Tortugas, Florida. Fla. Mar. Res., 46:1-31. [ Links ]
24 Jordán, E. 1989a. Efecto de la morfología del sustrato en el desarrollo de la comunidad coralina. An. Inst. Cienc. Mar y Limnol. Univ. Nal. Autón. México, 16(1): 105-118. [ Links ]
25 Jordán, E. 1989b. Gorgonian community structure and reef zonation patterns of Yucatán coral reefs. Bull. Mar. Sci., 45: 670-696. [ Links ]
26 Jordán, E. 1992. Recolonization patterns of Acroporapalmata in a marginal environment. Bull. Mar. Sci., 51:104-117. [ Links ]
27 Kinzie, R.A. 1973. The zonation of West Indian gorgonians. Bull. Mar. Sci., 23:93-155. [ Links ]
28 Knowlton, N. J.C. Lang and B.D. Keller. 1990. Case study of natural population collapse: post-hurricane predation on Jamaican Staghorn corals. Smithsonian Contr. Mar. Sci., 31: 1-25. [ Links ]
29 Kumpf, H.E. and H.A. Randall. 1961. Charting the marine environments of St. John, U.S. Virgin Islands. Bull. Mar. Sci., 11: 543-551. [ Links ]
30 Lasker, H. R. 1983. Vegetative reproduction in the octocoral Briareum asbestinum (Pallas). J. Exp. Mar. Biol. Ecol., 71(2): 57-169. [ Links ]
31 Lessios, H.A., J.D. Cubit, R.D. Robertson, M.J. Shulman, M.R. Parker, S.D. Garrity and S.C. Levings. 1984. Mass mortality of Diadema antillarum on the Caribbean coast of Panama. Coral Reefs, 3: 173-182. [ Links ]
32 Liddell, W.D and S.L. Ohlhorst. 1987. Patterns of reef community structure North Jamaica. Bull. Mar. Sci., 40: 311-329. [ Links ]
33 Littler, D. M, M. Littler, K.E Bucher and J. N. Norris. 1989. Marine plants of the Caribbean. Smithsonian Inst. Press, Washington D. C, 263 p. [ Links ]
34 Macintyre, I.G. and P.W. Glynn. 1976. Evolution of modern Caribbean fringing reef, Galeta Point, Panamá. American Association of Petroleum Geologists Bulletin, 60(7): 1054-1072. [ Links ]
35 Macintyre, I.G. and R.R. Grauss. 1982. Variation in growth forms of the reef coral Montastraea annularis (Ellis and Sollander): a quantitative evaluation of growths response to light distribution using computer simulation. 441-464. In Rützler, K and I. Macintyre (eds): The Atlantic barrier reef ecosystem at Carrie Bow cay Belize. I. Smithsonian. Contr. Mar. Sci., 12. [ Links ]
36 Márquez, G. 1987. Las islas de Providencia y Santa Catalina. Fondo Fen-Univ. Nacional de Colombia, Bogotá, 110 p. [ Links ]
37 Martínez, S.H. and G. Vernette. 1981. El complejo arrecifal de las islas del Rosario, zonación coralina, sedimentos y foraminíferos bentónicos. Rev. CIAF., 6(1-3): 329-345. [ Links ]
38 Multer, H.G and H.Zankl. 1985. Distribution and origin of Holocene patch reef in the West Indies. Proc. of the fifth Int. Coral Reef Symp. Tahiti., 3: 227-228. [ Links ]
39 Pfaff, R. 1969. Las Scleractínias y Milleporina de las islas del Rosario. Mitt. Inst. Colombo-Alemán de Invest. Cient., 3: 17-24. [ Links ]
40 Ortiz, V, and J.A. Sánchez. 1992. Las comunidades del bajo arrecifal profundo Imelda, Isla Barú. Caribe colombiano. VI. Estructura de la comunidad de corales negros (Antipatharia). Mem. VIII Sem. Nal. Cienc. Tec. Mar. Santa Marta, Colombia, I: 341-349. [ Links ]
41 Quintero, R. , J.A. Sánchez and A. Ramírez. 1993. Cartografía bioecológica de isla Tesoro. Caribe colombiano. Boletín Científico CIOH., 13: 45-64. [ Links ]
42 Ramírez, A. 1986. Ecología descriptiva de las llanuras madreporarias del Parque Nacional Corales del Rosario. Fondo FEN, Bogotá, 71 p. [ Links ]
43 Rigby, J. K and W. G. Macintire. 1966. The Isla de Lobos and Associated Reefs, Veracruz, Mexico. Coast. Stud. Inst.. Tech. Rep., 42: 3-42. [ Links ]
44 Robertson, K. and M. Cano. 1987. Teledección del sistema coralino de isla de Providencia, Colombia. Rev. CIAF., 251-260. [ Links ]
45 Rützler, K and I. Macintyre. 1982. The habitat distribution and community structure of the barrier reef complex at Carrie Bow Cay: 9-45. In K. Rützler and I. Macintyre (eds): The Atlantic barrier reef ecosystem at Carrie Bow cay Belize. I. Smithsonian. Contr. Mar. Sci., 12. [ Links ]
46 Sánchez, H. 1989. El Parque Nacional Natural Coralesdel Rosario (PNNCR). Bull. Inst. Bassind'Aquitaine, Bourdeaux., 45: 205-213. [ Links ]
47 Sánchez, J. A. 1992. Las comunidades del bajo arrecifal "Imelda" Isla Barú, Caribe colombiano. V. Estructura de la comunidad de gorgonáceos (Cnidaria: Octocorallia). Mem. VIII Sem. Nal. Cienc. Tec. Mar. Santa Marta. Colombia, I: 328-340. [ Links ]
48 Sánchez, J. A. and A. Ramírez. 1994. Descripción, composición y estructura de las comunidades coralinas de los arrecifes de isla del Tesoro. Caribe colombiano. B.Sc. Thesis, Univ. Jorge Tadeo Lozano, Bogotá, 130 p. [ Links ]
49 Sánchez, J. A., A. Ramírez and R. Quintero. 1992. Estudio de reconocimiento de la morfología y las comunidades bénticas de los arrecifes coralinos de isla del Tesoro (PNNCR). Caribe colombiano. Mem. VIII Sem. Nal. Cienc. Tec. Mar. I. CCO, Bogotá; 263-276. [ Links ]
50 Sarmiento, E., F. Flechas and G. Alvis. 1989. Evaluación cuantitativa y del estado actual de las especies coralinas del Parque Nacional Natural Corales del Rosario (PNNCR) Cartagena. Colombia. B.Sc. Thesis, Univ. Jorge Tadeo Lozano, Bogotá, 143 p. [ Links ]
51 Shinn, E.A, J.H. Hudson, D. Robbin and B. Lidz. 1981. Spur and grooves revisited: construction versus erosion, Looe Key reef, Florida. 476-483. In E.D. Gomez, CE. Birkeland, R.W. Buddemeier, R.E. Johannes, J.A. Marsh and R.T. Tsuda. (eds): Vol 1. Proc. 4th. Int. Coral. Reef Symp., Manila. [ Links ]
52 Stoddart, D. R. 1978. Mapping reefs and islands. 17-22. In D.R. Stoddart and R.E. Johannes (eds): Coral reefs: research methods. UNESCO Monograph on Océanographie Methodology 5, Page Brothers, London. [ Links ]
53 Van Duyl, L. F. 1985. Atlas of the living reefs of Curaçao and Bonaire (Netherlands Antilles). Thesis, Vrije Universiteit, Amsterdam, 37 p. [ Links ]
54 Van Veghel, M. L. J. 1994. Polymorphism in the Caribbean reef building coral Montastrea annularis. Ph.D. dissertation, Univ. of Amsterdam. 129 p. [ Links ]
55 Van Veghel, M. L. J., and R.P.M. Bak, 1993. Intraspecific variation of a dominant Caribbean reef building coral Montastrea annularis: genetic behavorial and morphometric aspects. Mar. Ecol. Prog. Ser., 92: 255-265. [ Links ]
56 Vernette, G. 1986. La plateforme continentale Caraïbe du Colombie. Importance du diapirisme argileux sur le morphologie, et la sédimentation.Thèse doctorale. Mém. del Inst. Géol. Bassin d'Aquitaine, 20: 1-387. [ Links ]
57 Vernette, G. 1989. Impact du diapirisme argileux sur les récifs de la plateforme colombienne des Caraïbes. Bull. Inst. Géol. Bassin. d'Aquitaine, Bordeaux, 45: 97-105. [ Links ]
58 Weil, E and N. Knowlton. 1994. A multi-character analysis of the Caribbean coral Montastraea annularis (Ellis and Solander, 1786) and its two sibling species, M. faveofata (Ellis and Solander, 1786) and M. franksi (Gregory, 1895). Bull. Mar. Sci., 55(1): 151-175. [ Links ]
59 Wells, S.J. 1973. New and old scleractinian corals from Jamaica. Bull. Mar. Sci., 23(1): 16-58. [ Links ]
60 Werding, B. and H. Sánchez. 1979. Situación general y estructuras arrecifales Informe faunístico y florístico de las islas del Rosario en la costa norte de Colombia. An. Inst. Inv. Mar. Punta de Betín, 11: 7-20. [ Links ]
APPENDIX
Systematic list of reef corals found in the Tesoro island coral reef, Colombian Caribbean, during 1992.
Phyllum COELENTERATAClass HIDROZOA Owen, 1843
Order MILLEPORINA Hickson, 1901
Family MILLEPORIDAE Fleming, 1901
Millepora alcicornis Linnaeus, 1758
Millepora complanata Linnaeus, 1758
Millepora spp.
Class ANTHOZOA Ehrenberg, 1834
Order SCLERACTINIA Bourne, 1900
Suborder ARCHENOCAENIIDA Alloiteau, 1952
Family ACROPORIDAE Verrill, 1901-1902
Acropora cervicornis (Lamarck, 1816)
Acropora palmata (Lamarck, 1816)
Family STYLOPHORIDAE Milne Edwadrs and Haime, 1857
Madracis decactis (Lyman, 1859)
Madracis miriabilis (Duchassaing and Michelotti, 1861)
Madracis formosa Wells, 1973
Suborder ASTRACOIDA Alloiteau, 1952
Superfamily ASTRAEOIDAE Alloiteau, 1952
Family FAVIIDAE Gregory, 1900
Favia fragum (Esper,1788)
Diploria labyrinthiformis (Linnaeus, 1758)
Diploria clivosa (Ellis and Solander,1786)
Diploria strigosa (Dana, 1846)
Colpophyllia natans (Houttuyn, 1772) (=C. breviserialis)
Montastraea cavernosa (Linnaeus, 1766)
Montastraea annularis (Ellis and Solander, 1786)
Montastraea faveolata (Ellis and Solander, 1786)
Montastraea franksi (Gregory, 1895)
Family COLUMNASTRAEIDAE Alloiteau, 1952
Stephanocoenia intersepta (Esper, 1795) (= S. michelinii)
Superfamily MUSSIOIDAE Alloiteau, 1952
Family MUSSIDAE Ortmann, 1890
Mussa angulosa (Pallas, 1766)
forma lacera (Pallas, 1766)
Mussa (=Scolymia) cubensis (Milne Edwards and Haime, 1849)
Isophyllia sinuosa (Ellis and Solander, 1786)
Isophyllastrea rigida (Dana, 1848)
Mycetophyllia lamarckiana Milne Edwadrs and Haime, 1849
forma hydnophoroida Zlatarski y Martinez, 1982
Mycetophyllia aliciae Wells, 1973
Mycetophyllia ferox Wells, 1973
Mycetophyllia danaana Milne Edwadrs and Haime, 1849
Mycetophyllia reesi Wells, 1973
Suborder MEANDRIIDA Alloiteau, 1952
Family DENDROGYRIIDAE Alloiteau, 1952
Dichocoenia stokesi Milne Edwadrs and Haime, 1848 (=D. stellaris)
Family MEANDRIIDAE Alloiteau, 1952
Subfamily MEANDRIINAE Vaughan and Wells, 1943
Meandrina meandrites (Linnaeus, 1758)
Subfamily EUPHYLLINAE Alloiteau, 1952
Eusmilia fastigiata (Pallas, 1766)
forma flabellata Wells, 1973
Suborder FUNGIIDA Duncan, 1884
Superfamily THAMNASTERIOIDAE Alloiteau, 1952
Familia AGARICIIDAE Gray, 1847
Agaricia agaricites (Linnaeus, 1758)
forma agaricites (Linnaeus, 1758) (=crassa; =masiva)
forma cannata Wells, 1973 (=bifaciata)
forma purpurea (Lesueur) (=unifaciata)
forma humilis Verrill 1901
forma danai Milne Edwards and Haime, 1851
Agaricia tenuifolia Dana, 1848
Agaricia fragilis Dana 1860
forma contrada Wells, 1973
Agaricia lamarcki Milne Edwards and Haime, 1851
Agaricia undata (Ellis and Solander 1786)
Leptoseris cuculiata (Ellis and Solander, 1786)
Superfamily SYNASTRAEOIDAE Alloiteau, 1952
Family SIDERASTRAEIDAE Vaughan and Wells, 1943
Siderastraea radians (Pallas, 1766)
Siderastraea siderea (Ellis and Solander, 1786)
Superfamily PORITIOIDAE Alloiteau, 1952
Familia POR1TIDAE Gray, 1842
Porites porites (Pallas, 1766)
forma furcata (Pallas, 1766)
Porites astreoides Lamarck, 1816